Abstract
Context: Macrothelypteris oligophlebia (Bak.) Ching (Thelypteridaceae) is a Chinese herbal medicine used traditionally for the treatment of diseases such as edema, boils, burns, and roundworms. However, research about the nephroprotective potential of this plant is not available.
Objective: Present study was designed to evaluate the protective effect of ethanol extract of M. oligophlebia rhizomes (EMO) on gentamicin (GM)-induced nephrotoxicity.
Materials and methods: Rats were intraperitoneal (i.p.) injected with GM (100 mg/kg) to induce nephrotoxicity and simultaneously EMO (250 and 500 mg/kg) was orally given to GM-treated rats for 8 days. Blood urea nitrogen (BUN), serum creatinine (Cr), malondialdehyde (MDA), nitric oxide (NO), superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) were evaluated in renal tissues. Histopathological analysis was used for evaluation of the renal damage.
Results: Administration with GM-induced renal dysfunction in rats. Pre-treatment with EMO (500 mg/kg) significantly decreased the levels of BUN, Cr, MDA and NO (decreased BUN from 12.71 ± 1.28 to 7.19 ± 0.23 mmol/l, Cr from 39.77 ± 5.34 to 19.17 ± 0.90 μmol/l, MDA from 5.60 ± 0.37 to 2.63 ± 0.24 nmol/ml, and NO from 868.17 ± 22.67 to 589.51 ± 8.83 μmol/ml), and also restored the activities of renal antioxidant enzymes (SOD, CAT, and GSH-Px) (restored SOD from 1.59 ± 0.17 to 2.94 ± 0.13 U/mg protein, CAT from 3.22 ± 0.34 to 10.57 ± 0.27 U/mg protein, and GSH-Px from 9.11 ± 1.29 to 20.72 ± 1.83 U/mg protein).
Discussion and conclusion: Our results suggest that the rhizomes of M. oligophlebia potentially have a protective role in renal tissue against oxidative stress in acute renal failure.
Introduction
Gentamicin (GM), an aminoglycoside class of bactericidal antibiotic, is effective against Gram-negative bacterial infections (CitationAl-Qarawi et al., 2008). However, the clinical use of GM is limited by its major drawback, acute renal failure (ARF), accounting for 10–20% of all cases of nephrotoxicity (CitationErdem et al., 2000). GM-induced nephrotoxicity is characterized by increased levels of serum creatinine (Cr) and blood urea nitrogen (BUN), decreased glomerular filtration rate (GFR) and morphological alterations (CitationSoliman et al., 2007; CitationBalakumar et al., 2010).
A growing body of experimental evidence both in vitro and in vivo demonstrates that GM-induced renal injury is believed to involve the generation and release of reactive oxygen species (ROS) in the renal cortex (CitationYanagida et al., 2004; CitationPolat et al., 2006; CitationYamam & Balikci, 2010). This is considered as one of the important mechanisms for GM-induced nephrotoxicity and other deleterious effects (CitationKhan et al., 2009). ROS, like super oxide anion, hydroxyl radical and hydrogen peroxide, exhibit beneficial effects on cellular responses and immune function at moderate concentration, but at high levels, ROS can produce cell injury including peroxidation of membrane lipids, proteins denaturation and DNA damage (CitationPham-Huy et al., 2008; CitationSandeep & Nair, 2010). ROS have been shown to affect several biological processes which are potentially important in glomerular disease and also in the pathogenesis of ischemic renal injury (CitationShah, 1984; CitationShah et al., 1987; CitationParlakpinar et al., 2006). To the best of our knowledge, GM also acts as an iron chelator which is capable of catalyzing free radical formation (CitationYanagida et al., 2004). Hence, drugs that serve as free radical scavengers or iron chelators may effectively prevent nephrotoxicity induced by GM.
