Abstract
Context: Eucalyptus camaldulensis Dehnh. (Myrtaceae) and Eucalyptus torelliana F. Muell are used in Nigerian traditional medicine for the treatment of cough associated with tuberculosis (TB) and other respiratory infections.
Objective: Hexane, chloroform, methanol extracts, and isolated compounds of E. camaldulensis and E. torelliana were screened for activity against Mycobacterium tuberculosis H37Rv (MtbH37Rv) to authenticate the traditional use of these plants.
Materials and methods: The microplate alamar blue assay (MABA) method was used to investigate the anti-M. tuberculosis activities. Bioassay-guided fractionation of the hexane extract of E. torelliana leaf was performed, and isolated compounds were characterized by MS, 1D- and 2D-NMR.
Results: The extracts inhibited the growth of MtbH37Rv [minimum inhibitory concentration (MIC) 4–64 µg/mL]. Spectroscopic characterization led to the identification of two compounds, hydroxymyristic acid methylester (1) and a substituted pyrenyl ester, a sterol (2). Compounds 1 and 2 had MIC of 49.45 and 46.99 µg/mL; IC50 >100 and 38.21 µg/mL; selectivity index (SI) >2.02 and 0.81, respectively, and a minimum bactericidal concentration (MBC) of 62.50 µg/mL.
Discussion and conclusions: The anti-TB activities of these plants on M. tuberculosis H37Rv support their use in traditional medicine for the treatment of coughs associated with TB and reveals the presence of anti-Mtb active compounds in the plants. These findings not only demonstrate a new potential area of therapeutic value of E. camaldulensis and E. torelliana, but also illustrate the role of esters as anti-Mtb active principles in ethnobotanical preparations and as lead compounds in the development of new and effective anti-Mtb drugs.
Introduction
Tuberculosis (TB) is one of the leading causes of death resulting from infectious diseases, and an estimated two million people die from tuberculosis annually (CitationWHO 2008). Mycobacterium tuberculosis (Mtb), the etiologic agent of TB, has infected one-third of the world’s population, making it a significant global health problem (CitationWHO 2008). Mycobacterial infections that were once thought to have been overcome have recently re-emerged due to the HIV/AIDS pandemic. The resurgence of TB now poses a serious threat to healthcare worldwide, especially with the emergence of multi-drug (MDR) and extensively drug-resistant (XDR) TB (CitationJones et al., 2008). The current anti-Mtb treatments involve a long course of a combination of antibiotics with serious adverse effects. This leads to poor patient compliance and, consequently, the development of resistance and has been a contributing factor to emerging drug-resistant Mtb strains. There is now an urgent need to discover and develop new anti-Mtb drugs particularly to target drug resistance, and improve the treatment of latent TB by targeting tubercle bacilli that are thought to remain within the lungs in a non-replicating state of persistence (CitationQuenelle et al., 2001). The use of traditional herbal remedies (ethnomedicines) for the treatment of TB is increasing in developing countries, and these plants are an excellent starting point for the development of new effective anti-Mtb drugs.
Eucalyptus (Myrtaceae) species are indigenous to Australia, but are naturalized in many parts of Africa, including Nigeria (CitationBhatti et al., 2007). Many Eucalyptus species are known for their medicinal uses, as the essential oil is a common ingredient in many medicinal and herbal preparations. The bark and the leaves of Eucalyptus species are used for cold and cough, influenza, toothaches, fevers, sore throat and other infections (CitationFarah et al., 2002; CitationKim et al., 2001). A poultice prepared from the leaves is applied to wounds and ulcers to reduce infection and stimulate healing. In Nigeria, Eucalyptus camaldulensis Dehnh. and E. torelliana F. Muell have been used for the treatment of coughs associated with TB and other forms of respiratory tract infections. The essential oils of E. camaldulensis and E. torelliana have been reported to have antimicrobial activities (CitationOyedeji et al., 1999). E. camaldulensis is a folk remedy for colds, colic, coughs, diarrhea, dysentery, hemorrhage, laryngalgia, laryngitis, pharyngitis, sore throat, spasm, trachalgia, and wounds. While the antimicrobial activities of Eucalyptus essential oils have been extensively investigated, there has been very little research into the antimicrobial effects of the other extracts of Eucalyptus species. We have reported the antimicrobial activities of E. camaldulensis and E. torelliana in our earlier investigations (CitationAdeniyi et al., 2006a,Citationb; Citation2009), and now for the first time report the anti-Mtb activity of E. camaldulensis and E. torelliana extracts.
