Abstract
Context: Different habitat conditions can be responsible for the production of secondary metabolites and for the antioxidant properties of plant products.
Objective: Thus, the aim of this study was to evaluate whether the antioxidant activity and tannin concentrations in the stem bark of Spondias tuberosa Arruda (Anacardiaceae) varied with collection site.
Material and methods: The bark was collected from 25 individual trees, distributed in five different landscape units, as follows: agroforestry gardens, areas of pastures, maize cultivation areas, mountain areas and mountain bases, with the former 3 being considered as anthropogenic habitats, and the latter 2 considered as habitats with native coverage. The study was conducted in the rural area of the city of Altinho, Pernambuco State (Northeast Brazil). The DPPH (1,1-diphenyl-2-picrylhydrazyl) method was used to measure the antioxidant activity and tannin concentrations were evaluated by using the radial diffusion method.
Results: The results demonstrated that there were no significant differences among the tannin concentrations of the individuals from the native (6.27% ± 1.75) or anthropogenic areas (4.63% ± 2.55), (H = 2.24; p > 0.05). In contrast, there were significant differences (H = 5.1723; p < 0.05) among the CE50 means of the antioxidant activities of the individuals from the native (32.10 µg/ml ± 5.27) and anthropogenic areas (27.07 µg/ml ± 2.29). However, correlations between the tannin concentrations and antioxidant activity of the extracts were not observed in the native (r = 0.39; p > 0.05) or in the anthropogenic areas (r = 0.38; p > 0.05).
Discussion and conclusion: Because the variation of the antioxidant capacity of S. tuberosa bark was not accompanied by a variation in the tannin concentration, this property may be related to the presence of other metabolite(s).
Introduction
Environmental, genotypic and phenotypic factors can be responsible for the qualitative-quantitative variations of secondary metabolites and the antioxidant capacity of plants (CitationWieser et al., 2002, Citation2003; CitationPeltonen et al., 2005; CitationSantos et al., 2006; CitationMonteiro et al., 2006; CitationMcCune & Johns, 2007; CitationKsouri et al., 2008). Different environmental factors, such as temperature and water availability, characterize different habitats and, consequently, can influence plant metabolism.
The combination of temperature, rainfall index and ozone concentration influences the production of antioxidants, especially ascorbate (CitationHofer et al., 2008). Many metabolites, especially secondary ones as phenols, can have their production influenced by several factors that act synergistically. For instance, longer periods of rainfall and low fertility in the soil act together in the increase of total phenol and tannin levels in Stryphnodendron polyphyllum Mart. (Fabaceae) (CitationJacobson et al., 2005; CitationSantos et al., 2006). However, in relation to tannin concentrations, the rainfall index and seasonal climate are the main factors associated with the increase in the production of this metabolite in the leaves of Myracrodruon urundeuva Allemão (Anacardiaceae) and the bark of Anadenanthera colubrina (Vell.) Brenan (Fabaceae) (CitationMonteiro et al., 2006).
Both age and sun exposure are factors that influence the production of antioxidant compounds in Fagus sylvatica L. (Fagaceae) leaves; older individuals and those exposed to more sunlight present a greater photoprotective capacity to the impact of oxidative stress (CitationWieser et al., 2003). CitationMcCune and Johns (2007), when evaluating the medicinal plants used by Canadian indigenous, concluded that locations with greater sun exposure, which tend to be drier or with poorer soil, contained species with enhanced antioxidant activity.
Thus, this study was aimed at evaluating whether the antioxidant activity and tannin levels of the stem bark of Spondias tuberosa Arruda (Anacardiaceae) varied according to the collection sites. To this end, we formulated three questions. Does the antioxidant activity of the stem bark of this species vary according with collection site? If so, is this variation accompanied by a variation in the tannin concentration? Is there a relationship between the tannin levels and antioxidant activity? S. tuberosa is a tree, endemic of the Caatinga, known locally as “umbu” and used for various purposes, especially food and medicinal (CitationLins Neto et al., 2010). Antidiabetic, hypocholesterolemic and anti-inflammatory uses are among the local therapeutic applications of this plant (CitationAlbuquerque et al., 2007; CitationLins Neto et al., 2010).
