Abstract
Context: Taraxacum officinale Weber (Asteraceae), known as dandelion, is used for medicinal purposes due to its choleretic, diuretic, antitumor, antioxidant, antiinflammatory, and hepatoprotective properties.
Objective: We sought to investigate the protective activity of T. officinale fruit extract against sodium nitroprusside (SNP)-induced decreased cellular viability and increased lipid peroxidation in the cortex, hippocampus, and striatum of rats in vitro. To explain the mechanism of the extract’s antioxidant activity, its putative scavenger activities against NO˙, DPPH˙, OH˙, and H2O2 were determined.
Methods: Slices of cortex, hippocampus, and striatum were treated with 50 μM SNP and T. officinale fruit ethanolic extract (1–20 µg/mL) to determine cellular viability by MTT reduction assay. Lipid peroxidation was measure in cortical, hippocampal and striatal slices incubates with SNP (5 µM) and T. officinale fruit extract (1–20 µg/mL). We also determined the scavenger activities of T. officinale fruit extract against NO˙, DPPH˙, OH˙, and H2O2, as well as its iron chelating capacity.
Results: The extract (1, 5, 10, and 20 μg/mL) protected against SNP-induced decreases in cellular viability and increases in lipid peroxidation in the cortex, hippocampus, and striatum of rats. The extract had scavenger activity against DPPH˙ and NO˙ at low concentrations and was able to protect against H2O2 and Fe2+-induced deoxyribose oxidation.
Conclusion: T. officinale fruit extract has antioxidant activity and protects brain slices against SNP-induced cellular death. Possible mechanisms of action include its scavenger activities against reactive oxygen species (ROS) and reactive nitrogen species (RNS), which are attributed to the presence of phenolic compounds in the extract.
Introduction
The generation of reactive oxygen species (ROS), which is a consequence of aerobic metabolism, and reactive intermediates occurs in various physiological and pathophysiological conditions (CitationJeon et al., 2008). Under normal conditions, ROS production is controlled by endogenous antioxidant systems present in the body that establish equilibrium between ROS and antioxidants. As such, overproduction of ROS or inadequate antioxidant content can cause oxidative stress (CitationKaur et al., 2006). ROS induce lipid peroxidation, damage biomolecules, such as DNA and proteins, and affect cellular viability (CitationJeon et al., 2008).
Oxidative stress resulting from an increase in free radical generation is an important factor in various neurodegenerative diseases (CitationBeal, 1996). The brain is highly susceptible to free radical damage because of its high utilization of oxygen and the presence of relatively low concentrations of antioxidant enzymes and free radical scavengers (CitationMuralidhara, 2008).
In fact, sodium nitroprusside (SNP) is a pro-oxidant agent that impairs cellular viability and induces lipid peroxidation in brain tissue (CitationRauhala et al., 1998). SNP has been suggested to cause cytotoxicity through the release of nitric oxide radical (NO˙), the generation of ferricyanide anions [(CN)5-Fe]−3 that can react with hydrogen peroxide (H2O2) via the Fenton reaction, and generating hydroxyl radicals (OH˙) in tissue preparations (CitationBeal, 1996). NO˙ induces oxidative stress, neuronal toxicity, and apoptotic cell death, which is accompanied by mitochondrial dysfunction (CitationRiobo et al., 2001; CitationFukushima et al., 2006; CitationRomero et al., 2010). NO˙ is a known mediator of inflammatory processes that play an important role in the pathogenesis of several neurodegenerative diseases (CitationBrown, 2007; CitationCalabrese et al., 2007); therefore, models of NO˙-mediated cytotoxicity may serve as tools to evaluate active compounds for further treatment (CitationRomero et al., 2010).
In an attempt to obtain protection against ROS and RNS, and to avoid the development of neurodegenerative diseases, different research groups have been interested in screening new therapies using antioxidant substances with scavenger activity (CitationWang et al., 2008).
In recent years, considerable attention has been directed towards the identification of plants with antioxidant activity that may be used for human consumption (CitationKaur et al., 2006). The therapeutic properties of some plant extracts used in traditional medicine have been linked to their capacity to interact with reactive species and produce antioxidant activities. Such antioxidant properties found in plant extracts have been attributed to polyphenols (CitationPeschel et al., 2006). Polyphenols act as antioxidant through several mechanisms, including free radical scavenging, metal ion chelation, hydrogen donation, and action as a substrate for radicals, such as superoxide anion and hydroxyl (CitationBarreira et al., 2008).