Materials and methods
Plant collection
The leaves and stem bark of E. camaldulensis and E. torelliana were collected within the campus of the University of Ibadan, Nigeria and authenticated by the Herbarium Staff of the Department of Botany, University of Ibadan and Forest Research Institute of Nigeria (FRIN) Herbarium. Voucher specimens were deposited at FRIN with the herbarium number (FHl 104908 and FHI 104909 for E. camaldulensis and E. torelliana, respectively). The leaves and stem bark were air-dried and pulverized using an electric grinder.
Extraction and isolation
A 2.5 kg pulverized sample each of the leaves and stem bark of E. camaldulensis and E. torelliana was extracted successively with n-hexane, chloroform, and methanol using a Soxhlet extractor. An aliquot (30.0 g) of n-hexane extract of E. torelliana leaf was fractionated by column chromatography (CC) eluting with a stepwise gradient of n-hexane:ethyl acetate (100:0–0:100). Fraction 2 (1300 mg, 95:5–90:10 n-hexane:ethyl acetate solvent system) was further fractionated by reverse phase preparative TLC on a C-18 column with methanol:water (98:2), and methanol:chloroform (98:2) as mobile phases and subfraction 2a (1150 mg, yellow, semi-solid, Rf = 0.51) was re-chromatographed by reverse phase flash chromatography over a C-18 silica gel column with a MeOH-H2O gradient from 70% MeOH in H2O to 100% MeOH to obtain compounds 1 (266 mg, Rf = 0.68, white solid) and 2 (66 mg, Rf = 0.85, yellow semi-solid), the purity of which were determined by analytical RHPLC (dC18, 3 µm, 4.6 × 150 mm column, 100% methanol mobile phase.
Structure elucidation of pure compounds
The molecular formulas for the pure compounds were determined by LC/MS method at ambient temperature on the Shimadzu IT-TOF (Ion Trap-Time of Flight) Prominence LC (LC20 AD) machine. NMR analysis was done on a 400 MHz Bruker® Avance® machine. The pure compounds were subjected to 1D (1H, 13C and DEPT-Q) and 2D (COSY and HSQC) experiments for the structure elucidation.
Anti-Mtb biological assays
Bacterial strain for anti-Mtb biological assays
MtbH37Rv (ATCC 27294) bacterium was grown in 100 mL of Middlebrook 7H9 broth (Difco, Detroit, MI) supplemented with 0.2% (V/v) glycerol (Sigma Chemical Co., Saint Louis, Mo.), 10% (v/v) OADC (oleic acid, albumin, dextrose, catalase; Difco), and 0.05% (v/v) Tween 80 (Sigma), also referred to as 7H9GC-T80.
Susceptibility testing of Mtb H37Rv ATCC 27294 and determination of minimum inhibitory concentration (MIC)
Anti-Mtb susceptibility testing of extracts was determined in the fluorometric microplate alamar blue assay (MABA) method as described by CitationCollins et al. (1997) in black, clear-bottomed, 96-well microplates, (black view plates; Packard Instrument Company, Meriden, Conn.) in order to minimize background fluorescence. Initial dilution of the extracts and compounds were prepared in dimethylsulfoxide (DMSO) (200 µL) and subsequent two-fold dilutions were performed in 100 µL of 7H12 medium in the microplates. The test concentrations ranged between 128–1 µg/mL for the crude extracts and 100–0.390 µg/mL for the compounds. Rifampicin (RMP, Sigma; concentrations at 4–0.0156 µg/mL) was used as a positive control and the solvent (DMSO) as a negative control.
Mtb H37Rv ATCC 27294 strain was diluted in 7H12 media to reach approximately 2 × 105 CFU/mL, and 100 µL was added to individual wells containing the extracts yielding a final volume of 200 µL and final inoculum of 1 × 105 CFU/mL in each well.