Materials and methods
Collection site and the selection of specimens
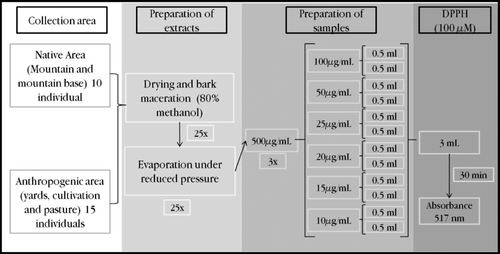
The collection of the individuals for this study was carried out, in July 2009, in a rural community known as “sítio Carão” (08° 35′ 16.1′′ S × 36° 05′ 36.1′′ W), Altinho − PE, northeast Brazil (8° 29′ 32′′ S × 36° 03′ 03′′ W). This region is characterized by Caatinga vegetation, with a hot, semi-arid climate (Bshs’) and an average temperature above 26°C. Rainfall is very irregular; in 2009, it rained 994.5 mm3 in Altinho (CitationIPA, 2010). The species was identified by biologist Luciana G. de Sousa and the voucher was incorporated into the Herbarium Vasconcelos Sobrinho of the Universidade Federal Rural de Pernambuco (UFRPE) under the number 48652.
Similar to other areas of the Caatinga (CitationSampaio, 2002; CitationAlbuquerque & Andrade, 2002; CitationAraújo et al., 2007), the community of Carão has experienced significant changes in the local vegetation due to the conversion of land use to pastures, permanent and/or temporary crops and timber resources (CitationAlmeida et al., 2011). These changes are responsible for the formation of landscape units associated with specific types of management (CitationLins Neto et al. 2010; CitationAlmeida et al., 2011). For the studied region, CitationAlmeida et al. (2011) recognized the following landscape units:
Mountain area − a mountain range adjacent to the community (690 m of altitude), with a predominance of shrubs and trees approximately 10 m tall and trees reaching over 50 cm of stem diameter at ground level. Portions of this area were cleared approximately 60 years ago for the planting of subsistence crops, however, in the inclined areas that lead to the flat part of the mountain area, these activities have ceased, and the vegetation has been regenerating for approximately 50 years.
Mountain base − a transitional area between the mountain area and the regions with agriculture and grazing, which are on the flat areas, with altitudes that vary between 460 and 520 m. This area has been regenerating for approximately 15 years, after the abandonment of farming. It is covered with shrubs approximately 3 m in height and some trees.
Pasture − an area used for raising cattle and goats, where the vegetation was cleared approximately 30 years ago. These areas tend to be flat, with herbaceous vegetation and altitudes ranging from 440 to 460 m.
Cultivation areas − these areas differ from the pasture in that they are used for the cultivation of maize, beans and cactus pear [Opuntia fícus-indica Mill (Cactaceae)]. Among the agricultural practices is the preparation of the soil for planting, which consists of yearly plowing and cutting of the few woody and herbaceous elements.
Agroforestry gardens − areas closer to households where, as in the cultivation and pasture units, the individuals of S. tuberosa and some other species are cultivated because of their desirable characteristics, such as medicinal, food and shade uses.
Based on this characterization, we classified the landscape units into the native areas (the mountain area and mountain base) and the anthropogenic areas (the pasture, cultivation and agroforestry gardens). From each of these landscape units were randomly selected five individuals, for a total of 25, with 10 being from the native areas and 15 from the anthropogenic areas. The selection was based on 20 individuals previously marked by CitationLins Neto (2008) in each unit.
Samples of the stem bark were collected from each individual, as this is the part of the plant used to treat diseases in the region (CitationAlbuquerque et al., 2007; CitationLins Neto et al., 2010). All of the plant material was collected on the same day in July, 2009, that occasionally, was the wettest month of the year, with 213.5 mm3 of rainfall in the city of Altinho (CitationIPA, 2010).
