Abstract
Context: Celsia coromandelina Vahl (Scrophulariaceae) is a shrub found throughout Bangladesh and India, and it is distributed widely in the plains of West Bengal. It is used by the tribal people to treat diarrhea, dysentery, insomnia, skin eruption, fever, syphilis, helminthes infection, and to control fertility.
Objective: The objective of this study was to fractionate stigmasterol derivative and to investigate the effects of petroleum ether extract of C. coromandelina (PECC) aerial parts on the onset of reproductive maturity and the ovarian steroidogenesis in immature female mice.
Materials and methods: PECC was prepared by hot extraction process and one compound was isolated by preparative TLC from it. PECC was completely freed from solvent and administered in immature female mice intraperitoneally once on every alternate day for nine doses. The sexual maturity was observed by means of vaginal opening, first estrus (days), rate of body growth, changes in weight of ovary, uterus and pituitary. The content of ascorbic acid, cholesterol, Δ5-3β-hydroxy steroid dehydrogenase (Δ5-3β-HSD) and glucose 6-phosphate dehydrogenase (G 6-PDH) activities in ovaries and carbonic anhydrase activity in uterus were measured by means of biochemical technique in control and treated mice. The activity of PECC was compared with standard marker compound ethinyl estradiol.
Results: The isolated compound was characterized as stigmasterol derivative. PECC treatment caused a remarkable delay (30.27 and 18.56%, respectively, by low dose) in sexual maturity compared to vehicle control as evidenced by the age of vaginal opening and appearance of first estrus (cornified smear). PECC treatment also caused a significant fall (58.6 and 50.0%, respectively, by low dose) in Δ5-3β-HSD and G 6-PDH activities involved in ovarian steroidogenesis compared to vehicle control. Total cholesterol and ascorbic acid content in ovaries and carbonic anhydrase activity in uterus were increased significantly (low dose by 49.3, 424.6 and 82.4%, respectively) along with a reduction in the weight of ovary, uterus and pituitary in comparison to that of control.
Discussion and conclusion: Overall, these results demonstrate that PECC has a good antifertility effect and is responsible for the delayed development of sexual maturity, suppression of ovarian steroidogenesis and elevation of carbonic anhydrase activity in uterus of immature mice. This supports the claim by tribal people as a potential remedy for birth control.
Introduction
Celsia coromandelina Vahl (Scrophulariaceae) is a shrub found throughout Bangladesh, India and distributed widely in the plains of West Bengal (CitationChatterjee, 1996; CitationRahman, 2006). C. coromandelina and its different parts are used by the tribal people for treatment of various disorders such as diarrhea, dysentery, insomnia, skin eruption, fever, syphilis, helminthes infection, and control of fertility (CitationChopra et al., 1992; CitationNadkarni, 2000; CitationPal et al., 2005, Citation2006). The leaf juice is used externally to relieve burning sensations of hands and feet. It is also used in bleeding piles because of its astringent properties (CitationKirtikar & Basu, 2001). The aerial parts of this plant have antioxidant properties (CitationPal et al., 2009). One of the major active constituents in C. coromandelina is sterol (CitationChatterjee, 1996).
Normal puberty is associated with the onset and progressive activation of the hypothalamic pituitary-gonadal (HPG) axis and the resultant development of secondary sexual characteristics. Delayed puberty describes the clinical condition in which the pubertal events start late or are attenuated or arrested (CitationKalantaridou & Chrousos, 2002). Now, it is well known that gonadotrophins influence the ovarian steroidogenesis which is closely connected with the growth of sexual organs in early life and the onset of puberty (CitationArmstrong et al., 1982; CitationGupta et al., 2004). It is also reported that premature puberche shows an exaggerated ovarian androgen synthesis from the early stages of puberty which is evidenced from gonadotrophin-releasing hormone agonist testing (CitationIbáñez et al., 1997). C. coromandelina and its aerial parts are an ancient tribal remedy for prevention of birth (CitationChatterjee, 1996). But the detailed mechanism of action of this plant and its effects on sexual maturity as an antifertilty agent in early stages of life has not yet been established. The present study has been undertaken to fractionate stigmasterol derivative from the petroleum ether extract of aerial parts of C. coromandelina (PECC) and to study the effects of PECC on the appearance of puberty and ovarian steroidogenesis in immature female mice to substantiate the folklore claim.