Wells containing extracts only were used to detect auto-fluorescence of extracts. Additional control wells consisted of bacteria-only and medium-only. Plates were incubated aerobically at 37°C. At day 7 of the incubation, 20 µL of Alamar Blue solution (Trek Diagnostic Systems Cleveland, Ohio) and 12.5 μL of 20% Tween 80 were added to all of the wells, and the plates were re-incubated at 37°C for 24 h. Fluorescence was measured in a Victor III multilabel fluorometer (Perkin Elmer Life Sciences Inc., Boston, MA) in bottom-reading mode with excitation at 530 nm and emission at 590 nm. Anti-TB activity was recorded as percentage inhibition of ≥90% relative to the mean of replicate bacteria-only controls after incubation for 7 days. The MICs were determined after incubation for 7 days at 37°C, defining percent inhibition as 1-(test well fluorescence units/mean FU fluorescence units of triplicate wells containing only bacteria) × 100. The MIC values refer to the lowest concentration at which samples exhibited an inhibition of ≥90% relative to the mean of replicate bacteria-only controls.
Cytotoxicity assay
Evaluation of the cytotoxic activity of compounds 1 and 2 in Vero cells (African green monkey kidney cells) was performed as described previously (Cantrell et al., 1996) using the CellTiter 96 aqueous nonradioactive cell proliferation assay (Promega Corp., Madison, WI). The IC50 was defined as the reciprocal dilution resulting in 50% inhibition of the Vero cells. In addition, selectivity index (SI) values were determined as the ratio of cytotoxicity over the MIC. The cytotoxicity was determined by exposing the Vero cells to different concentrations of the extracts. Stock solutions of the extracts were prepared at 10 mg/mL, the positive control rifampin (RMP) at 50 mg/mL in DMSO. Geometric three-fold dilutions were performed in 96-well clear cell culture plates using the cell culture medium MEM (Gibco, Grand Island, NY) supplemented with 10% of fetal bovine serum (HyClone, Logan, UT). Final DMSO concentrations did not exceed 1% v/v. Sample dilutions were distributed in duplicate in 96-well tissue culture plates (Becton Dickinson Labware, Lincoln Park, NJ) at a volume of 50 µL per well. An equal volume containing 5 × 105 Vero cells (CCL-81; American Type Culture Collection, Rockville, MD) was added to each well and incubated at 37°C in an atmosphere of 5% CO2 in air. After 72 h, cell viability was measured using the CellTiter 96 aqueous nonradioactive cell proliferation assay (Promega Corp., Madison, WI) according to the manufacturer’s instructions. Absorbance 490 nm was read in a Victor III reader (Perkin Elmer Life Sciences Inc., Boston, MA). The IC50 was determined using a curve-fitting program.
Results
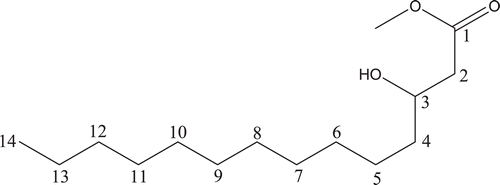
The crude extracts of E. camaldulensis and E. torelliana inhibited the growth of the Mtb H37Rv strain by ≥90% in the concentrations tested (). MICs were determined and the results are shown in . The hexane extracts of E. camaldulensis stem bark and E. torelliana leaf exhibited significant anti-Mtb activities with an MIC value of 4 and 8 μg/mL, respectively. These results suggested the presence of good antimicrobial potency and a high concentration of active principles in these plants. Effective inhibition is due to the high content of active principles in the extracts. Phytochemical screening of these plants revealed the presence of tannins, saponins and other triterpenes (cardiac and anthraquinone glycosides). Bioassay-guided fractionation of the active hexane extract of E. torelliana leaf yielded compounds 1 and 2 which had MIC value of 49.45 and 46.99 µg/mL; IC50 of >100 and 38.21 µg/mL; SI of >2.02 and 0.81, respectively and minimum bactericidal concentration (MBC) 62.50 µg/mL for both compounds. Molecular formula determined by LC/MS analysis gave the molecular formula as C15H30O3 and the molar (monoisotopic) mass as 258.21 for compound 1 with ionization in the negative mode.
Table 1. Anti-Mtb activity of lyophilized crude extracts of Eucalyptus camaldulensis (Ec) and E. torelliana (Et) on Mtb H37RvATCC 27294.
Table 2. Anti-Mtb activity of pure compounds 1 and 2 against Mycobacterium tuberculosis H37RvATCC 27294.