Taraxacum officinale Weber (Asteraceae), commonly known as dandelion, has long been used for medicinal purposes due to its choleretic, diuretic, antitumor, antioxidant, antiinflammatory, antinociceptive, and hepatoprotective properties (CitationBaba et al., 1981; CitationKisiel & Barszcz, 2000; CitationHu & Kitts, 2003; CitationJeon et al., 2008). Flavonoids and phenolic compounds, such as luteolin, caffeic acid, and cholorgenic acid, are found in extracts of T. officinale (CitationHu & Kitts, 2003; CitationDomitrovic et al., 2010; CitationKoh et al., 2010). These compounds, which are generally found in plants, protect cells from oxidative stress by preventing the formation of free radicals or by detoxifying free radicals, resulting in the prevention of a variety of pathophysiological processes (CitationMates & Sanchez-Jimenez, 2000).
Considering the previously reported antioxidant and pharmacological properties of the extract as well as the involvement of ROS and RNS in several brain disorders, we investigated whether a fruit extract of T. officinale possesses the capacity to protect brain slices against SNP-induced decreases in cellular viability. The antioxidant activity of the extract in brain structures was determined by measuring lipid peroxidation induced by SNP, a pro-oxidant agent. To explain the mechanisms by which T. officinale fruit extract displays antioxidant activity, we conducted in vitro experiments to demonstrate its putative scavenger activity against different free radicals, such as NO˙, DPPH˙, OH˙, and H2O2, and its iron chelating capacity.
Methods
Chemicals
Thiobarbituric acid (TBA), malonaldehyde-bis-dimethyl acetal (MDA), 1,1-diphenyl-1-picrylhydrazyl(DPPH),3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), deoxyribose, ortho-phenanthroline and iron chloride (FeCl3) were purchased from Sigma (St. Louis, MO,USA). Sodium nitroprusside (SNP) was obtained from Merck (Darmstadt, Germany) and iron sulphate (FeSO4) from Reagen (Rio de Janeiro, RJ, Brazil). All other reagents were obtained from local suppliers.
Animals
Male Wistar rats weighing 250 (± 35.36) g, approximately 3 months of age, from our own breeding colony were kept in cages with continuous access to water and food, in a room with controlled temperature (22 ± 3°C) and in 12 h light/dark cycle with lights on at 7:00 am. The animals were maintained and used in accordance with the guidelines of the Committee on Care and Use of Experimental Animal Resources, School of Veterinary Medicine and Animal Science of Federal University of Santa Maria, Brazil.
Plant material
The fresh fruit of T. officinale Weber (Asteraceae), known as dandelion, were used as the plant material and obtained from Cruz Alta University (Rio Grande do Sul, Brazil). The collection of T. officinale fruit was carried out during the winter and early spring. The taxonomic identification was confirmed by Department of Botany of Cruz Alta University, and registered under the number 157 in the Herbarium of Cruz Alta University.
Preparation of the extract
For the preparation of the ethanolic extract, fruits (1 g) were extracted with ethanol (70%) for 2 weeks at room temperature. The extract was evaporated and concentrated to dryness under reduced pressure using a rotary evaporator and redissolved in ethanol (EtOH; 70%) to a concentration of 2 mg/mL.
Determination of total phenolic content
The total phenolic content was determined by adding 0.5 mL of the extract to 2.0 mL of 7.5% sodium carbonate and 2.5 mL of 10% Folin-Ciocalteu reagent. The reaction mixture was incubated at 45°C for 15 min. Afterwards, the absorbance was measured at 765 nm using a spectrophotometer. Gallic acid was used as a phenol standard (CitationSingleton et al., 1999). The total phenolic content was expressed as micrograms of gallic acid equivalent/mg extract.