Materials and methods
Preparation of extract
The aerial parts of C. coromandelina were collected from Panua, Bankura district region of West Bengal, India, in the month of August 2009. This was authenticated by Dr. H. J. Chowdhury, Joint Director, Central National Herbarium, Botanical Survey of India, Howrah, India. The voucher specimen no 144/DDM/IFTM/08/2009 has been submitted to IFTM University for further reference. After collection, plant parts were washed properly with water to remove foreign materials. The plant materials were dried in shade, powdered to coarse particles, sieved through 40 mesh size and then extracted with petroleum ether (40–60°) in a Soxhlet apparatus. Petroleum ether extract was evaporated to complete dryness under vacuum. The yield of petroleum ether extract of C. coromandelina aerial parts (PECC) was 3.5% w/w with respect to the dry powder. For pharmacological testing, PECC was dissolved in Arachis oil.
Phytochemical screening
Small amount of dried extract (PECC) was appropriately treated to prepare sample solution and then subjected to phytochemical tests. The phytochemical screening of PECC was performed using the following reagents and chemicals: alkaloids with Dragendorff’s reagent; flavonoids with the use of Mg and HCl; tannins and phenolic compounds with ferric chloride and potassium dichromate solutions; steroids with Libermann Burchard reagent; terpenoids with tin and thionyl chloride; amino acids with ninhydrin solution and saponins with ability to produce suds; glycosides with chloroform and concentrated sulphuric acid. These were identified by the characteristic color changes as per standard procedures (CitationGhani, 2003).
Fractionation and chemical investigation of PECC
PECC on preparative TLC using silica gel G as stationary phase and chloroform-methanol (4:1, v/v) as mobile phase, gave fraction A1 [Rf value 0.61 (), m.p. 165°C, λmax (ethanol) 240 nm] having the characteristics I.R. (FT/IR 4200 type A) peaks at 3427 cm−1 (OH), 2925 cm−1 (aromatic), 2855 cm−1 (CH), 1738 cm−1 (C=O, unsaturation), 1461 cm−1, 1375 cm−1, 1259 cm−1, 1058 cm−1, 757 cm−1 suggesting the structural similarity with stigmasterol derivative (CitationDryer, 1994; CitationPal et al., 2005), which was confirmed by comparison with standard authentic sample obtained from authentic source (Central Drug Laboratory, Kolkata, India) (m.p., I.R. and comparative TLC). Qualitative tests (Liberman Burchard, Salkowski) for steroid were also positive for fraction A1.
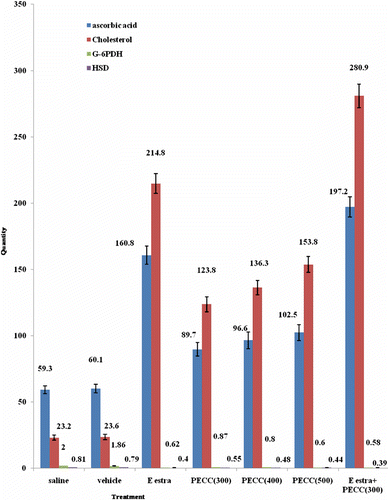
Figure 1. Effects of petroleum ether extract of aerial parts of C. coromandelina (PECC) on content of ascorbic acid, cholesterol and the activities of G 6-PDH and D5-3b- HSD in mouse ovary. Results presented as mean ± SEM from 6 animals. Statistical analysis was done by ANOVA followed by post hoc test of significance, Dunnett’s t-test. *P < 0.05 as compared with vehicle control. PECC: petroleum ether extract of aerial parts of C. coromandelina. E estra: Ethinyl estradiol.