Herbal medicine has a long history in the treatment of renal injury. A number of crude herbal extract, such as Phyllanthus amarus Schum. and Thonn. (Euphorbiaceae), Sida rhomboidea. Roxb (Malvaceae), and Nigella sativa Linn. (Ranunculaceae) have been reported to prevent GM-induced nephrotoxicity (CitationBegum et al., 2006; CitationAdeneye & Benebo, 2008; CitationThounaojam et al., 2010).
Macrothelypteris oligophlebia (Bak.) Ching (Thelypteridaceae) is widely distributed in southwestern China. Its rhizomes have been used as a folk medicine mainly for the treatment of diseases such as edema, boils, burns, and roundworms (CitationEditorial Board of China Herba, 1998). Study on the chemical constituents of this species has been conducted previously and several flavonoids were isolated, including kaempferol, rutin and kaempferol-3-O-β-rutinoside (CitationHori et al., 1987). However, reports on the pharmacological effects of M. oligophlebia have not been documented. The present study was designed to investigate the nephroprotective activity of ethanol extract of M. oligophlebia rhizomes against GM-induced ARF in vivo.
Materials and methods
Chemicals
Commercial kits for measure BUN and Cr were obtained from Jiancheng Biotechnology Institute (Nanjing, China). Bovine serum albumin and gentamicin (GM) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other reagents were of analytical grade and purchased from Sinopharm chemical Reagent Co. Ltd. (Shanghai, China).
Plant material
The air-dried rhizomes of M. oligophlebia were collected during June 2009 in the Jiangxi province, China and identified by Prof. Ceming Tan, Forest Plants Specimen Mansion. A voucher specimen (MTF0812) was deposited in College of Pharmacy, Huazhong University of Science and Technology.
Preparation of extract
The air-dried rhizomes of M. oligophlebia (1.5 kg) were ground and extracted with 75% ethanol at 95°C for 2 h. The resulting solvent was concentrated in an evaporator to obtain a crude extract and the yield was 10.4 g/100 g of the raw material.
For animals test, an appropriate weight of ethanol extract of M. oligophlebia rhizomes (EMO) was dissolved with distilled water to get two dosages (250 and 500 mg/kg body weight).
Phytochemical studies
The ethanol extract of M. oligophlebia rhizomes were subjected to column chromatography over silica gel and Sephadex LH-20 repeatedly. The isolated compounds were identified according to the spectral techniques and compared with the previous data reported in the literature.
Test animals
Male Wister rats, weighing 200 ± 20 g each, were purchased from the Research Center of Laboratory Animals, Tongji Medical Center, Huazhong University of Science and Technology, Wuhan, China. The animals were placed under a 12 h light-dark cycle at controlled temperature (23 ± 2°C) and humidity (60 ± 5%). They were fed with standard mice chow and tap water ad libitum and acclimated 7 days before experiment. All animals were fasted 12 h prior to experiment but free access to water. All experiments were conducted in accordance with the Chinese Community guidelines for the use of experimental animals and approved by the Huazhong University of Science and Technology Committee on Animal Care and Use.
Gentamicin-induced acute renal toxicity in rats
Animals were divided into four groups, each group comprises of eight rats. Group I (control group) animals were injected intraperitoneal (i.p.) with saline for 8 days. Group II (GM-treated group) animals were injected i.p. with GM (100 mg/kg body weight) for 8 days (CitationNitha & Janardhanan, 2008; CitationThounaojam et al., 2010). Group III and IV animals were administered orally with EMO (250 and 500 mg/kg body weight) 2 h prior to injection i.p. with GM for the same period.
After the last once of the treatment, all animals were sacrificed. Blood samples were collected to determine the serum levels of BUN and Cr. Kidney of each rat was quickly removed, harvested two pieces through a midline incision; one piece was fixed in formalin for pathological examination, the other piece was frozen at −80°C for further enzymatic analysis.
Assessment of renal function
BUN and Cr levels in serum samples were assayed by using standard diagnostic kits.
