Abstract
Context: Wogonin is a flavone derivative isolated from Scutellaria baicalensis Georgi (Labiatae) root, which is a traditional Chinese drug used as an anti-inflammatory and for management of dysmenorrhea.
Objective: The effect of wogonin on the uterus has not yet been examined. We investigated the relaxant effects of wogonin on contractile activity of isolated uterine strips of rats.
Materials and methods: The effect of wogonin on spontaneous uterine contraction, and uterine contraction induced by agonists, K+-depolarization and oxytocin in Ca2+-free solution was observed. To clarify the type of potassium channel, we tested the effects of 4-aminopyridine, tetraethylammonium and glibenclamide.
Results: Wogonin reduced the contractile amplitude of uterine strip smooth muscle of rats in a dose-dependent manner. The concentration of wogonin for reducing the contraction amplitude by 50% (IC50) on spontaneous contractions was 60.5 μM. Wogonin also inhibited the contraction induced by three agonists (oxytocin, prostaglandin F2α and acetylcholine). For the uterine strips pretreated with oxytocin in Ca2+-free solution or K+-depolarization, wogonin showed relaxant effect on the induced uterine contractions. In addition, whereas the inhibitive effect of wogonin on the contraction of uterine smooth muscle in rats could be partly blocked by 4-aminopyridine and tetraethylammonium, it was not influenced by glibenclamide.
Discussion and conclusion: Wogonin significantly inhibited the contraction of rat uterine smooth muscle probably through the inhibition of the inflow of extracellular calcium into cells via cell membrane, and intracellular release of calcium ions. In addition, the relaxant effect induced by wogonin might be due in part to the opening of voltage-dependent and large conductance Ca2+-activated K+ channels.
Introduction
Dysmenorrhea, classified in primary and secondary forms, is a common problem for women of child-bearing age, and may occur in up to 50% of menstruating women. Primary dysmenorrhea refers to menstrual cramping pain in the lower abdomen caused by enhanced uterine contractions, in the absence of underlying pelvic pathology (CitationTonini, 2002). Nonsteroidal anti-inflammatory drugs (NSAIDs) are the main treatment in clinical medicine for dysmenorrhea, but they have many side effects (CitationLefebvre et al., 2005).
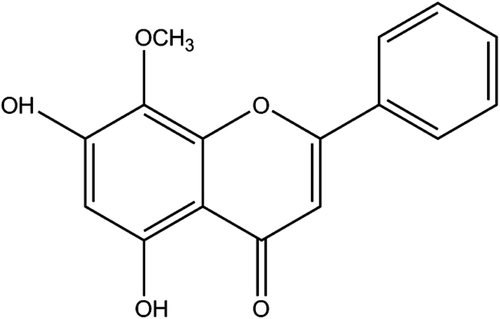
Scutellaria baicalensis Georgi (Labiatae) is a medicinal herb widely used for the treatment of various inflammatory diseases, hepatitis, tumors, allergic reactions, diarrhea, and dysmenorrhea in East Asian countries including China, Korea, Taiwan, and Japan (CitationKubo et al., 1994; CitationLin et al., 2005). One of the active components in the plant is 5, 7-dihydroxy-8-methoxyflavone, also known as wogonin (). Wogonin is a flavonoid and has been shown to exert antioxidant (CitationGao et al., 1999), antiviral (CitationMa et al., 2002; CitationGuo et al., 2007), antithrombotic (CitationKimura et al., 1997) and anti-inflammatory (CitationChen et al., 2001; CitationGuo et al., 2007) activities. However, the relaxant effect of wogonin on the uterus contractions has not yet been examined. In this paper, we analyzed the relaxant effect and physiological mechanisms of wogonin on the rat uterine contractions.
Materials and methods
Plant material and extraction
Wogonin was extracted from dried S. baicalensis. The dried roots of S. baicalensis were purchased from SanYan Medicine Corporation (Taipei, Taiwan). Authenticity of the plant species was validated by the specific morphological and anatomical features and thin-layer chromatography (CitationLin et al., 1990). The voucher specimen was deposited at the laboratory in the Graduate Institute of Pharmacognosy, Taipei Medical University.