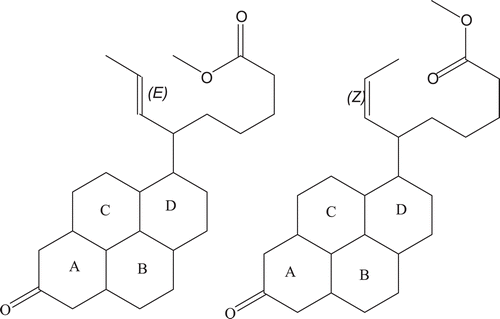
Structure elucidation by ID (1H and 13C) and 2D (COSY and HSQC) NMR analysis showed that compound 1 is a hydroxy fatty acid ester (). It is a saturated compound with melting point of 39°C. The molecular formula for compound 2 was predicted to be C25H38O3 while the molar (monoisotopic) mass was 386.29. Structure elucidation by ID (1H and 13C) and 2D (COSY and HSQC) NMR analysis () showed that compound 2 is a substituted pyrenyl ester.
Table 3. Proton NMR (1H) of compound 1.
Table 4. Proton NMR (1H) of compound 2.
Carbon-13 NMR of compound 2
13C NMR (CDCl3, 400MHz): δ 51.23(C-1), 168.93 – 169.57(C-1′, carboxylic acid), 32.36 – 32.74(C-2), 22.27 – 22.69(C-2′), 36.38 – 36.48(C-3), 23.22 – 23.37(C-3′), 26.11 – 26.29(C-4), 23.93 – 24.16(C-4′), 42.12 – 42.18(C-5), 24.32 – 24.38(C-5′), 38.12 – 38.15(C-6), 24.69 – 24.88(C-6′), 213.58 – 213.63(C-7, ketonic), 76.68 – 77.31(C-7′), 48.04 – 48.13(C-8), 112.30 – 112.95(C-8′), 36.98 – 37.35(C-9), 29.22 – 30.37(C-10), 39.13 – 39.57(C-11), 34.09(C–12), 55.232(C-13), 55.223(C–14), 55.44(C-15), 40.83(C-16) and 19.47 – 19.71 (methyl carbon).
Compound 1–hydroxymyristic acid methyl ester
A total number of 13 proton signals were observed. The proton NMR revealed several sets of highly shielded protons. The proton signals occurring at 0.919–0.96 ppm appeared as a doublet centered between 0.893–0.908 ppm. The latter protons are consistent with methyl signals of a straight-chain hydrocarbon and it is thus assigned. On the HSQC experiments, the protons centered on 0.893–0.908 had direct couplings with carbons having chemical shift values of 16, 18, 22, 28 and 32. Thus, there is a probability that these protons may still be representative of some other highly shielded protons other than the methyl protons. Successive increment of CH2 leads to further deshielding of the protons, hence, subsequent protons are assigned chemical shift values from 1.2 and 1.3–1.8. The proton centered on 1.2 ppm is assigned to carbon number 13 and this couples directly with the 0.919–0.96 ppm doublets (on C-14) protons. However, the multiplet centered between 1.3–1.8 ppm could be either of C-12, C-5 or C-6 protons judging from the coupling patterns and the direct coupling with carbon having chemical shift values of 37 ppm. Some of the other protons occurring at 2.775 and 3.21–3.25 ppm could be due to the successive CH2 groups beyond C-6 protons. Highly diagnostic, however, were the C-2 protons that had direct coupling (HSQC experiment) with a carbon having a chemical shift of 58 ppm. These protons occurred as multiplets and coupled with 1H signals centered on 1.3–1.8 ppm. However, the weak, broad singlet occurring at 3.558 ppm could not be assigned readily as it did not give any consistent direct coupling with a carbon atom. The hydroxyl ester side chain provided some clear-cut signals that were assigned with little ambiguity. The singlet occurring at 3.671 ppm is consistent with methyl protons present on a highly de-shielded center and, thus, this is assigned to the methyl group linked to the ester. The carbonyl group provided another strong deshielding influence on some proton signals and hence C-2 protons are assigned chemical shift value of 2.49 ppm. The hydroxyl group, however, showed a better deshielding effect; hence, the C-3 protons are assigned 3.86–3.90 ppm. A weak signal was also observed at around 5.9 ppm that occurred as a singlet. This variable signal may likely be due to the hydroxyl proton. With the mass spectrum result giving a base peak at 257.2123 m/z, and other physicochemical properties of compound 1 (appearance - white solid, soluble in organic solvents and melting point ∼39°C) giving values related to myristic acid, suggests that compound 1 is a myristic acid methyl ester (http://www.nmrdb.org/cheminfo/servlet/org.). Compound 1 is a wax and waxes are esters of fatty acids with long chain monohydric alcohols (one hydroxyl group). Natural waxes are often mixtures of such esters, and may also contain hydrocarbons.