Measurement of renal antioxidants
Kidneys were rinsed immediately with 0.9% cold physiological saline and then homogenized with ice cold phosphate buffer (1:10, w/v) using a homogenizer. The obtained homogenate was centrifuged at 800 rpm for 10 min at 4°C, the resulting supernatant was further centrifuged at 10,500 rpm for 20 min at 4°C to yield the post mitochondrial supernatant, which was used to determine the superoxide dismutase (SOD), malondialdehyde (MDA), catalase (CAT), nitric oxide (NO) and glutathione peroxidase (GSH-Px) (CitationPaglia & Valentine, 1967; CitationMarklund & Marklund, 1974; CitationZhong et al., 2009; CitationMohan et al, 2010; CitationKoppula & Choi., 2011). Protein concentrations in tissue homogenates were determined by the method described previously using bovine serum albumin as the standard (CitationBradford, 1976).
Histopathological evaluation
The tissue samples were fixed in 10% neutral buffered formalin for 1 day, dehydrated in ascending graded series of alcohol and embedded in paraffin, 6 μm of the tissue sections were stained with hematoxylin and eosin. The kidney tissues were examined under light microscope to evaluate their pathological symptoms such as lymphocyte infiltration, necrosis and cytoplasmic vacuolization.
Statistical analysis
Data were represented as mean ± standard deviation (S.D.). Results were analyzed by one-way ANOVA followed by the Dunnett post hoc test. Differences were considered significant at p < 0.05.
Results
Phytochemical studies
The isolated compounds were identified as matteucinol, naringenin, naringenin-4′-O-glucoside, protoapigenone, 5,7-dihydroxy-6,8-dimethyl flavanone and protoapigenin-4′-O-β-d-glucoside. All these components belong to the flavonoid group.
Effect of EMO treatment on renal function
As shown in , serum levels of BUN and Cr were marked higher in GM-treated group as compared to the control group, indicating the formation of severe nephrotoxicity. Pre-treatment with EMO significantly attenuated the increased levels of BUN and Cr induced by GM.
Table 1. Effect of EMO on BUN, Cr, SOD, MDA, CAT, NO and GSH-Px activities in rats treated with GM.
Effect of EMO treatment on kidney tissue enzymes
The activities of SOD, NO, CAT, MDA, and GSH-Px in each group were shown in . In comparison with the control group, rats that only treated with GM significantly elevated the concentrations of MDA and NO, and decreased the activities of SOD, CAT and GSH-Px. Administration of EMO to rats resulted in a marked reduction in MDA and NO levels, and an increase in SOD, CAT and GSH-Px activities.
Histopathological observations
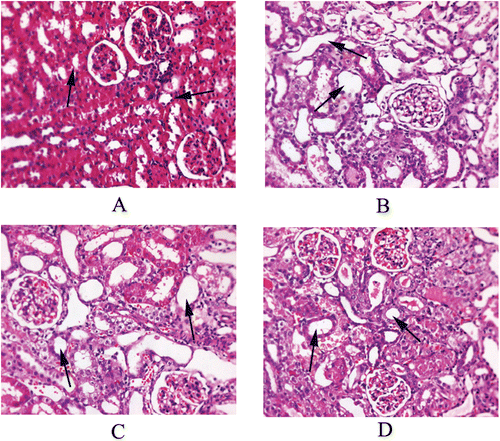
Histology of kidney sections from rats treated with GM alone showed obvious features of ARF (), as compared to the control group (. From the picture, marked tubular necrosis in renal cortex was observed. Pre-treatment with 500 mg/kg dose of EMO provided the best histological protection against GM-induced renal tubular damage.
Figure 1. Representative imagines of kidney from rats. (A) Control group: section shows normal tubules; (B) Gentamicin (100 mg/kg) treated group: tubule shows extensive and marked necrosis; (C) Gentamicin + EMO (250 mg/kg) treated group: tubules show limited damage; (D) Gentamicin + EMO (500 mg/kg) treated group: tubule reveals slight degenerative change. EMO, ethanol extract of Macrothelypteris oligophlebia rhizomes.