In brief, dried S. baicalensis roots were cut into small pieces, immersed, and extracted with 10-fold v/w 50% aqueous ethanol twice at room temperature for 2 weeks. After filtration, the residues were reflux-extracted with 4-fold v/w of 50% aqueous ethanol twice for 6 h. The 50% aqueous ethanol extracts were subjected to column chromatography on silica gel eluted with CHCl3 and CHCl3-MeOH, and chromatographed on silica gel eluted with hexane-acetone to yield wogonin. The compound was identified by direct comparison of its electrospray ionization (ESI) mass, 1H- and 13C-nuclear magnetic resonance (NMR) spectroscopic data with authentic samples. Purity tests of wogonin were performed by high-performance liquid chromatography (HPLC). The HPLC system consisted of a Shimadzu model LC-10AT system (Kyoto, Japan) equipped with a Shimadzu model SIL-9A autoinjector and a Shimadzu model SPD-10 a detector (Shimadzu, Kyoto, Japan). The samples were separated on a Supelocosil TMLC18 (4.6 mm × 250 mm, 5 μm) with CH3OH-CH3COOH-H2O (5:5:90, A) and CH3OH-CH3COOH-H2O (90:5:5, B) as mobile phase at flow rate of 1.0 ml/min gradient elution, with UV detection at 275 nm, 26°C in 0–40 min (A:B = 6:4–2:8). The results showed the three kinds of active components could be separated completely in 30 min. The purity of all compounds exceeded 99.0% (CitationLi et al., 2004).
Reagents
Oxytocin (OXT), acetylcholine HCl (ACh), dimethyl sulfoxide (DMSO), 4-aminopyridine (4-AP), tetraethylammonium (TEA) and glibenclamide (Glib) were purchased from Sigma, St. Louis, MO, USA. Diethylstilbestrol was purchased from China Chemical & Pharmaceutical, (Taiwan), and prostaglandin F2α (PGF2α) from Ono Pharmaceutical (Japan). The stock solutions of all drugs were diluted to the desired concentrations with a physiological salt solution.
Animals
We used non-pregnant female Wistar rats weighing 250–350 g purchased from the Center of Experimental Animals, National Taiwan University, Taipei, Taiwan. All experiments were performed in accordance with guidelines for animal experiments of Taipei Medical University and the guiding principles for the care and use of laboratory animals approved by the Chinese Society of Laboratory Animal Sciences, Taiwan. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Isolation of rat uterus
Diethylstilbestrol (0.5 mg/kg) was subcutaneously injected to female Wistar rats 24 h before operation to induce estrus and to increase drug sensitivity of uterus. Thereafter, animals were sacrificed by decapitation, and the uterus was excised and cut into two muscle strips (each 1 cm). The uterine strips were immediately removed and placed in a flask containing Locke’s solution of the following composition (in mM): NaCl 154, KCl 5.63, NaHCO3 1.79, CaCl2·2H2O 2.55, and glucose 5.55. Experiments commenced within 5 min of removal of tissue samples. Preparations were placed in isolated organ baths, incubated in Locke’s solution at 37 ± 1°C and bubbled with gas (95% O2, 5% CO2). The preload was 1 g, and the equilibration period was no less than 45 min (CitationZafra-Polo et al., 1993). Contractions were recorded by force displacement transducers (Kent Scientific Corporation, USA) using MP100 Biological System workstation software (Biopac Systems, Inc, USA) on a PC.
Experimental procedure
Experimental observation of wogonin effect on spontaneous contractions
Rat uterine strips were equilibrated in Locke’s solution for 50 min. Cumulative amounts of wogonin (1–100 µM) were added every 15 min. Control experiments were performed with the vehicle (0.1% DMSO). The amplitude of spontaneous contractions on the uterine smooth muscle was recorded.
Experimental observation of wogonin effect on agonist-induced contractions
Uterine strips were incubated in the Locke’s solution and equilibrated for 50 min, then treated with OXT (0.01 U/ml bath concentration), PGF2α (0.1 μM bath concentration) and ACh (1 μM bath concentration), respectively, for 15 min, and further treated with wogonin (1–100 µM), respectively, every 15 min in the presence of OXT, PGF2α and ACh. Control experiments were performed with the vehicle (0.1% DMSO).
Experimental observation of wogonin effect on OXT-induced Ca2+-free contractions
Uterine smooth muscle strips were equilibrated for 1 h in Locke’s solution. The solution was then replaced with Ca2+-free solution containing 3 mM EDTA, and incubation continued for 50 min. Subsequently, the solution was replaced by a Ca2+-free solution containing 1 mM EDTA, and the uterus was incubated for an additional 20–30 min. After a sustained contractile response to OXT (0.01 U/ml) was obtained, cumulative concentrations of wogonin (1–100 µM) was added every 15 min, respectively, in the presence of OXT. Tensions of uterine smooth muscle strips were recorded over a 10 min period after administration.