Compound 2 – a substituted pyrenyl ester
A total of nine proton signals were observed, with three giving clear-cut and consistent 1H-1H and HSQC coupling patterns. The mass spectrum gave a base peak of 387.2000 m/z and some physicochemical properties give indication to a predicted structure consistent with a substituted prenylated ester. The proton signals gave some highly shielded values. The proton centered on 0.84 ppm occurred as a triplet and it coupled with protons having chemical shift value of 0.89 ppm. These protons are assigned to protons on rings B, C or D. Some of the other protons on the rings are assigned chemical shift value of 1.23 ppm. Several other singlets were observed in the proton NMR-spectrum. Highly diagnostic are three of the singlets. The singlet occurring at 2.41 pm is assigned to the carbon adjacent to C=O linkage on ring A whereas the singlets 3.27 and 3.44 ppm are consistent for protons on the ethylenic side chain. The suspected compound being a methyl ester, the singlet occurring at 3.62 ppm is assigned to the methyl protons. However, the singlet occurring at 7.29 ppm depicts an aromatic proton. Since the suspected molecule has no aromatic skeleton, this could be a contamination. The 13C spectrum gave carbon signals with some multiplicities. Since 13C is often recorded as completely J modulated spectra, there is the possibility that the compound contains a mixture of isomers. Further evidence for suggesting the presence of isomers is the ethylenic linkage that provides the possibility of a cis-trans isomerism on the molecule. Some diagnostic carbon signals are present in 13C NMR spectra recorded. The signals occurring around 19.467–19.711 is representative of methyl carbons. Some of the carbon signals gave direct coupling with some of the protons. For example, carbons centered on 26.109–26.915 ppm coupled directly with 1H centered on 2.13 ppm. These protons also had direct couplings with carbons having chemical shifts of 29.216–30.371 ppm; 39.131–39.567 ppm and 42.124–42.182 ppm. The carbons centered on 42.124–42.182 ppm also had direct coupling (HSQC experiment) with protons centered on 0.84 and 0.89 ppm. These carbons are most likely those present in rings B, C and D. The second compound gave a signal at 213.581 and 213.625 ppm, which is consistent with ketonic group in ring A. The two sets of ketonic signals once again confirm the presence of a mixture of isomers. In addition, diagnostic is the carboxylic acid carbonyl carbon with chemical shift values of 168.931 and 169.574 ppm.
Discussion
The eradication of Mtb has been a major objective of treatment strategies worldwide. Unfortunately, none of the treatment regimens have been able to achieve a 100% eradication rate. Effective eradication of Mtb is difficult due to the unusual structure and chemical composition of the mycobacterial cell wall, which hinders the entry of antibiotics into the cell thus making many antibiotics ineffective (CitationBrennan & Nikaido, 1995). Another reason underlying this failure is the rapid emerging resistance among many strains of Mtb towards various known antibiotics. Due to rapidly emerging resistant strains of TB, there is a great interest in developing new anti-mycobacterial drugs from plants.
Plant-derived anti-mycobacterial compounds belong to an exceptionally wide diversity of chemical classes, including alkaloids, terpenoids, coumarins, peptides and phenolics (CitationWeigenand et al., 2004; CitationTan et al., 2008; CitationDeng et al., 2008). The anti-Mtb activity exerted by the crude extracts of E. camaldulensis and E. torelliana is due to the presence of tannins and triterpenes (saponins, cardiac and anthraquinones glycosides) all of which have been reported to possess good anti-mycobacterial activities against Mtb and other mycobacterial species (CitationAdeniyi et.al., 2004; CitationMacabeo et al., 2005; CitationKim et al., 2009; CitationGordien et al., 2009). The ability of these Eucalyptus extracts to inhibit the growth of Mtb is significant because the mycobacterial cell wall outer membrane is thought to act as a barrier to many substances including antibiotics.