In vitro analysis of the antioxidant activity
Plant extract
Dry and pulverized bark was macerated for six days, at a ratio of 1:20 w/v in 80% methanol. The material was then filtered through filter paper, and the extraction alcohol was evaporated under reduced pressure, generating a dry extract. Based on this dry extract, three independent samples were prepared in methanol with a concentration of 500 µg/mL. For each of these 3 samples, 6 dilutions were made, at concentrations of 100, 50, 25, 20, 15 and 10 µg/mL.
Measurement of the antioxidant activity by the DPPH method
The antioxidant activity was assessed using the DPPH (1,1-diphenyl-2-picrylhydrazyl) assay, according to a protocol adapted from CitationCotelle et al. (1996) and CitationMcCune and Johns (2002, Citation2007). For this purpose, a DPPH solution was prepared at 100 μM in methanol. From each dilution of the extract (see above), 0.5 mL was added to 3.0 mL of the DPPH solution in a test tube; these analyses were carried out in duplicate. After 30 min of incubation, the absorbance of the solution was determined by a spectrophotometer at 517 nm (). Ascorbic acid was used as a positive control at concentrations of 5, 10, 15, 20, 25, 30, 40 and 50 µg/mL.
Figure 1. The experimental design of the measurements of the antioxidant activity in vitro, using DPPH (1,1-diphenyl-2-picrylhydrazyl).
Based on the absorbance readings, the CE50 was obtained, which represents the concentration of extract or ascorbic acid (positive control) required to decrease the initial concentration of DPPH by 50%. To calculate the CE50, a graphic was prepared where the sample concentrations (µg/mL) or positive control were displayed in the abscissa and the percentage of DPPH remaining (% DPPHREM) was placed in the ordinate, obtaining a first order exponential curve and its equation (CitationSousa et al., 2007). The %DPPHREM was calculated according to the following formula:
where [DPPH]T = t corresponds to the concentration of DPPH after reaction with the extract, and [DPPH]T = 0 is the initial concentration of DPPH, that is, 40 μg/mL (100 μmol/L) (CitationSousa et al., 2007).
Analysis of tannins
Plant extracts
The dry and pulverized bark was standardized by sieving through a 16-mesh (1 mm in diameter) screen. The plant material was extracted for 1 hour at room temperature, using 50% methanol, at a ratio of bark:solvent of 50:1 (mg:mL).
Preparation of assay plates
The radial diffusion method (CitationHagerman, 1987), adapted by CitationCabral et al. (2010), was used to determine the tannin concentrations. This analysis is based on the same principles of immunoassay methods because of the similarity, in some aspects, that exist between the interactions of antigen–antibody and protein–tannin, forming multivalent complexes that precipitate and are visible. The area of the complex, in this case, is linearly proportional to the tannin quantity (CitationHagerman, 1987; CitationCabral et al., 2010).
A gel was prepared using a solution of 50 mM acetic acid and 60 μM ascorbic acid and adjusting the pH to 5.0 by the addition of sodium acetate. Agarose (type I) (Sigma–Aldrich, MO) was added to 1%, and this mixture was stirred and heated until it boiled for the homogenization of the agarose. After cooling to 45°C, bovine serum albumin (BSA) fraction V, free of fatty acids (Sigma–Aldrich), was added to 0.1%.
Ten milliliters of the solution was distributed into 9.0 cm Petri plates and left on a level surface to obtain a uniform layer of gel until total solidification. Wells of 4 mm in diameter were created, 2.0 cm apart from each other and from the edges of the plates. Three successive portions of 8 μL from each extract were applied to the wells of the diffusion gel. All analyses were performed in authentic triplicates.
For the preparation of the standard curve, 2, 4, 8, 12, 16 and 20 μL of an aqueous solution of tannic acid (25 mg/mL) was distributed into the wells in triplicate. Portions greater than the capacity of the wells were fractionally added to avoid overflow.
To analyze the size of the obtained diameters, the plates were scanned using a scanner. The diameter of the rings were measured through Corel Draw© X3 program Version 13, by drawing a circle, using the model of the program and taking the average of two perpendicular diameters for each ring. Readings for the standard curve were inserted in a scatter plot to obtain the line equation through linear regression with aid of Excel software version 2003 (CitationCabral et al., 2010).