Experimental observation of wogonin effect on contractions induced by K+-depolarization
The organ was immersed in Jalon–Ringer solution and equilibrated for 50 min. This solution was replaced by a high K+ condition (KCl 56.3 mM) that caused a rapid contraction, followed by slight relaxation and a prolonged contraction plateau. When the plateau was reached, cumulative concentrations of wogonin (1–100 μM) were administered, and concentration-related relaxations were observed.
Experimental observation of wogonin effect involved in the potassium channels
In order to test the involvement of potassium channels in the mechanism of action of wogonin, we used three kinds of potassium channel blockers including 4-AP [a voltage-dependent potassium channel (KV) blocker; 5 mM], TEA [a large conductance Ca2+-activated potassium channel (BKCa) blocker; 1 mM] and Glib [an adenosine triphosphate (ATP)-dependent potassium channel (KATP) blocker, 30 μM]. These blockers were respectively applied to the uterine strips once at the beginning of experiment. After 15 min, wogonin was added every 15 min in a cumulative way. The cumulative concentration-response curves of wogonin for each case were then constructed and compared with those obtained with uterine smooth muscle strips that had not been treated with these inhibitors.
Statistical analysis
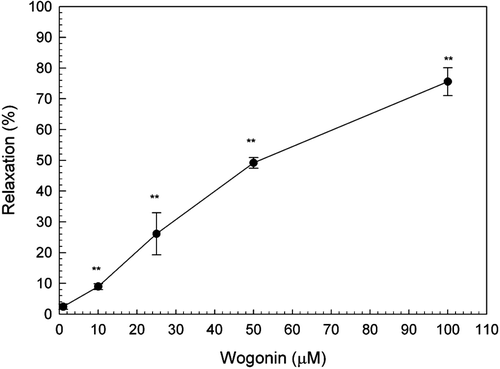
The results are expressed as the mean ± SE of several preparations (n) from different animals. The amplitude of contractions occurring over a 10 min period before adding wogonin was taken as the maximum contraction amplitude. Relaxation was expressed as a percentage of reduction in the contraction amplitude from the maximum to the value after wogonin treatment. According to the dose–response curves, the concentration of wogonin for producing 50% of relaxation (IC50) was estimated. Statistical analysis of the results was performed using one-way analysis of variance (ANOVA) and Student’s t-test. Significant differences with controls are shown as *p < 0.05 and **p < 0.01.
Results
Effects of wogonin on spontaneous contractions of the rat uterus
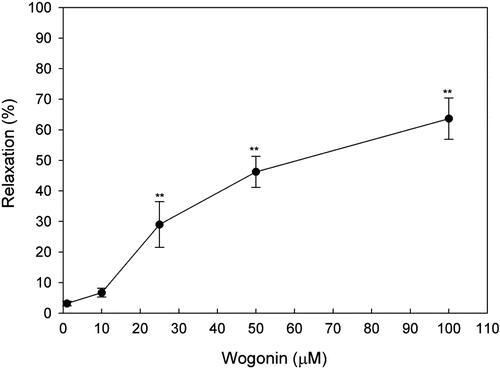
Application of wogonin, in a cumulative fashion, inhibited spontaneous contractions of the rat uterus in a dose-dependent manner, whereas the addition of vehicle alone (0.1% DMSO) had no effect. Different concentrations of wogonin could attenuate contractile activity of uterine strips and degrade the amplitude of contraction wave. The IC50 value of wogonin on the amplitude of spontaneous uterine contractions was 62.03 µM ().
Effects of wogonin on uterine contractions induced by agonists
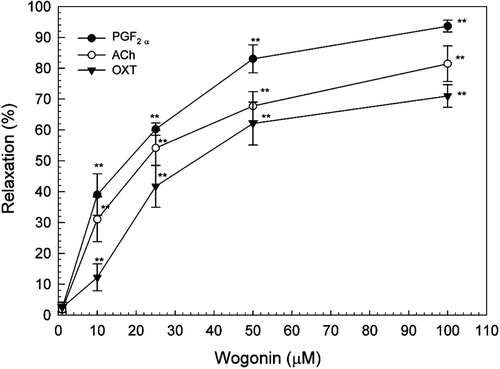
Wogonin exhibited a dose-dependent relaxant effect on the rat isolated uterus precontracted with PGF2α (0.1 μM), ACh (1 μM) and OXT (0.01 U/ml). The IC50 values of wogonin on PGF2α-, ACh-, and OXT-induced contractions were 17.97, 22.80 and 35.65 μM, respectively. This indicates that wogonin had stronger effect in inhibiting PGF2α-induced uterine contractions ().