Because the anti-Mtb activity of the extract can be more broadly ascribed to non-polar components, the isolated compounds represent important, but not the only active constituents. This can be seen by the fact that the MIC concentration (8 μg/mL) of the crude extract that is lower than MIC concentrations (49.5 and 46.9 μg/mL, respectively) of the pure compounds 1 and 2 isolated from the same crude extract. These results also suggest that rather than using one purified compound, a standardized extract may be of more therapeutic importance in the treatment of TB. The anti-Mtb activity exhibited by the isolated pure compounds is supported by other reports on previous isolation of biologically active compounds from medicinal plants. Such reports include the isolation of totarol (anti-mycobacterial terpenoid) from Juniperus communis L. (Cuppressaceae) (CitationGordien et al., 2009); four new anti-TB compounds were isolated from Sapium haematospermun (CitationWordemichael et al, 2004); and five polyynes were isolated from the roots of Angelica sinensis (Deng et al., 2004).
The MBC of the purified compounds from our investigation was 62.5 µg/mL, while the IC50 was >100 µg/mL and 38.21 μg/mL and SI was >2.02 and 0.81 for compounds 1 and 2, respectively. Compound 1 had an SI value >2.02, and thus potentially has selective toxicity for TB whereas compound 2 with SI value of less than 1 suggests that the compound may be more toxic for mammalian cells than for TB, hence, it has no selective toxicity. Other previous studies of Eucalyptus species have reported on the antimicrobial (CitationOyedeji et al., 1999; CitationDelaquis et al., 2002; CitationSartorelli, 2007) and anti-mycobacterial (CitationSherry & Warnke, 2004) activities of the Eucalyptus essential oils. There are very few reports on the antimicrobial activities of Eucalyptus extracts (CitationBabayi et al., 2004; CitationAdeniyi et al., 2009a,Citationb; CitationCock, 2009), and to the best of our knowledge no report so far on the anti-mycobacterial activity of Eucalyptus extracts.
The results presented here indicate that the extracts of E. camaldulensis and E torelliana used in Nigerian traditional medicine have anti-Mtb activities in vitro. These activities support their local use for the treatment of cough associated with TB and various respiratory tract infections, and reveal the presence of anti-Mtb active compounds in the plants. These findings not only reveal a new potential area of therapeutic value of E. camaldulensis and E. torelliana, but also highlight the role of esters as anti-Mtb active principles in ethnobotanical preparations and as lead compounds in the development of new and effective anti-Mtb drugs.
Declaration of interest
This study was funded in part by Senate Research Grant SRG/COM/2000/7B to BAA and MacArthur Foundation Grant to TOL. The contents are solely the responsibility of the authors and do not necessarily represent the views of the funding agency.
References
- Adeniyi BA, Groves MJ, Gangadharam PR. (2004). In vitro anti-mycobacterial activities of three species of Cola plant extracts (Sterculiaceae). Phytother Res, 18, 414–418.
- Adeniyi BA, Odufowoke RO, Olaleye SB. (2006a). Anti-bacterial and gastroprotective properties of Eucalyptus torelliana F. Muell crude extract. Intl J Pharmacol, 2, 362–365.
- Adeniyi BA, Lawal TO, Olaleye SB. (2006b). Antimicrobial and gastroprotective activities of Eucalyptus camaldulensis crude extracts. J Biol Sci, 6, 1141–1145.
- Adeniyi CB, Lawal TO, Mahady GB. (2009a). In vitro susceptibility of Helicobacter pylori to extracts of Eucalyptus camaldulensis and Eucalyptus torelliana. Pharm Biol, 47, 99–102.
- Adeniyi BA, Onwubuche BC, Anyiam FM, Ekundayo O, Mahady GB. (2009b). Anti- Helicobacter pylori activities of Eucalyptus grandis: Effects on susceptibility, urease activity and cell surface hydrophobicity. Pharm. Biol, 47:13–17.
- Babayi H, Kolo I, Okogun JI, Ijah UJ. (2004). The antimicrobial activities of methanolic extracts of Eucalyptus camaldulensis and Terminalia catappa against some pathogenic microorganisms. Biokemistri, 16, 106–111.
- Bhatti HN, Igbal Z, Chatta SA, Bhukari IH (2007): Oil potential and chemical composition of Eucalyptus cebra. Int J Agri Biol, 9, 136–138.