Cellular viability
Cellular viability in cortex, hippocampus and striatum slices was quantified by measuring the reduction of MTT to a dark violet formazan product by mitochondrial dehydrogenases (CitationMosmann, 1983). Slices (0.4 mm) of the brain structures were obtained by transversally cuts using a McIlwain chopper. SNP was used in the concentration of 50 μM to induce a decrease in cellular viability (CitationÁvila et al., 2008). Slices were preincubated with the T. officinale fruit extract (1, 5, 10 and 20 µg/mL) or vehicle (EtOH) and SNP (50 µM) for 2 h at 37°C in oxygenated PBS buffer (124 mM NaCl, 5 mM KH2PO4, 10 mM Na2HPO4, 5 mM NaH2PO4, 10 mM Glucose). After incubation, the slices were washed twice with 0.5 mL of buffer. MTT reduction assay was performed in plates containing 500μL of buffer, and the reaction was started by adding 0.5 mg/mL MTT. After 1 h of incubation at 37°C, the medium was removed and the slices dissolved in dimethylsulfoxide (DMSO). The rate of MTT reduction was measured spectrophotometrically at 570 nm. The slices were homogenized in SDS 1% and NaOH 0.1 N solution and separated to carry out protein measurement according to the method of Lowry (CitationLowry et al., 1951).
Lipid peroxidation (LPO)
Animals were killed by decapitation and the brain was removed. The whole brain was used or dissected into three specific structures (cortex, hippocampus and striatum) and then homogenized (1:10) in Tris-HCl 10 mM buffer, pH 7.4 and centrifuged at 4,000x g at 4°C for 10 min. The low speed supernatant fraction obtained (S1) was used for TBARS measurements. The antioxidant effect of T. officinale fruit extract was evaluated against SNP (5 µM) through measurements of thiobarbituric acid reactive substances (TBARS) production, according to CitationOhkawa et al. (1979). The S1 fractions were preincubated at 37°C for 1 h in a buffered medium containing the T. officinale fruit extract (1, 5, 10 and 20 μg/mL) or vehicle (ethanol) and SNP (5 µM). The basal TBARS levels were determined in the absence of SNP (autooxidation of the homogenates). Then, SDS 8.1%, acetic acid/ HCl buffer and thiobarbituric acid 0.6% were added to the tubes and incubated at 95°C for 1 h. TBARS formation was determined spectrophotometrically at 532 nm, using malondialdehide (MDA) as standard.
Free radical scavenging assays
Scavenging activity of NO˙
NO˙ scavenging activity was determined using the Griess reagent (Green et al., 1981). SNP (5 mM, in PBS) was incubated at 25°C with different concentrations of T. officinale fruit extract (1, 5, 10, 20, 50, 75 and 100 µg/mL) or vehicle (EtOH). After 120 min, 0.5 mL of incubation solution was sampled and mixed with 0.5 mL of Griess reagent [0.1% N-(naphthyl) ethylenediamine dihydrochloride, 1% sulphonilic acid in 5% of phosphoric acid]. The absorbance was measured at 550 nm. The values were compared with the control to determine the percentage of inhibition of nitrite reaction with Griess reagent, depicted by the T. officinale extract as an index of its NO˙ scavenger activity (CitationMarcocci et al., 1994). NO˙ scavenging activity was expressed as the inhibition percentage of free radical by the sample and was calculated using the following equation:
where A0 was the absorbance of the control (blank, without extract) and Asample was the absorbance in the presence of different extract concentrations. Tests were carried out in duplicate.
DPPH˙ scavenging activity
The measurement of the T. officinale fruit extract scavenger activity against the DPPH˙ radical was performed in accordance with CitationCho et al. (2002). DPPH˙ solution (85 μM) was added to a medium containing different concentrations of the extract (10, 20, 30, 40, 50, 75 and 100 µg/mL) or vehicle (EtOH) and incubated at room temperature for 30 min. The decrease in the absorbance measured at 518 nm depicted the scavenger activity of the extract against DPPH˙ radical. Ascorbic acid was used as positive control to determine the maximal decrease in DPPH˙ absorbance. DPPH˙ scavenging activity was expressed as the inhibition percentage of free radical by the sample and was calculated using the following equation:
where A0 was the absorbance of the control (blank, without extract) and Asample was the absorbance in the presence of different extract concentrations. Tests were carried out in duplicate. The IC50 values (the concentration necessary to inhibit 50% radical formation) of ascorbic acid and plant extract were calculated using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA) and are expressed as mean (95% confidence intervals).