Effect of wogonin on OXT-induced uterine contractions in the Ca2+-free solution
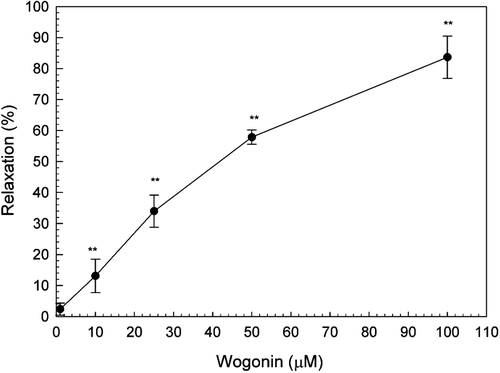
A uterine smooth muscle strip was incubated in a Ca2+-free solution containing 3 mM EDTA for 50 min, and then in a Ca2+-free solution containing 1 mM EDTA for an additional 20–30 min. A sustained contractile response to OXT (0.01 U/ml) was obtained, and cumulative amounts of wogonin were added. It was found that with the increase in the concentration of wogonin, uterine contractions were gradually inhibited (). The inhibitory effect of wogonin was significant at the doses of 10, 25, 50 and 100 μM (p < 0.01).
Effect of wogonin on uterinei contractions induced by K+-depolarization (high K+ condition)
The effect of wogonin on a high concentration of potassium (KCl 56.3 mM)-induced uterine contractions was examined. As shown in () the uterine contractions induced by K+ depolarization were gradually inhibited with increasing concentrations of wogonin reaching the significant level at 10, 25, 50 and 100 μM (p < 0.01). The IC50 of the wogonin was 52.37 μM.
Effects of potassium channel blockers on wogonin-induced relaxation in uterine smooth muscle
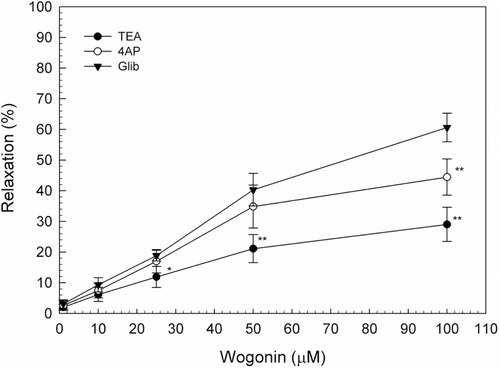
To clarify the type of potassium channel, we tested the effect of 4-AP (a KV blocker; 5 mM), TEA (a BKCa blocker; 1 mM) and Glib (a KATP blocker; 30 μM). TEA and 4-AP could partially block the inhibitive effect of wogonin on the contraction of uterine smooth muscle in rats. Comparing with the control group the influences of TEA and 4-AP had significant difference in the contractile amplitude after variance analysis (p < 0.01), while Glib had no obvious blocking effect on the inhibition of contractile activity of uterine smooth muscle in rats ().
Discussion
There are several studies which evaluated the effect of Chinese medicinal prescriptions for the dysmenorrhea. CitationCalixto et al. (1991) investigated the effect of Leonotis nepetaefolia R. Br. (Labiatae) on rat uterus contractions in vitro; Perez-Vallina et al. (1995) investigated the effect of nonsteroid anti-inflammatory drugs on rat uterus contractions induced by PGF2α or KCl in vitro. Our group has investigated the effect of Chinese medicinal prescriptions for dysmenorrhea (CitationHsu et al., 2003, 2006). Our results in this study ( and ) demonstrate that wogonin, one of the major components of the herb S. baicalensis, exerted significant relaxant effects on spontaneous and agonist (OXT, PGF2α and ACh)-induced uterine contractions. It may contribute to its important role in treating dysmenorrhea.
When the intracellular Ca2+ ion concentration in uterus smooth muscle exceeds 10−6 M, uterine contractions will be induced (CitationFritsch & Murdoch, 1997). There are four different Ca2+ influx channels which regulate the intracellular Ca2+ ion concentration: receptor-operated channels (ROCs), voltage-operated channels (VOCs), second messenger-operated channels and store-operated channels (SOCs) (CitationBolton, 1979; CitationD’Ocon et al., 1991; CitationHurwitz, 1986; CitationTriggle et al., 1989).