Animals and treatment
Eighty-four albino female mice (22 ± 2 g; Swiss strain) in closed colonies and randomly bred, weaned at 31 days of age were obtained from B. N. Ghosh & Company, India, and screened for the present study. The animals were fed standard pellet diet (Hindustan Lever Ltd, India) consisting of vitamin mixture (1%), mineral mixture (4%), corn oil (10%), sucrose (20%), cellulose (0.2%), casein 95% pure (10.5%), starch (54.3%) and water was provided ad libitum. The experimental design was subjected to the scrutiny of Institutional Ethical Committee (reg. no. IFTM/837ac/0158). The animals were handled as per the guidance of the Committee for the Purpose of Control and supervision on Experimental animals (CPCSEA), New Delhi. All efforts were made to minimize both the suffering and number of animals used. From the acute toxicity study, the LD50 value of PECC was found to be more than 2.0 g/kg intraperitoneally (CitationPal et al., 2005). The mice were divided into seven groups containing 12 animals each. The animals of Group I and II received saline (0.9% NaCl w/v) and vehicle, Arachis oil (5 mL/kg); Group III, IV, V received PECC (300, 400 and 500 mg/kg, respectively). Group VI and VII received ethinyl estradiol (standard marker compound) (0.03 mg/kg, i.p.) and ethinyl estradiol plus PECC (300 mg/kg), respectively. Normal saline, Arachis oil, ethinyl estradiol and PECC were administered i.p. from 40 days age of mice on every alternate day for nine doses. Initial body weight before treatment and final body weight during sacrifice were noted.
Six animals from each group were carefully observed to determine sexual maturity and left without any disturbance. Reproductive maturity were made in two measures; days at vaginal opening and days at first estrus. The mice were examined between 10 and 11 a.m. and 6 and 7 p.m. to determine vaginal opening and after vaginal introitus, a daily vaginal lavage was taken from each mouse to determine the age at first estrus (cornified smear). Twenty-four hours after the last injection (i.e. at the age of 57 days) and 18-h fasting condition, 6 mice from each group were sacrificed by cervical dislocation. Ovaries, uterus and pituitary were dissected out without any delay, detached adherent fatty materials and weight taken and preserved in ice for further processing.
Biochemical estimation
Ovaries were homogenized with chloroform-ethanol mixture (2:1), non-polar part was taken after extraction and total cholesterol was estimated (Kingsley & Roscoe, 1949). To 5 mg tissue of ovaries 45 µL ice cold 5% metaphosphoric acid was added and then it was centrifuged for 20 min at 3500g. After that 30 µL supernatant, 15 µL acetate buffer and 15 µL 2,6-dichlorophenolindophenol sodium (0.1 mg/mL) were taken and mixed. Absorbance was measured taking water as blank at 520 nm. Standard curve was prepared with known concentration and ascorbic acid content was quantified (CitationOmaye et al., 1979). Then, ovaries were mixed with 0.1 M phosphate buffer (pH 7.4) (CitationRabin et al., 1961) and 0.5 M Tris-HCl (pH 8.3) (CitationLohr & Waller, 1974) to estimate Δ5-3β-hydroxy steroid dehydrogenase and glucose 6-phosphate dehydrogenase activity, respectively. Protein content was estimated with Folin-phenol reagent (CitationGupta et al., 2003a). The enzyme activity was defined in unit per mg of protein.
The uterus was homogenized with 3.5 mL normal saline. Then 8 mL of 40% ethanol and 4 mL of chloroform were quickly added. The mixture was stirred in a centrifuge tube for 3 min to a thin sludge and allowed to stand for 20 min. The mixture was centrifuged for 10 min at 3500g and the supernatant was taken for the estimation of carbonic anhydrase. Veronal buffer (3 mL of 0.22 M veronal, pH 7.95), 3 drops of bromothymol blue and 0.3 mL of enzyme and 2 mL of water were mixed in a 15 mL stopper weighing bottle and placed in ice water for 15 min. Ice cold water (5 mL) saturated CO2 (0.071 M) was added anaerobically from a long nozzle all glass syringe. The time was observed for the pH to drop to 6.3, determined with the aid of a bromothymol standard at this pH. The solutions were mixed in less than 1 s without bubbling or loss of CO2. Similarly, blank sample was prepared without the addition of enzyme. The enzyme activity was calculated by using the following formula: the rate of enzymatic hydration of CO2 = (t0− t)/(t− 1) 6.9 × 10−5 mol/L/s, where t and t0 are time of reaction in the presence and the absence of enzyme, respectively (CitationMazumder et al., 2003a).