Determination of oxidative damage to deoxyribose
The deoxyribose degradation assay was performed according to CitationPuntel et al. (2005). Deoxyribose oxidation was induced with both H2O2 and FeSO4 alone and in combination. Briefly, the reaction medium was prepared containing the plant extract (1, 5, 10 and 20 μg/mL) or vehicle (EtOH), 3 mM deoxyribose, potassium phosphate buffer (0.05 mM, pH 7.4), 50 μM FeSO4, and 500 μM H2O2. Solutions of FeSO4 and H2O2 were made prior to use. Reaction mixtures were incubated at 37°C for 30 min and stopped by the addition of 0.8 mL of trichloroacetic acid (TCA; 2.8%), followed by the addition of 0.4 mL of thiobarbituric acid (TBA; 0.6%). Next, the medium was incubated at 100°C for 20 min and the absorbance was recorded at 532 nm (CitationHalliwell & Gutteridge, 1981). Standard curves of MDA were constructed for each experiment. The values are expressed as percentage of control values.
Iron chelating property and reducing power
The iron chelating property of the extract (CitationMinotti & Aust, 1987; Bucher et al., 1993) was determined according to CitationPuntel et al. (2005). The method is based on the reaction of free Fe2+ with ortho-phenantroline, forming a colored complex. First, the mixture containing Fe2+ (150 μM) and different concentrations of the extract (1, 5, 10, 20, 50, 75 and 100 µg/mL) or vehicle (EtOH) was used to form a complex. Afterwards, a solution of ortho-phenantroline was added to the mixture (62.5 μg/mL, equivalent to 0.25%). The formation of complexes between Fe2+ and the extract was estimated by a decrease in the color reaction at 510 nm when compared to a control tube without extract. The values are expressed as a percentage of chelating activity.
To examine the reducing power of the T. officinale fruit extract, the same method (ortho-phenantroline) was used. The method is based on the power of the extract in reducing free Fe3+ to form Fe2+. Fe 2+ reacts with ortho-phenantroline forming a colored complex. FeCl3 (150 μM) solution and different concentrations of the extract (1, 5, 10, 20, 50, 75 and 100 µg/mL) or vehicle (EtOH) were used. Afterwards, a solution of ortho-phenantroline was added to the mixture (62.5 μg/mL equivalent to 0.25%). The extract reducing power was estimated by an increase in the color reaction at 510 nm when compared to a control tube without extract. The values were expressed in absorbance. The solutions were prepared freshly for each experiment.
Statistical analysis
Statistical significance was assessed by one-way ANOVA, followed by Duncan post hoc test. Alternatively, the reducing power test was analyzed by two-way ANOVA followed by the Bonferroni post hoc test. Results are expressed as mean ± SEM. The differences were considered significant when p < 0.05.
Results
Total phenolic content
Total phenolic content, as assayed by the method of Folin-Ciocalteau, was expressed in microgram equivalents of gallic acid per milligram of each fraction of extract. We detected 180 ± 0.0157 μg gallic acid equivalents of phenols in 1 mg of ethanolic fruit extract.
Cellular viability
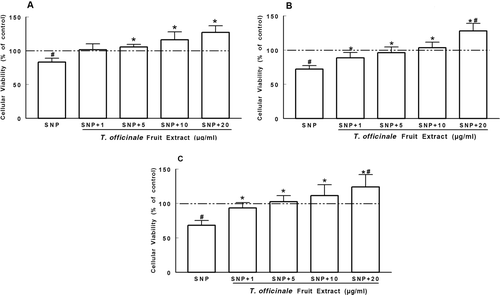
SNP induced a decrease in cellular viability in the cortex (about 83%), striatum (about 72%), and hippocampus (about 68.2%) when compared to the control (p < 0.05, ). The extract (5–20 µg/mL) protected cortical slices against SNP-induced decreased cellular viability (p < 0.05, . However, in the striatum and hippocampus, the protective effect of the extract was seen at concentrations of 1–20 µg/mL (p < 0.05, ).
Figure 1. Protective effect of T. officinale fruit extract on the decrease of cellular viability induced by SNP. Slices of cortex (A), striatum (B) and hippocampus (C) were preincubated with T. officinale fruit extract (1, 5, 10 and 20 µg/mL) or vehicle (EtOH) and SNP (50 µM) for 2 h at 37°C in oxygenated PBS buffer. Cellular viability was evaluated by the MTT reduction method. Results are expressed as the percentage of control (dotted line). # Indicates statistical difference from control group. * Indicates statistical difference from SNP group by one-way ANOVA, followed by Duncan’s post hoc test (p < 0.05). All experiments were performed in duplicate (n = 6).