Discussion
Preliminary phytochemical studies on EMO revealed that flavonoids were its characteristic constituents. Flavonoids are polyphenolics that commonly occur in herbal medicine. They have been proposed to have the ability to scavenge free radicals such as peroxide, hydroperoxide or lipid peroxyl and thus inhibit the oxidative stress that give rise to deteriorative disease (CitationJadeja et al., 2009; CitationMishra et al., 2010). These six known flavonoids obtained from EMO were isolated for the first time from this plant. Naringenin and protoapigenone have been shown to possess antioxidant, nephroprotective, neuroprotective and antitumor activities (CitationBadary et al., 2005; CitationYoudim & Bakhle, 2006; CitationHuang et al., 2010). In this study, the nephroprotective activity of ethanol extract of M. oligophlebia rhizomes was evaluated for the first time.
The nephroprotective activity of EMO was assessed on renal injury induced by GM in rats. GM is widely used to investigate nephroprotective activity in animal models. One mechanism of GM-induced nephrotoxicity is believed to involve in renal oxidative stress (CitationKhan et al., 2009; CitationBalakumar et al., 2010; CitationYaman & Balikci, 2010). Moreover, this underlying mechanism has been supported by the fact that administration of several components with antioxidant properties, free radical scavengers and antioxidant enzyme are capable of preventing GM-induced nephrotoxicity (CitationBalakumar et al., 2010). This, for instance, including vitamin E (CitationVarzi et al., 2007), N-acetylcysteine (CitationMazzon et al., 2001), SOD mimetic (CitationCuzzocrea et al., 2002), and curcumin (CitationFarombi & Ekor, 2006). Furthermore, our results confirm the hypothesis that ARF is related to renal oxidative injury.
In the present study, we have shown that administration of GM at a dose of 100 mg/kg eventuated in renal dysfunction indicated by the elevated levels of Cr and BUN as well as histopathological evaluation which revealed marked and extensive tubular necrosis throughout the cortex. Oral administration of EMO was found to have a protective potential against GM-induced nephrotoxicity. Increased levels of Cr and BUN, accompanied by the tubular necrosis in renal tissue were significantly prevented by EMO, suggesting an almost normalization of kidney function.
The activities of the renal antioxidant enzymes, such as SOD, CAT and GSH-Px, obviously depleted in the GM-treated alone group compared to the control group. This deficiency in renal antioxidant defense enzymes could aggravate the renal oxidative damage. SOD is an enzyme associated with copper, zinc, and manganese which are essential for enzyme activity (CitationSharma, 1985). It is said that SOD has the distinct ability to neutralize superoxide, one of the most damaging free radical substances in nature. The decreased SOD activity is not enough to scavenge the superoxide anion produced during the normal metabolic process and cause the initiation and propagation of lipid peroxidation in GM-treated group (CitationNitha & Janardhanan, 2008). The activities of CAT and GSH-Px also decreased in the GM-treated group, which resulted in the decreased ability of renal to scavenge toxic hydrogen peroxide and lipid peroxidation (CitationBadary et al., 2005; CitationNitha & Janardhanan, 2008). Therefore, the significant increase in the level of MDA, as marker of lipid peroxidation, was observed in the GM-treated group. Pre-treatment with EMO to rats reduced the depletion of SOD, CAT and GSH-Px activities, resulting in reduction of the renal MDA. In addition, our experimental data indicated that the increasing generation of NO was also reduced by EMO, and renal NO played a vital role in the regulation of GFR (CitationSafa et al., 2010). The results obtained in this study suggested that EMO was capable of ameliorating GM-induced renal damage.
Conclusion
In summary, the results of the present study demonstrated that ethanol extract of M. oligophlebia rhizomes showed significant nephroprotective activity against GM-induced ARF in rats. Additionally, further studies are essential to find out the active compounds from EMO that are responsible for its nephroprotective role.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (30973864); Hubei Province Natural Science Foundation (2009CDA067).