Statistical analysis
The results were analyzed for statistical significance using one way ANOVA followed by Dunnett’s t-test as a post hoc test of significance taking vehicle treated animals as control (CitationBolton, 1995; CitationEl-Halawany et al., 2010) in all cases except the carbonic anhydrase activity which was analyzed by ANOVA followed by Tukey’s test (CitationMendes et al., 2010). P < 0.05 and P < 0.001 was considered significant and highly significant respectively when compared with vehicle control.
Results and discussion
PECC on preliminary phytochemical analysis was found to contain steroid, saponin, and glycoside. From the chemical investigation, it is suggested that the compound isolated from PECC is stigmasterol derivative. Pharmacological results are presented in and and . From , it is clear that PECC treatment significantly delayed the onset of reproductive maturity (low dose by 30.27 and 18.56%, respectively) in 40 days old immature female mice as evidenced by the days at vaginal opening and onset of first estrus in comparison to control groups. In the same situation, a delay in onset of sexual puberty is accompanied by no significant lowering of rate of body growth (weight gain).
Table 1. Mean age in days of female mice at two measures of sexual maturity and rate of body growth after treatment with petroleum ether extract of aerial parts of C. coromandelina (PECC).
Table 2. Weights of ovary, uterus and pituitary after treatment with petroleum ether extract of aerial parts of C. coromandelina (PECC).
The weights of ovary, uterus and pituitary were decreased significantly (low dose by 45.6, 50.0 and 46.7%, respectively) () together with elevation of level of cholesterol and ascorbic acid (low dose by 49.3 and 424.6%, respectively) () in PECC treated animals in a dose dependent manner. The activities of G 6-PDH and Δ5-3β-hydroxy steroid dehydrogenase (HSD) were decreased considerably (P < 0.05) by PECC (low doses by 58.6 and 50.0%, respectively) in comparison to vehicle treated mouse ovary (). The carbonic anhydrase enzyme activity in the uterus of PECC treated animals was found significantly elevated (low dose by 82.4%) with respect to that of control. Standard marker compound, ethinyl estradiol at the dose of 0.03 mg/kg also exhibited significant antisteroidogenic activity. Simultaneous administration of ethinyl estradiol with PECC (300 mg/kg) caused a highly significant (agonist activity) (P < 0.001) delay in vaginal opening and appearance of first estrus, elevation in ascorbic acid, cholesterol level in ovary and increase in carbonic anhydrase activity in uterus of treated mice in comparison to that of vehicle control.
In the present investigation, PECC, in a dose-dependent manner, significantly retarded the onset of puberty as it is proved by the age at vaginal opening (CitationElbetieha et al., 1998; CitationIyare et al., 2010) and appearance of first estrus (). These abnormalities in the estrus cycle and the reduction in the weight of the ovary, uterus and pituitary in the present communication may be associated with the suppression of ovarian steroidogenic activities () (CitationDhanju et al., 2001).