Lipid peroxidation
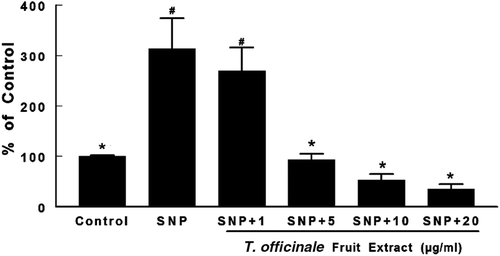
SNP induced a 300% increase in TBARS levels in S1 of whole brain (p < 0.05, ). At concentrations of 5, 10, and 20 µg/mL, the extract significantly reduced TBARS levels when compared to the SNP-treated group (p < 0.05, ).
Figure 2. Effects of T. officinale fruit extract on SNP-induced lipid peroxidation in whole brain of rats. Brain homogenates were pre-incubated at 37°C for 1h in a buffered medium containing T. officinale fruit extract (1, 5, 10 and 20 μg/mL) or vehicle (EtOH) and SNP (5 µM). Lipid peroxidation was evaluated through measurements of thiobarbituric acid reactive substances (TBARS) production. Results are expressed as percent of control (without SNP and extract). 100% of control corresponds to 3.92 ± 0.37 nmol MDA/g of tissue. # Indicates statistical difference from control group. * Indicates statistical difference from SNP group by one-way ANOVA, followed by Duncan’s post hoc test (p < 0.05). All experiments were performed in duplicate (n = 5).
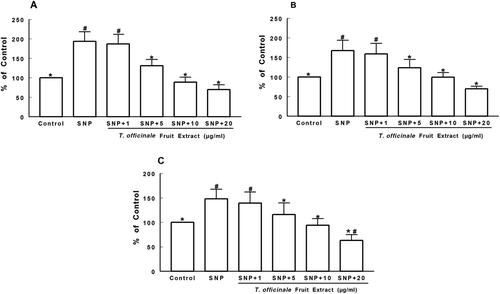
shows the antioxidant activity of the extract against lipid peroxidation in the cortex, striatum, and hippocampus of rats. SNP induced a significant increase in TBARS formation in all brain structures to approximately 200% above the control level (p < 0.05, –). The extract significantly reduced SNP-induced TBARS formation to control levels from 5 µg/mL in all brain structures analyzed (p < 0.05, –). In the hippocampus, the highest concentration used (20 µg/mL) showed a significant difference when compared with the control (p < 0.05, . Basal TBARS levels were also decreased in all brain structures analyzed by the extract at 5 µg/mL (data not shown).
Figure 3 Effects of T. officinale fruit extract on SNP-induced TBARS production in cortex (A), striatum (B) and hippocampus (C) of rats. The homogenates of brain structures were preincubated at 37°C for 1h in a buffered medium containing T. officinale fruit extract (1, 5, 10 and 20 μg/mL) or vehicle (EtOH) and SNP (5 µM). Lipid peroxidation was evaluated through measurements of thiobarbituric acid reactive substances production (TBARS). Results are expressed as percent of control (without SNP and extract). 100% of control corresponds to 1.63 ± 0.21 nmol MDA/g of tissue (A), 1.63 ± 0.34 nmol MDA/g of tissue (B) and 1.58 ± 0.06 nmol MDA/g of tissue (C). # Indicates statistical difference from control group. * Indicates statistical difference from SNP group by one-way ANOVA, followed by Duncan’s post hoc test (p < 0.05). All experiments were performed in duplicate (n = 5).
Free-radical scavenging activity
The capacity of T. officinale fruit extract to scavenge NO˙ and DPPH˙ is shown in . The extract showed significant NO˙ scavenging activity at concentrations of 5–100 µg/mL (. At a concentration of 75 µg/mL, T. officinale extract inhibited NO˙ formation by 39.35%.
Figure 4. Scavenging activity of T. officinale fruit extract on NO˙ (A) and DPPH˙ (B). For NO˙ analysis, SNP (5 mM) was incubated at 25°C for 120 min with T. officinale fruit extract (1, 5, 10, 20, 50, 75 and 100 µg/mL) or vehicle (EtOH) and NO˙ scavenging activity was determined using the Griess reagent. For DPPH˙ scavenger activity, T. officinale (10, 20, 30, 40, 50, 75 and 100 µg/mL) or vehicle (EtOH) were incubated at room temperature for 30 min and the decrease in the absorbance measured at 518 nm depicted the scavenger activity of the extract against DPPH˙ radical. Results are expressed as percent of inhibition in relation to control without extract. The mean control value is 21.85 ± 1.33 μM of nitrite (A) and 0.904 ± 0.0138 ABS (B). a, b, c and d indicate statistical significance among different concentration of the extracts by one-way ANOVA, followed by Duncan’s post hoc test (p < 0.05). All experiments were performed in duplicate (n = 4).