Recently, some other factors have been described as contributing to this contraction such as increase of intracellular concentration of inositol triphosphate (IP3) or arachidonic acid production, which then increases the intracellular Ca2+ concentration (CitationPhillippe & Chien, 1995). On the other hand, rat uterus OXT-induced contraction results from increases of cytosolic Ca2+ originating from IP3-sensitive intracellular stores and of calcium influx via ROCs (CitationTrujillo et al., 2000). In our experiment using a Ca2+-free solution, the Ca2+ influx channels will not be the causes for the increase of intracellular Ca2+ concentration. Therefore, our experimental results of wogonin effect on OXT-induced uterine contractions in the Ca2+-free solution () show that wogonin is able to alleviates uterine contractions through inhibiting the release of Ca2+ from intracellular stores.
The intracellular Ca2+ ion concentration is also affected by membrane potential because depolarization of membrane potential opens the voltage-gated Ca2+ channels and then the extracellular Ca2+ ions migrate into the cell, resulting in the induction of uterine contractions (CitationD’Ocon et al., 1991). High K+ condition changes membrane potential, and therefore we also investigated the effects of wogonin on K+ depolarization-induced uterine contractions in this study. The findings shown in reveal that wogonin at 10, 25, 50 and 100 μM could significantly inhibit the uterine contraction induced by K+ (56.3 mM KCl) depolarization. This suggests that wogonin might have a role to stabilize the membrane potential of the uterine smooth muscle cells, and subsequently decrease uterine contractions by decreasing the membrane action potential.
At lease three types of potassium ion currents have been described in rat myometrium: a fast transient current and two calcium-dependent non-inactivating currents. A single-channel recording experiment revealed that large-conductance calcium-dependent potassium channels appear in the myometrium of both pregnant and non-pregnant (CitationTriggle, 1996) and their activation results in cell hyperpolarization and suppression of the concentration of intracellular Ca2+. Therefore, a potassium channel opener is a strong uterine relaxant.
Potassium channels are composed of diverse groups of membrane channels including a voltage-dependent potassium channel (KV), a large conductance Ca2+-activated potassium channel (BKCa) and an ATP-dependent potassium channel (KATP). Opening of potassium channels causes hyperpolarization of the plasma membrane by increasing potassium conductance and reduces cell excitability by shifting the membrane potential away from the threshold for excitation (CitationKoji et al., 1999). In the present study, we used potassium channel blockers, including 4-AP, TEA and Glib. These results () showed that wogonin-induced relaxation of the uterus was blocked by TEA and 4-AP but not by Glib. The present findings suggested that the relaxation effect induced by wogonin may be partially due to BKCa and KV in isolated uterine smooth muscle strips.
Conclusion
The present findings clearly show that wogonin has multiple effects on the uterine smooth muscles. The mechanism may be related to that it can inhibit extracellular calcium inflowing into cells via cell membrane and intracellular release of calcium ions. In addition, wogonin induced relaxation responses in uterus smooth muscle may be dependent on the activation of Kv and BKCa potassium channels. These results indicate that wogonin is one of the active ingredients of S. baicalensis and has the potential to be developed into an effective drug for the treatment of primary dysmenorrhea.
Acknowledgments
We are grateful to the research grant from Alumni Foundation of Taipei Medical University, Taipei, Taiwan.
Declaration of interest
The authors report no conflicts of interest.
References
- Bolton TB. (1979). Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev, 59, 606–718.
- Calixto JB, Yunes RA, Rae GA. (1991). Effect of crude extracts from Leonotis nepetaefolia (Labiatae) on rat and guinea-pig smooth muscle and rat cardiac muscle. J Pharm Pharmacol, 43, 529–534.
- Chen YC, Shen SC, Chen LG, Lee TJ, Yang LL. (2001). Wogonin, baicalin, and baicalein inhibition of inducible nitric oxide synthase and cyclooxygenase-2 gene expressions induced by nitric oxide synthase inhibitors and lipopolysaccharide. Biochem Pharmacol, 61, 1417–1427.
- D’Ocon P, Blasco R, Candenas L, Ivorra D, López S, Villaverde C, Castedo L, Cortes D. (1991). Inhibition of calcium entry induced by cularines and isocrasifoline in uterine smooth muscle. Eur J Pharmacol, 196, 183–187.
- Fritsch MK, Murdoch FE. (1997). Estrogens, progestins, and contraceptives. In: Brody TM, Larner J, Minneman KP, eds. Human Pharmacology: Molecular to Clinical. Missouri: Mosby Year Book, 499–518.