This observation is also related with the increase level of cholesterol, which acts as a precursor for the biogenesis of steroid hormones in ovaries (CitationMarcus & Coulston, 1996; CitationRang et al., 1999) and may indicate that cholesterol was not utilized in this case (CitationKrum et al., 1964). The increase level of ascorbic acid in PECC treated mice also suggest depressed ovarian steroidogenic activity and hypofunctioning of ovary () (CitationDeane, 1952). To clearly understand these facts, G 6-PDH and Δ5-3β-hydroxy steroid dehydrogenase, the two key enzymes involved ovarian steroidogenic activity was estimated (CitationSuzuki et al., 1984). These two enzymes are directly connected with the biosynthesis of ovarian steroidal hormones. Any alteration in the activity of G 6-PDH and Δ5-3β-HSD causes abnormalities in hormonal production in ovaries. The role of G 6-PDH of pentose phosphate pathway in the biosynthesis of estrogen in the sexually immature animal has been established long ago (CitationKnorr et al., 1970). Therefore, in the present investigation, a depression of G 6-PDH and Δ5-3β-HSD activity after treatment with PECC suggests a diminution of ovarian steroidogenesis in a dose-dependent manner (CitationMajumder et al., 1997; CitationPal et al., 2003; CitationGupta et al., 2003b). It is also established that gonadotrophins accelerates the rate of production of NADPH essential for hydroxylation reaction in the formation of steroid hormones from cholesterol through the activation of G 6-PDH metabolism in pentose phosphate pathway (CitationMckerns, 1965).
A large number of workers agree with the point that sexual maturity is closely connected with ovarian steroidogenesis (CitationArmstrong et al., 1982). Therefore, it may be decided based on our experimental data that the delay on the onset of sexual maturity after treatment with PECC is possibly due to the suppression and inhibition of steroidogenic activity in ovary. Further studies and clarification are required to find out the possible site of action of PECC either directly on the ovary or via gonadotrophin secretion.
The increase in carbonic anhydrase activity in uterus of treated animals (PECC and standard marker compound) is possibly due to the increase level of progesterone (CitationCrossland, 1980). The elevated level of progesterone inhibits the secretion of LH and thus prevents ovulation and it also makes the cervical mucus less suitable for the passage of sperm. It also changes the endometrium in such a way as to discourage implantation (CitationRang et al., 1999) and thus prevents fertilization.
Phytochemical tests as well as characterization of fractionated compound indicate the presence of stigmasterol derivative in PECC (CitationNakamura, 1998; CitationMazumder et al., 2003b). It is already reported that various sterols (CitationHiremath et al., 1994, Citation1999; CitationMadhavan et al., 2009) have the property to exhibit antifertility activities. Hence, it may be considered in reference to earlier reports that stigmasterol, the major component in PECC may be responsible for the delayed appearance of puberty and suppression of ovarian steroidogenesis by petroleum ether extract of C. coromandelina (CitationGupta et al., 2003b, Citation2004), which can account for the traditional uses of it in birth control.
Conclusions
The present study indicates that the petroleum ether extract of aerial parts of C. coromandelina (PECC) has good antifertility effects and is responsible for the delayed development of sexual maturity and suppression of ovarian steroidogenic activity in immature mice. Again, the results related to carbonic anhydrase activity in uterus of control and treated mice are definitely encouraging, as this may be exploited in future investigation where carbonic anhydrase level would serve as an index for determining the antifertility efficacy of a drug. Stigmasterol, the major component in PECC may be responsible for the antifertility activity of PECC in immature mice. Further studies on the isolation of other active compound(s) (if present) responsible for this effect are in progress. This study supports the claim by tribal people as a potential remedy for birth control.
Declaration of interest
The authors declare no conflicts of interest.
References
- Armstrong DG. (1982). 3 beta-hydroxy-delta 5-steroid dehydrogenase activity in the rapidly growing ovarian follicles of the domestic fowl (Gallus domesticus). J Endocrinol, 93, 415–421.
- Bolton S. (1995). Statistics. In: Gennaro AR (ed). Remington: The Science and Practice of Pharmacy, Vol. 2. Easton, PA: Mack Publishing Co., pp. 113–114.
- Chatterjee TK. (1996). The plants having antifertility activities. In: Herbal Options. Kolkata, India: Eastern Traders, pp. 80–94.
- Chopra RN, Nayer SL, Chopra IC. (1992). Glossary of Indian Medicinal Plants. New Delhi, India: Publication and Information Directorate, CSIR.