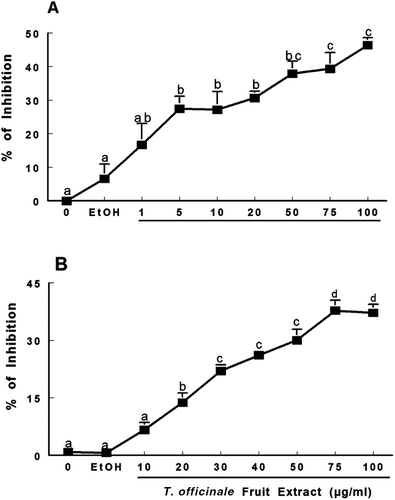
Moreover, the extract possesses significant DPPH˙ radical scavenging activity at 20–100 µg/mL (. At a concentration of 75 µg/mL, the extract showed 37.7% inhibition. The IC50 (the concentration necessary to inhibit 50% radical formation), values were 123.94 (98.22–147.66) and 8.77 (6.23–10.23) µg/mL for T. officinale fruit extract and ascorbic acid, respectively.
Deoxyribose oxidation
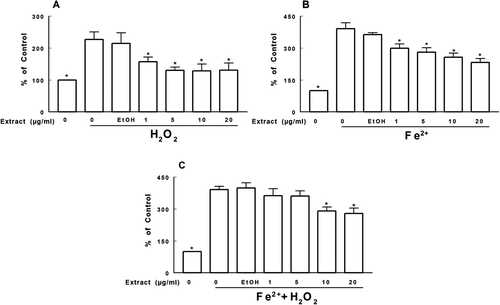
The capacity of the extract to inhibit deoxyribose oxidation is shown in . The extract was able to protect against deoxyribose oxidation induced by H2O2 or Fe2+ at concentrations of 1, 5, 10, and 20 µg/mL (p < 0.05; –). A similar protective effect was observed only at concentrations of 10 and 20 µg/mL of the extract when both H2O2 and Fe2+ were added to the medium (p < 0.05, .
Figure 5. Effect of T. officinale fruit extract on the deoxyribose oxidation induced by H2O2 (A), Fe2+ (B) and Fe2+ + H2O2 (C). T. officinale fruit extract (1, 5, 10 and 20 μg/mL) or vehicle (EtOH) were incubated with 3 mM deoxyribose, 50 μM FeSO4 and/or 500 μM H2O2 at 37°C for 30 min. Results are expressed as percent of control. The mean control value is 1.065 ± 0.193 nM of MDA/g of deoxyribose. * Indicates a statistically significant difference from H2O2 (A), Fe2+ (B) or Fe2+ + H2O2 (C) by one-way ANOVA, followed by Duncan’s post hoc test (p < 0.05). All experiments were performed in duplicate (n = 4).
Iron chelating property and reducing power
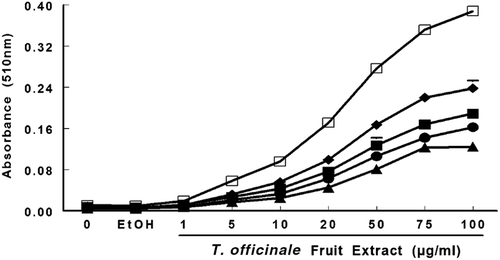
shows the reducing power of T. officinale fruit extract. The extract demonstrated reducing power in a concentration- and time-dependent manner as indicated by a significant effect in two-way ANOVA analysis (F32, 72 = 510, p ≤ 0.0001; ). Moreover, one-way ANOVA showed a significant effect of the extract at 20 µg/mL (p < 0.01) and 1 h of incubation. However, after 24 h, a significant effect of the extract was observed at 1 µg/mL (p < 0.001). The extract did not have iron (Fe2+)-chelating activity (data not shown).