Declaration of interest
The authors declare that there are no conflicts of interest.
References
- Adeneye AA, Benebo AS. (2008). Protective effect of the aqueous leaf and seed extract of Phyllanthus amarus on gentamicin and acetaminophen-induced nephrotoxic rats. J Ethnopharmacol, 118, 318–323.
- Al-Qarawi AA, Abdel-Rahman H, Mousa HM, Ail BH, El-Mougy SA. (2008). Nephroprotective action of Phoenix dactylifera in gentamicin-induced nephrotoxicity. Pharm Biol, 46, 227–230.
- Badary OA, Abdel-Maksoud S, Ahmed WA, Owieda GH. (2005). Naringenin attenuates cisplatin nephrotoxicity in rats. Life Sci, 76, 2125–2135.
- Balakumar P, Rohilla A, Thangathirupathi A. (2010). Gentamicin-induced nephrotoxicity: Do we have a promising therapeutic approach to blunt it? Pharmacol Res, 62, 179–186.
- Begum NA, Dewan ZF, Nahar N, Mamun MR. (2006). Effect of n-hexane of Nigella sativa on gentamicin-induced nephrotoxicity in rats. Bangl J Pharmacol, 1, 16–20.
- Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 72, 248–254.
- Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Di Paola R, Britti D, De Sarro A, Pierpaoli S, Caputi A, Masini E, Salvemini D. (2002). A role for superoxide in gentamicin-mediated nephropathy in rats. Eur J Pharmacol, 450, 67–76.
- Editorial Board of China Herba. (1998). China herbal. Shanghai, China: Shanghai Scientific and Technical Publishers.
- Erdem A, Gündogan NU, Usubütün A, Kilinç K, Erdem SR, Kara A, Bozkurt A. (2000). The protective effect of taurine against gentamicin-induced acute tubular necrosis in rats. Nephrol Dial Transplant, 15, 1175–1182.
- Farombi EO, Ekor M. (2006). Curcumin attenuates gentamicin-induced renal oxidative damage in rats. Food Chem Toxicol, 44, 1443–1448.
- Hori K, Satake T, Saiki Y, Murakami T, Chen CM. (1987). Chemical and chemotaxonomical studies of Filices. LXIX. The novel coumarins of Macrothelypteris torresiana CHING var. calvata Hollt. (= M.oligophlebia Ching). Pharm Soci Jap, 107, 491–494.
- Huang XH, Xiong PC, Xiong CM, Cai YL, Wei AH, Wang JP, Liang XF, Ruan JL. (2010). In vitro and in vivo antitumor activity of Macrothelypteris torresiana and its acute/subacute oral toxicity. Phytomedicine, 17, 930–934.
- Jadeja R, Thounaojam M, Ansarullah Ramachandran, AV, Devkar R. (2009). Phytochemical constituents and free radical scavenging activity of Clerodendron glandulosum. Coleb methanolic extract. J Compl Integr Med, 6, 1–23.
- Khan SA, Priyamvada S, Farooq N, Khan S, Khan MW, Yusufi AN. (2009). Protective effect of green tea extract on gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Pharmacol Res, 59, 254–262.
- Koppula S, Choi DK. (2011). Cuminum cyminum extract attenuates scopolamine-induced memory loss and stress-induced urinary biochemical changes in rats: a noninvasive biochemical approach. Pharm Biol, 49, 702–708.
- Marklund S, Marklund G. (1974). Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem, 47, 469–474.
- Mazzon E, Britti D, De Sarro A, Caputi AP, Cuzzocrea S. (2001). Effect of N-acetylcysteine on gentamicin-mediated nephropathy in rats. Eur J Pharmacol, 424, 75–83.
- Mishra N, Dubey A, Mishra R, Barik N. (2010). Study on antioxidant activity of common dry fruits. Food Chem Toxicol, 48, 3316–3320.