- Crossland J. (1980). The sex hormones. In: Lewis’s Pharmacology. New York: Churchill Livingstone, pp. 732–752.
- Deane HW. (1952). Histochemical observations on the ovary and oviduct of the albino rat during the estrous cycle. Am J Anat, 91, 363–413.
- Dhanju CK, Sangha GK, Sekhon PK. (2001). Biochemical status of ovaries after induction of superovulation on different days of estrus cycle in mice. Indian J Exp Biol, 39, 777–780.
- Dryer JR. (1994). Applications of Absorption Spectroscopy of Organic Compounds, 9th Edn, New Delhi, India: Prentice-Hall of India Pvt. Ltd.
- Elbetieha A, Al-Hamood MH, Alkofahi A, Bataineh H. (1998). Reproductive toxicity potentials of Salvia fruticosa (Labiatae) in rats. J Ethnopharmacol, 53, 515–520.
- El-Halawany AM, Chung MH, Abdallah HM, Nishihara T, Hattori M. (2010). Estrogenic activity of a naringinase-treated extract of Sophora japonica cultivated in Egypt. Pharm Biol, 48, 177–181.
- Ghani A. (2003). Medicinal Plants of Bangladesh, 2nd Revised Edn. Dhaka, Bangladesh: The Asiatic Society of Bangladesh.
- Gupta M, Mazumder UK, Pal DK, Bhattacharya S. (2003a). Anti-steroidogenic activity of methanolic extract of Cuscuta reflexa roxb. stem and Corchorus olitorius Linn. seed in mouse ovary. Indian J Exp Biol, 41, 641–644.
- Gupta M, Mazumder UK, Pal DK, Bhattacharya S. (2003b). Effect on onset of puberty and ovarian steroidogenesis following administration of methanolic extract of Cuscuta reflexa Roxb stem and Corchorous olitorius Linn seed treated mice. J Ethnopharmacol, 89, 55–59.
- Gupta M, Mazumder UK, Vamsi ML, Sivakumar T, Kandar CC. (2004). Anti-steroidogenic activity of the two Indian medicinal plants in mice. J Ethnopharmacol, 90, 21–25.
- Hiremath SP, Badami S, Swamy HK, Patil SB, Londonkar RL. (1994). Antifertility activity of Striga orobanchioides. Biol Pharm Bull, 17, 1029–1031.
- Hiremath SP, Rudresh K, Badami S, Patil SB, Patil SR. (1999). Post-coital antifertility activity of Acalypha indica L. J Ethnopharmacol, 67, 253–258.
- Iyare EE, Adegoke OA, Nwagha UI. (2010). Mechanism of delayed puberty in rats whose mothers consumed Hibiscus sabdariffa during lactation. Pharm Biol, 48, 1170–1176.
- Kalantaridou SN, Chrousos GP. (2002). Clinical review 148: Monogenic disorders of puberty. J Clin Endocrinol Metab, 87, 2481–2494.
- Kirtikar KR, Basu BD. (2001). Indian Medicinal Plants, 3rd Edn. Vol. 8., New Delhi, India: Sri Satguru Publications.
- Knorr DW, Vanha-Perittula T, Lipsett MB. (1970). Structure and function of rat testis through pubescence. Endocrinology, 86, 1298–1304.
- Krum AA, Morris MD, Bennett LL. (1964). Role of cholesterol in the in vivo biosynthesis of adrenal steroids by the dog. Endocrinology, 74, 543–547.
- Lohr GW, Waller HD. (1974). Glucose-6-phosphate dehydrogenase. In: Bergmeyer HU (ed). Methods of Enzymatic Analysis, Vol. 2. Florida: Verlag Chemie, pp. 636–643.
- Ibáñez L, Potau N, Zampolli M, Street ME, Carrascosa A. (1997). Girls diagnosed with premature pubarche show an exaggerated ovarian androgen synthesis from the early stages of puberty: evidence from gonadotropin-releasing hormone agonist testing. Fertil Steril, 67, 849–855.