Figure 6. Reducing power of T. officinale extract was evaluated by spectrophotometer detection of Fe3+ to Fe 2+ transformation. T. officinale fruit extract (1, 5, 10, 20, 50, 75 and 100 µg/mL) or vehicle (EtOH) were incubated with 150 μM FeCl3, 62.5 μg/mL ortho-phenantroline. The extract reducing power was estimated by an increase in the color reaction at 510 nm when compared to a control in different times, 1 hour (▴); 2 hours (• ); 3 hours (▪); 6 hours (♦) and 24 hours (□). The mean control value is 0.01 ± 0.00051 ABS. All experiments were performed in duplicates (n = 3).
Discussion
Phytochemicals isolated from fruit, vegetables, and herbs have been extensively studied both in vivo and in vitro to characterize potential antioxidant and bioactive properties (CitationHu & Kitts, 2003). Phenolic compounds have significant importance because they are responsible for scavenging free radicals and sequestering transition metal ions (CitationHu & Kitts, 2003). The content of phenolic compounds in ethanolic extract was 180 ± 0.015 µg/mg plant extract as expressed in gallic acid equivalents. Considering that similar levels were found in the flower extract, which also possesses antioxidant activity (CitationHu & Kitts, 2005), we believe that the protective effects of the fruit extract against SNP-induced decreases in cellular viability are probably due to the phenolic compounds.
Cellular viability is affected by oxidative stress, since the production of reactive species causes damage to the inner and outer mitochondrial membranes and opens the mitochondrial permeability transition pores, thereby inducing apoptosis (CitationSas et al., 2007). SNP is described a pro-oxidant agent that impairs mitochondrial function and decreases MTT reduction (CitationNie et al., 2006).
In this study, the exposure of cortical, hippocampal, and striatal slices to SNP, a NO˙ donor, decreased cellular viability. This result is consistent with previous observations in brain slices (CitationÁvila et al, 2008) and cortical neurons, since SNP induces cell death and apoptosis-related mitochondrial dysfunction (CitationFukushima et al., 2006; CitationRomero et al., 2010). The neurotoxic effect of SNP has been attributed to the production of ROS and release of NO˙.
Recent studies have demonstrated a role of NO˙, which is an important neuronal messenger in the central nervous system, in the pathophysiology of disorders, such as Alzheimer’s and Parkinson’s diseases (CitationCastillo et al., 2000). High NO˙ levels generated in response to cytokines or the excitatory neurotransmitter glutamate can result in neuronal cell death (CitationCoyle et al., 1993; CitationBolanos et al., 1997). Several mechanisms have been proposed for NO˙-induced cell death, including oxidative stress and mitochondrial alterations (CitationRiobo et al., 2001; CitationFukushima et al., 2006; CitationRomero et al., 2010). NO˙, is a free radical that combines with superoxide radical (O2-) to form peroxynitrite (ONOO−), a potent oxidant capable of inducing lipid peroxidation (CitationSah et al., 1995). In this study, SNP reduced cellular viability, and this effect was prevented by the extract in all brain structures. This may be attributed to the antioxidant activity of the extract, acting as a scavenger of the reactive species generated by SNP.
The antioxidant effect of the extract was demonstrated by its efficient ability to reduce lipid peroxidation generated by SNP in whole brain and brain structures. This antioxidant activity may be attributed to the phenolic compounds present in the extract, as they have the ability to accept electrons, which can combine with free radicals generated by SNP, thereby protecting lipids against oxidation. Similar antioxidant ability has been shown by T. officinale flower extracts, which suppressed lipid oxidation in vitro in linoleic acid emulsions (CitationHu & Kitts, 2005).
In the present study, the fruit extract effectively reduced nitrite formation, indicating decreased levels of NO˙ and suggesting a possible scavenger activity against reactive nitrogen species (RNS). Hu and Kitts reported an activity against RNS in ethanolic flower extracts, which was attributed to the phenolic content (CitationHu & Kitts, 2005). In previous studies, the ethanolic extract of T. officinale flowers demonstrated suppressive effects on NO˙ production in macrophage cells (CitationJeon et al., 2008). Two flavonoid compounds, luteolin and luteolin-7-O-glucoside, in T. officinale suppress the production of NO˙ in LPS-activated RAW264.7 macrophage cells, which was attributed to the suppression of inducible nitric oxide synthase (iNOS; CitationHu & Kitts, 2003).