- Mohan M, Kamble S, Gadhi P, Kasture S. (2010). Protective effect of Solanum torvum on doxorubicin-induced nephrotoxicity in rats. Food Chem Toxicol, 48, 436–440.
- Nitha B, Janardhanan KK. (2008). Aqueous-ethanolic extract of morel mushroom mycelium Morchella esculenta, protects cisplatin and gentamicin induced nephrotoxicity in mice. Food Chem Toxicol, 46, 3193–3199.
- Paglia DE, Valentine WN. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med, 70, 158–169.
- Parlakpinar H, Tasdemir S, Polat A, Bay-Karabulut A, Vardi N, Ucar M, Yanilmaz M, Kavakli A, Acet A. (2006). Protective effect of chelerythrine on gentamicin-induced nephrotoxicity. Cell Biochem Funct, 24, 41–48.
- Pham-Huy LA, He H, Pham-Huy C. (2008). Free radicals, antioxidants in disease and health. Int J Biomed Sci, 4, 89–96.
- Polat A, Parlakpinar H, Tasdemir S, Colak C, Vardi N, Ucar M, Emre MH, Acet A. (2006). Protective role of aminoguanidine on gentamicin-induced acute renal failure in rats. Acta Histochem, 108, 365–371.
- Safa J, Argani H, Bastani B, Nezami N, Rahimi Ardebili B, Ghorbanihaghjo A, Kalagheichi H, Amirfirouzi A, Mesgari M, Soleimany Rad J. (2010). Protective effect of grape seed extract on gentamicin-induced acute kidney injury. Iran J Kidney Dis, 4, 285–291.
- Sandeep D, Krishnan Nair CK. (2010). Amelioration of cisplatin-induced nephrotoxicity by extracts of Hemidesmus indicus and Acorus calamus. Pharm Biol, 48, 290–295.
- Shah SV. (1984). Effect of enzymatically generated reactive oxygen metabolites on the cyclic nucleotide content in isolated rat glomeruli. J Clin Invest, 74, 393–401.
- Shah SV, Baricos WH, Basci A. (1987). Degradation of human glomerular basement membrane by stimulated neutrophils. Activation of a metalloproteinase(s) by reactive oxygen metabolites. J Clin Invest, 79, 25–31.
- Sharma RP. (1985). Interactions of cis-platinum with cellular zinc and copper in rat liver and kidney tissues. Pharmacol Res Commun, 17, 197–206.
- Soliman KM, Abdul-Hamid M, Othman AI. (2007). Effect of carnosine on gentamicin-induced nephrotoxicity. Med Sci Monit, 13, BR73–BR83.
- Thounaojam MC, Jadeja RN, Devkar RV, Ramachandran AV. (2010). Sida rhomboidea Roxb leaf extract ameliorates gentamicin induced nephrotoxicity and renal dysfunction in rats. J Ethnopharmacol, 132, 365–367.
- Varzi HN, Esmailzadeh S, Morovvati H, Avizeh R, Shahriari A, Givi ME. (2007). Effect of silymarin and vitamin E on gentamicin-induced nephrotoxicity in dogs. J Vet Pharmacol Ther, 30, 477–481.
- Yaman I, Balikci E. (2010). Protective effects of Nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp Toxicol Pathol, 62, 183–190.
- Yanagida C, Ito K, Komiya I, Horie T. (2004). Protective effect of fosfomycin on gentamicin-induced lipid peroxidation of rat renal tissue. Chem Biol Interact, 148, 139–147.
- Youdim MB, Bakhle YS. (2006). Monoamine oxidase: isoforms and inhibitors in Parkinson’s disease and depressive illness. Br J Pharmacol, 147 Suppl 1, S287–S296.
- Zhong SZ, Ge QH, Qu R, Li Q, Ma SP. (2009). Paeonol attenuates neurotoxicity and ameliorates cognitive impairment induced by d-galactose in ICR mice. J Neurol Sci, 277, 58–64.