- Brennan PJ, Nikaido H. (1995). The envelope of mycobacteria. Annu Rev Biochem, 64, 29–63.
- Brooks JF, Carroll KC, Butel JS, Morse SA. (2007). Medical Microbiology, 24th Edn. New York: MacGraw Hill, pp 320–327.
- Cock IE. (2009). Antimicrobial activity of Eucalyptus major and Eucalyptus baileyana methanolic extracts. The Internet Journal of Microbiology, 6:1 (ISSN: 1937–8289).
- Collins L, Franzblau SG. (1997). Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother, 41, 1004–1009.
- Delaquis PJ, Stanich K, Girard B, Mazza G. (2002). Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int J Food Microbiol, 74, 101–109.
- Deng S, Wang Y, Inui T, Chen SN, Farnsworth NR, Cho S, Franzblau SG, Pauli GF. (2008). Anti-TB polyynes from the roots of Angelica sinensis. Phytother Res, 22, 878–882.
- Farah A, Fechtal M, Chouch A, Zarira S. (2002). The essential oil of Eucalyptus camaldulensis and its natural hybrid (clone 583) from Morocco. Flav Fragr J, 17, 395–397.
- Gordien AY, Gray AI, Franzblau SG, Seidel V. (2009). Antimycobacterial terpenoids from Juniperus communis L. (Cuppressaceae). J Ethnopharmacol, 126, 500–505.
- http://www.nmrdb.org/cheminfo/servlet/org.cheminfo.hook.appli.HookServlet
- Jones KD, Hesketh T, Yudkin J. (2008). Extensively drug-resistant tuberculosis in sub-Saharan Africa: An emerging public-health concern. Trans R Soc Trop Med Hyg, 102, 219–224.
- Kim CE, Griffiths WJ, Taylor PW. (2009). Components derived from Pelargonium stimulate macrophage killing of Mycobacterium species. J Appl Microbiol, 106, 1184–1193.
- Kim JP, Lee IK, Yun BS, Chung SH, Shim GS, Koshino H, Yoo ID. (2001). Ellagic acid rhamnosides from the stem bark of Eucalyptus globulus. Phytochemistry, 57, 587–591.
- Macabeo AP, Krohn K, Gehle D, Read RW, Brophy JJ, Cordell GA, Franzblau SG, Aguinaldo AM. (2005). Indole alkaloids from the leaves of Philippine alstonia scholaris. Phytochemistry, 66, 1158–1162.
- Tan MA, Takayama H, Aimi N, Kitajima M, Franzblau SG, Nonato MG. (2008). Antitubercular triterpenes and phytosterols from Pandanus tectorius Soland. var. laevis. J Nat Med, 62, 232–235.
- Oyedeji AO, Ekundayo O, Olawoye ON, Adeniyi BA, Koenig WA. (1999). Antimicrobial activity of essential oils of five Eucalyptus species growing in Nigeria. Fitoterapia, 70, 526–528.
- Quenelle DC, Winchester GA, Staas JK, Barrow EL, Barrow WW. (2001). Treatment of tuberculosis using a combination of sustained-release rifampin-loaded microspheres and oral dosing with isoniazid. Antimicrob Agents Chemother, 45, 1637–1644.
- Sartorelli P, Marquioreto AD, Amaral-Baroli A, Lima ME, Moreno PR. (2007). Chemical composition and antimicrobial activity of the essential oils from two species of Eucalyptus. Phytother Res, 21, 231–233.
- Sherry E, Warnke PH. (2004). Successful use of an inhalational phytochemical to treat pulmonary tuberculosis: A case report. Phytomedicine, 11, 95–97.
- Weigenand O, Hussein AA, Lall N, Meyer JJ. (2004). Antibacterial activity of naphthoquinones and triterpenoids from Euclea natalensis root bark. J Nat Prod, 67, 1936–1938.
- Woldemichael GM, Gutierrez-Lugo MT, Franzblau SG, Wang Y, Suarez E, Timmermann BN. (2004). Mycobacterium tuberculosis growth inhibition by constituents of Sapium haematospermum. J Nat Prod, 67, 598–603.
- World Health Organization (2008). Global Tuberculosis Control: Surveillance, Planning and Financing. WHO, Geneva.