- Gao Z, Huang K, Yang X, Xu H. (1999). Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim Biophys Acta, 1472, 643–650.
- Guo Q, Zhao L, You Q, Yang Y, Gu H, Song G, Lu N, Xin J. (2007). Anti-hepatitis B virus activity of wogonin in vitro and in vivo. Antiviral Res, 74, 16–24.
- Hsu CS, Yang JK, Yang LL. (2003). Effect of a dysmenorrhea Chinese medicinal prescription on uterus contractility in vitro. Phytother Res, 17, 778–783.
- Hsu CS, Yang JK, Yang LL. (2006). Effect of “Dang-Qui-Shao-Yao-San” a Chinese medicinal prescription for dysmenorrhea on uterus contractility in vitro. Phytomedicine, 13, 94–100.
- Hurwitz L. (1986). Pharmacology of calcium channels and smooth muscle. Annu Rev Pharmacol Toxicol, 26, 225–258.
- Kimura Y, Okuda H, Ogita Z. (1997). Effects of flavonoids isolated from scutellariae radix on fibrinolytic system induced by trypsin in human umbilical vein endothelial cells. J Nat Prod, 60, 598–601.
- Koji O, Yoshihito I, Hiroyuki S. (1999). Estradiol inhibits Ca2+ and K+ channels in smooth muscle cells from pregnant rat myometrium. Eur J Pharmacol, 376, 101–108.
- Kubo M, Asano T, Shiomoto H, Matsuda H. (1994). Studies on rehmanniae radix. I. Effect of 50% ethanolic extract from steamed and dried rehmanniae radix on hemorheology in arthritic and thrombosic rats. Biol Pharm Bull, 17, 1282–1286.
- Lefebvre G, Pinsonneault O, Antao V, Black A, Burnett M, Feldman K, Lea R, Robert M; SOGC. (2005). Primary dysmenorrhea consensus guideline. J Obstet Gynaecol Can, 27, 1117–1146.
- Li HB, Jiang Y, Chen F. (2004). Separation methods used for Scutellaria baicalensis active components. J Chromatogr B Analyt Technol Biomed Life Sci, 812, 277–290.
- Lin GB, Xie JX, Yao P. (2005). Parmacodynamic study of Fufanghuangqin (FFHQ). Lishizhen Medicine and Materia Medica Research, 16, 862–863.
- Lin SJ, Hsieh-Wang JJ, Tsai MJ, Tseng CF, Wen KC. (1990). Studies on the thin-layer chromatographic identification of Scutellatiae radix and this herb in some Chinese herbal preparations. Ann Rept NLFD, 8, 288–290.
- Ma SC, Du J, But PP, Deng XL, Zhang YW, Ooi VE, Xu HX, Lee SH, Lee SF. (2002). Antiviral Chinese medicinal herbs against respiratory syncytial virus. J Ethnopharmacol, 79, 205–211.
- Pérez Vallina JR, Cantabrana B, Hidalgo A. (1995). Calcium-and G-protein-related spasmolytic effects of nonsteroidal anti-inflammatory drugs on rat uterus contractions in vitro. Pharmacology, 50, 324–332.
- Phillippe M, Chien EK. (1995). Potassium chloride effects on the hormonal signal transduction mechanisms underlying phasic myometrial contractions. J Endocrinol, 146, 485–493.
- Tonini G. (2002). [Dysmenorrhea, endometriosis and premenstrual syndrome]. Minerva Pediatr, 54, 525–538.
- Triggle DJ. (1996). Depolarization as a regulatory signal at voltage-gated calcium channels. Zhongguo Yao Li Xue Bao, 17, 193–196.
- Triggle DJ, Langs DA, Janis RA. (1989). Ca2+ channel ligands: Structure-function relationships of the 1,4-dihydropyridines. Med Res Rev, 9, 123–180.
- Trujillo MM, Ausina P, Savineau JP, Marthan R, Strippoli G, Advenier C, Pinto FM, Candenas ML. (2000). Cellular mechanisms involved in iso-osmotic high K+ solutions-induced contraction of the estrogen-primed rat myometrium. Life Sci, 66, 2441–2453.
- Zafra-Polo MC, Tormos MJ, Cortes D, Anselmi E. (1993). Comparative study of the rat uterine smooth muscle relaxant activity of three bisbenzyltetrahydroisoquinolines with tetrandrine. J Pharm Pharmacol, 45, 563–566.