- Madhavan V, Prem Kumar BH, Murali A, Yoganarasimhan. (2009). Antifertility activity of Drosera burmannii. Pharm Biol, 47, 128–131.
- Marcus R, Coulston AM. (1996). The vitamins. In: Gilman AG, Goodman LS, Rall TW, Murad F. (ed). Goodman & Gilmann’s The Pharmacological Basis of Therapeutics, New York: McGraw-Hill Health Professions Divisions, pp. 1547–1572.
- Mazumder UK, Gupta M, Pal DK, Bhattacharya S. (2003a). Induction of carbonic anhydrase by Cuscuta reflexa Roxb stem and Corchorus olitorius Linn. seed in mice. Indian J Pharm Sci, 65, 401–403.
- Mazumder UK, Gupta M, Pal D, Bhattacharya S. (2003b). Chemical and toxicological evaluation of methanol extract of Cuscuta reflexa Roxb. stem and Corchorus olitorius Linn. seed on hematological parameters and hepatorenal functions in mice. Acta Pol Pharm, 60, 317–323.
- Majumder PK, Dasgupta S, Mukhopadhaya RK, Mazumdar UK, Gupta M. (1997). Anti-steroidogenic activity of the petroleum ether extract and fraction 5 (fatty acids) of carrot (Daucus carota L.) seeds in mouse ovary. J Ethnopharmacol, 57, 209–212.
- Mckerns KW. (1965). Gonadotropin regulation of the activities of dehydrogenase enzymes of the ovary. Biochim Biophys Acta, 97, 542–550.
- Mendes CC, Marinho CM, Moreira-Junior VF, Queiroz FM, Dantas GL, Macedo MF, Oliveira CN, Schwarz A. (2010). Evaluation of Bauhinia monandra aqueous and ethanol extracts in pregnant rats. Pharm Biol, 48, 780–785.
- Nadkarni KM. (2000). The Indian Meteria Medica, Vol. 1, Mumbai, India: Popular Prakashan.
- Nakamura T, Goda Y, Sakai S, Kondo K, Akiyama H, Toyoda M. (1998). Cardenolide glycosides from seeds of Corchorus olitorius. Phytochemistry, 49, 2097–2101.
- Omaye ST, Turnbull JD, Souberlich HE. (1979). Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. In: Cormick DBM, Wright LD. (ed). Methods in Enzymology, Vol. 62. New York: Academic Press, pp. 3–47.
- Pal DK, Gupta M, Mazumder UK, Bhattacharya S. (2003). Proceedings The 12th North American ISSX Meeting, Abstract no 419, Providence, Rhode Island, U.S.A., 12–16 Oct, Published in Drug Metabolism Reviews, 35, 210.
- Pal D, Nandi M. (2005). CNS activities of Celesia coromandeliane Vahl. in mice. Acta Pol Pharm, 62, 355–361.
- Pal DK, Mazumder A, Bandyopadhya P, Jena A, Pandeya R. (2006). Evaluation of anthelmintic activities of aerial parts of C. coromandeliane Vahl and M. pentaphylla Linn. Ancient Sci Life, 25, 28–32.
- Pal DK, Halder P, Bhuniya A. (2009). A study on the in vitro antioxidant activity of aerial parts of Celsia coromandeliane Vahl and bark of Mesua ferrea Linn. Pharmacologyonline, 3, 200–203.
- Rabin BL, Leipsner G, Deane HW. (1961). A rapid, sensitive assay procedure for adrenal steroid-3 beta-ol-dehydrogenase activity. Endocrinology, 69, 619–625.
- Rang HP, Dale MM, Ritter, JM. (1999). The reproductive system. In: Pharmacology. New York: Churchill Livingstone, pp. 436–453.
- Rahman MO. (2006). Scrophulariaceae Taxa in Bangladesh. Bangladesh J Plant Taxon, 13, 139–154.
- Suzuki S, Endo Y, Tanaka S, Iizuka R. (1984). Indirect immunofluorescence studies on the steroid-producing activity of hamster ova. Am J Obstet Gynecol, 148, 76–85.