Data analysis
Because the data were not normally distributed, the Kruskal–Wallis test was used to assess whether or not the collection site (habitat) influenced the antioxidant activity and tannin concentrations of the extracts. The Spearman correlation test was used to determine whether there was a relationship between the tannin concentrations and antioxidant activity. The software BioEstat 5.0 was employed in all tests (CitationAyres et al., 2007).
Results and discussion
On average, the individuals collected from the anthropogenic areas had the highest values of antioxidant activity (). Among the landscape units, the area closest to households provided the greatest activity, which presented, on average, smaller CE50 values (25.62 µg/mL) (). Statistically, there was a significant difference between the total mean of the CE50 of the antioxidant activity of the individuals from the anthropogenic (27.07 µg/ml ± 0.73) and native areas (32.10 µg/ml ± 2.82) (H = 5.1723; p < 0.05). These results corroborated other studies showing that different collection sites are a factor that can influence the antioxidant activity of plants (CitationWildi & Lütz, 1996; CitationMcCune & Johns, 2007; CitationAnli & Vural, 2009).
Table 1. The CE50 of the antioxidant activity (expressed in µg/mL) and the tannin concentration (expressed as the percentage of Spondias tuberosa Arruda bark) collected in different landscape units of the Caatinga, Northeast Brazil.
The soil fertility is another one of these factors. CitationMcCune and Johns (2007) noted that medicinal plants collected at sites with lower soil fertility and water availability had the highest antioxidant activities (smallest CE50). However, this result disagrees with ours in the sense that the areas with the smallest values of CE50 were those of households and pastures, which in a previous study conducted by CitationLins Neto (2008), presented a higher fertility when compared to the other areas ().
Furthermore, this difference in the antioxidant activity among the collection sites can be explained by the fact that, although environmental factors affect the antioxidant activity of plants, not all of the compounds responsible for this activity necessarily need to be affected: the environment can influence some antioxidant compounds and not others (CitationWieser et al., 2003). CitationAnli and Vural (2009) observed that some phenolic compounds may be found in significantly different concentrations, while others are not, depending on the collection site, consequently altering the antioxidant capacity.
Although our study evaluated only the bark of S. tuberosa, the collection site also has been reported to influence the ascorbic acid concentrations in the fruits of individuals from the same community (CitationLins Neto, 2008). Ascorbic acid has a known antioxidant activity, and similar to our findings, it was observed that individuals collected in areas near households presented significant differences, with greater concentrations of ascorbic acid, when compared to other collection sites (CitationLins Neto, 2008).
Overall, the analysis of the tannin concentrations demonstrated that there was no significant difference between the individuals from the native (6.27% ± 0.46) and anthropogenic areas (4.64% ± 1.05), (H = 2.24; p > 0.05). Despite the lack of a statistical difference, it is worthwhile to mention that the highest concentrations of tannin, on average, were found in the native area units. Among them, the Forest area had the highest concentrations (6.73%) and the lowest antioxidant activity ().
Although some previous studies have demonstrated that environmental effects and collection sites influence the production of phenolic compounds, especially those related to tannins (CitationMonteiro et al. 2006; CitationAlonso-Amelot et al., 2007; CitationAnli & Vural 2009), our results corroborate other studies, which have reported that abiotic stresses increase the concentration of phenolic compounds, though not necessarily tannins, resulting in an increase in the antioxidant activity (CitationNavarro et al., 2006; CitationKsouri et al., 2007, Citation2008).
The difference in tannin concentrations, although not significant, may be related to the differences in the soil fertility found by CitationLins Neto (2008), given that the most fertile areas presented the smallest values of tannin concentrations in our study. Fertile soils seem not to influence the increase of tannin concentrations, presenting at times an inverse relationship with some compounds that are necessary for plant development, such as nitrogen, phosphorus, sulfur and potassium (CitationKoricheva et al., 1998; CitationFidelis, 2003; CitationGobbo-Neto & Lopes, 2007).
Although several studies have correlated phenolic compounds, including tannins, with antioxidant activity (CitationLima et al., 2004; CitationMcCune & Johns, 2007; CitationRamirez et al., 2009; CitationZhang et al., 2010), a correlation between the tannin concentrations and the CE50 were observed neither in the native (r = 0.39; p > 0.05) nor in the anthropogenic areas (r = 0.38; p > 0.05). These findings substantiate several previous studies (CitationSousa et al., 2007; CitationAnli & Vural, 2009; CitationKähkönen et al., 1999). CitationSousa et al., (2007), when analyzing five medicinal plants, observed that there was no positive correlation between the quantity of total phenols and the antioxidant activity for some species, suggesting that other constituent more effectively contributes to the sequestration of free radicals. CitationKähkönen et al. (1999) analyzed 92 different plant extracts, sub-grouped into fruits, herbs, vegetables, grains and trees, and observed no significant correlation between the concentration of total phenols and the antioxidant activity in any of the studied subgroups, concluding that antioxidant activity cannot be predicted based on phenolic content. A study on grape varieties from Turkey demonstrated that, although varieties collected in a given region presented the highest phenolic concentrations and the greatest antioxidant capacities than those from other regions, it does not seem reasonable to claim a direct relationship between the antioxidant capacity and the total phenolic content of the varieties (CitationAnli & Vural, 2009). Other studies have shown that phenolic compounds can differ qualitatively and quantitatively, both among cultivars and among different parts of the plant (CitationCartea et al., 2011).
The best explanation for the lack of a relationship between the antioxidant capacity and the tannin concentration, under the same conditions, is the fact that certain compounds with antioxidant properties respond differently to these conditions. CitationLavola et al. (2003), when studying Pinus sylvestris, concluded that the environmental factors that positively affect the pathway of the secondary metabolism of flavonoids also negatively affect, or are neutral, in the tannin pathway.
CitationWieser et al. (2003) studied the leaves of Fagus sylvatica in different gradients of exposure to solar radiation, and observed that the individuals that received more exposure to this factor presented a significant increase in the concentration of ascorbate and α–tocopherol; however, the concentration of glutathione did not differ when compared to plants in the shade, showing that some compounds may be more sensitive to certain factors. Similarly, the collection site was shown to influence the production of antioxidant phenolic compounds, such as catechin, epicatechin, vanillic acid and syringic acid, in grape varieties collected in different regions of Turkey (CitationAnli & Vural, 2009).
This influence can occur within the same group of metabolites, as reported by CitationSiatka and Kašparová (2010). These authors showed that, although there is a correlation between the antioxidant activity and the concentration of total phenols in the flowers of Bellis perennis L., a correlation between the concentration between total flavonoids and antioxidant activity was not observed, showing that not all of the flavonoids were responsible for this activity. Based on this idea, a portion of the tannins of the species studied in this report may not have the same antioxidant capacity, given that the radial diffusion method does not qualitatively distinguish tannins (CitationHagerman, 1987).
Conclusions
Our results indicate that, for the studied areas, the collection sites did not influence the tannin concentrations of Spondias tuberosa, although the collection site was responsible for the differences in the antioxidant activity. Because the variation in the antioxidant capacity of S. tuberosa bark was not accompanied by a corresponding variation in the tannin concentration, this variation may be related to the presence of other metabolite(s), whose production and/or accumulation is likely more sensitive to different habitats.
Acknowledgments
The authors would like to thank Prof. Msc. Ernani Machado de Freitas Lins Neto for the support in the selection and collection of plant material and Msc. Daniela Lyra de Vasconcelos Cabral for their help in analyzing the tannins. We thank CNPq (“Edital Universal”) and FACEPE for its financial support and grants to U.P. Albuquerque and T. A. S. Araújo.
Declaration of interest
The authors declare they have no conflict of interest.
References
- Albuquerque UP, Andrade LHC. (2002). Uso de recursos vegetais da Caatinga: O caso do agreste do estado de Pernambuco (Nordeste do Brasil). Interciencia, 27, 336–345.
- Albuquerque UP, Medeiros M, Almeida ALS, Monteiro JM, Lins Neto EMF, Melo JG, Santos JP. (2007). Medicinal plants of the Caatinga (semi-arid) vegetation of NE Brazil: A quantitative approach. J Ethnopharmacol, 114, 325–354.
- Alonso-Amelot ME, Oliveros-Bastidas A, Calcagno-Pisarelli MP. (2007). Phenolics and condensed tannins of high altitude Pteridium arachnoideum in relation to sunlight exposure, elevation, and rain regime. Biochem Syst Ecol 35, 1–10.
- Almeida ALS, Albuquerque UP, Castro CC. (2011). Reproductive biology of Spondias tuberosa Arruda (Anacardiaceae), a fructiferous endemic species in caatinga (dry forest), under distinct management conditions in Northeastern, Brazil. Journal of Arid Environments, 75, 330–337.
- Ertan Anli R, Vural N. (2009). Antioxidant phenolic substances of Turkish red wines from different wine regions. Molecules, 14, 289–297.
- Araújo EL, Castro CC, Albuquerque UP. (2007). Dynamics of Brazilian Caatinga: A review concerning plants environment and people. Funcional Ecossystems and communities, 1, 15–28.
- Ayres M, Ayres MJ, Ayres DL, Santos AAS. (2007). BioEstat 5.0: aplicações estatísticas nas áreas das ciências biológicas e médicas. Brasília, Brazil: Sociedade Civil Mamirauá, CNPq.
- Cabral DLV, Peixoto Sobrinho TJS, Amorim ELC, Albuquerque UP. (2010). Relationship of biometric parameters on the concentration of tannins in two medicinal plants a case study. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, 9, 368–376.
- Cartea ME, Francisco M, Soengas P, Velasco P. (2011). Phenolic compounds in Brassica vegetables. Molecules, 16, 251–280.
- Cotelle N, Bernier JL, Catteau JP, Pommery J, Wallet JC, Gaydou EM. (1996). Antioxidant properties of hydroxy-flavones. Free Radic Biol Med, 20, 35–43.
- Fidelis, IDS. (2003). Crescimento, armazenamento, homeopatia, produção de metabólitos secundários e teste biológico do extrato de Sphagneticola trilobata (L.) Pruski em coelhos diabéticos. Universidade Federal de Viçosa. Avaliable at: ftp://ftp.bbt.ufv.br/teses/fitotecnia/2003/176815f.pdf. Accessed on 10 September 2009.
- Gobbo-Neto L, Lopes NP. (2007). Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Química Nova, 30, 374–381.
- Hagerman AE. (1987). Radial diffusion method for determining tannin in plant extracts. J Chem Ecol, 13, 437–448.
- Hofer N, Alexou M, Heerdt C, Löw M, Werner H, Matyssek R, Rennenberg H, Haberer K. (2008). Seasonal differences and within-canopy variations of antioxidants in mature spruce (Picea abies) trees under elevated ozone in a free-air exposure system. Environ Pollut, 154, 241–253.
- IPA. (2010). Instituto Agronômico De Pernambuco. Avaliable at: http://www.ipa.br/indice_pluv.php#calendario_indices. Accessed on 10 September 2009.
- Jacobson TKB, Garcia J, Santos SC, Duarte JB, Farias JG, Kliemann HJ. (2005). Influência de fatores edáficos na produção de fenóis totais e taninos de duas espécies de barbatimão (Stryphnodendron sp.). Pesquisa Agropecuária Tropical, 35, 163–169.
- Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. (1999). Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem, 47, 3954–3962.
- Koricheva J, Larsson S, Haukioja E, Keinänen M. (1998). Regulation of woody plant secondary metabolism by resource availability: Hypothesis testing by means of meta-analysis. Oikos, 83, 212–226.
- Ksouri R, Megdiche W, Debez A, Falleh H, Grignon C, Abdelly C. (2007). Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol Biochem, 45, 244–249.
- Ksouri R, Megdiche W, Falleh H, Trabelsi N, Boulaaba M, Smaoui A, Abdelly C. (2008). Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C R Biol, 331, 865–873.
- Lavola A, Aphalo PJ, Lahti M, Julkunen-Tiitto R. (2003). Nutrient availability and the effect of increasing UV-B radiation on secondary plant compounds in Scots pine. Environmental and Experimental Botany, 49, 49–60.
- Lima VLAG, Melo EA, Maciel MIS, Silva GSB, Lima DES. (2004). Fenólicos totais e atividade antioxidante do extrato aquoso de broto de feijão-mungo (Vigna radiata L.). Revista de Nutrição, 17, 53–57.
- Lins Neto EMF. (2008). Usos tradicionais e manejo incipiente de Spondias tuberosa arruda. no semi-rido do Nordeste do Brasil. Dissertação de mestrado, Pós-Graduação em Botânica, Universidade Federal Rural de Pernambuco. 100 p.
- Lins Neto EMF, Peroni N, Albuquerque UP. (2010). Traditional knowledge and management of Umbu (Spondias tuberosa, Anacardiaceae): An endemic species from the semi–arid region of Northeastern Brazi. Econ Bot, 64, 11–21.
- McCune LM, Johns T. (2002). Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest. J Ethnopharmacol, 82, 197–205.
- McCune LM, Johns T. (2007). Antioxidant activity relates to plant part, life form and growing condition in some diabetes remedies. J Ethnopharmacol, 112, 461–469.
- Monteiro JM, Lins Neto EMF, Araujo EL, Amorim ELC, Albuquerque UP. (2006). The effects of seasonal climate changes in the Caatinga on tannin levels in Myracrodruon urundeuva and Anadenanthera colubrina. Revista Brasileira de Farmacognosia, 16, 338–344.
- Navarro JM, Flores P, Garrido C, Martinez V. (2006). Changes in the contents of antioxidants compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chemistry, 96, 66–73.
- Peltonen PA, Vapaavuori E, Julkunen-tiitto R. (2005). Accumulation of phenolic compounds in birch leaves is changed by elevated carbon dioxide and ozone. Global Change Biology, 11, 1305–1324.
- Ramirez MR, Geracitano L, Barros DM, Henriques AT. (2009). Efectos beneficiosos de extractos de frutas rojas y de sus antocianos. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 8, 456–468.
- Sampaio EVSB. (2002). Uso das plantas da Caatinga. Pp. 4990. In: Sampaio EVSB, Giulietti AM, Virgínio J, Gamarra-Rojas CFL. (orgs.). Vegetação e flora da Caatinga. Recife, Brazil: APNE/CNIP.
- Santos SC, Costa WF, Batista F, Santos LR, Ferri PH, Ferreira HD, Seraphin JC. (2006). Brazilian Seasonal variation in the content of tannins in barks of barbatimão species. J Pharmacog, 16, 552–556.
- Siatka T, Kašparová M. (2010). Seasonal variation in total phenolic and flavonoid contents and DPPH scavenging activity of Bellis perennis L. flowers. Molecules, 15, 9450–9461.
- Sousa CMM, Rocha e Silva H, Vieira Jr GM, Ayres MCC, Costa CLS, Araújo DS, Cavalcante LCD, Barros EDS, Araújo PBM, Brandão MS, Chaves MH. (2007). Fenóis totais e atividade antioxidante de cinco plantas medicinais. Química Nova, 30, 351–355.
- Zhang SJ, Lin YM, Zhou HC, Wei SD, Lin GH, Ye GF. (2010). Antioxidant tannins from stem bark and fine root of Casuarina equisetifolia. Molecules, 15, 5658–5670.
- Wieser G, Tegischer K, Tausz M, Häberle KH, Grams TE, Matyssek R. (2002). Age effects on Norway spruce (Picea abies) susceptibility to ozone uptake: A novel approach relating stress avoidance to defense. Tree Physiol, 22, 583–590.
- Wieser G, Hecke K, Tausz M, Häberle K-H, Grams TEE, Matyssek R. (2003). The influence of microclimate and tree age on the defense capacity of European beech (Fagus sylvatica L.) against oxidative stress. Annals of Forest Science, 60, 131–135.
- Wildi B, Lütz C. (1996). Antioxidant composition of selected high alpine plant species from different altitudes. Plant Cell Environ, 19, 138–146.