In addition, the fruit extract shows significant scavenger activity against DPPH˙ radicals. Similarly, the T. officinale flower ethanolic extract showed scavenging activity against DPPH˙ radicals in a concentration-dependent manner (CitationJeon et al., 2008). The DPPH˙ scavenging ability of the extract may be attributed to its ability to donate hydrogens. The donation of a hydrogen to the free radical and then reduction to an unreactive species is one mechanism by which an antioxidant can remove a free radical (CitationWang et al., 2008). DPPH˙ radical scavenging activity in the T. officinale flower extract was attributed to a reducing activity derived from the phenolic content of the extract (CitationHu & Kitts, 2005). Thus, the T. officinale fruit extract also acted as a scavenger of DPPH˙ radicals.
Moreover, in the present study, low concentrations of the dandelion fruit extract protected deoxyribose from H2O2- and Fe2+ -induced oxidation. Several plant extracts/constituents exert their antioxidant activities by chelating catalytic metals (CitationNiu et al., 2000). For example, flavonoids possess high Fe2+-chelating activity. Their scavenging potential and metal chelating ability are dependent on their unique phenolic structure as well as the number and position of their hydroxyl groups (CitationPazos et al., 1999). Nevertheless, we observed that the dandelion fruit extract did not show significant Fe2+-chelating activity. However, T. officinale flower extract is a potential transitional metal ion chelator (CitationHu & Kitts, 2005). Flavonoids present in the fruit extract are unlikely to have the required structure as in the flower extract, which could explain this result.
The activity of the extract in preventing deoxyribose oxidative degradation induced by Fenton reagents was also established. The Fenton reaction (Fe2+ + H2O2→ Fe3+ + OH− + OH˙) generates OH˙ radicals, which induce severe oxidative damage to cellular components (CitationHalliweel, 1995). The mechanism of antioxidant action of polyphenols is usually attributed to OH˙-scavenging activity (CitationSah et al., 1995). However, the antioxidant efficiency of polyphenols against deoxyribose degradation is not caused by the neutralization of OH˙ radicals, but by chelating Fe2+ (CitationLopes et al., 1999).
The extract reduced Fe3+ to Fe2+ ions, demonstrating concentration- and time-dependent reducing power. Studies have reported that several polyphenols can reduce Fe3+ to Fe2+ (CitationMoran et al., 1997). The reducing properties are generally associated with the presence of reducing molecules, which exert antioxidant activity by donating a hydrogen atom and breaking the free radical chain (CitationWang et al., 2008). Electron-donating agents contribute to antioxidant activity by donating an electron to the free radical, which results in the neutralization of the radical. The reduced species then acquires a proton from the solution (CitationWang et al., 2008).
Taken together, our data demonstrate that T. officinale fruit extract is a potent antioxidant at low concentrations, as evident by the decrease in lipid peroxidation and protection against SNP-induced cellular dysfunction. One possible mechanism by which T. officinale fruit extract protects against oxidative stress is through ROS- and RNS-scavenger activity, which is attributed to phenolic compounds. The phenolic compounds in T. officinale fruit extract act as neuroprotective antioxidants or reducing agents.
As the extract was protective against the decrease in cellular viability and the increase in lipid peroxidation induced by SNP in brain structures, further studies on the neuroprotective activity of T. officinale fruit extract in vivo are needed. Further investigations are also necessary to determine the specific phenolic compounds present in the extract and their quantity.
Conclusion
In conclusion, T. officinale fruit extract has antioxidant activity at low concentrations and protects against cell death in the brain. One possible mechanism of action of T. officinale fruit extract is as a scavenger of ROS and RNS. These effects might be attributed to the phenolic content present in the extract.
Declaration of interest
This study was supported by a FINEP research grant “Rede Instituto Brasileiro de Neurociência (IBN-Net)” # 01.06.0842-00 and INCT for Excitotoxicity and Neuprotection-MCT/CNPq. D.C. received a fellowship from CNPq/PIBIC/UFSM. C.W.N., J.B.T.R and F.A.A.S. receivd a fellowship from CNPq. Additional support was provided by CAPES.
References
- Avila DS, Gubert P, Palma A, Colle D, Alves D, Nogueira CW, Rocha JB, Soares FA. (2008). An organotellurium compound with antioxidant activity against excitotoxic agents without neurotoxic effects in brain of rats. Brain Res Bull, 76, 114–123.
- Baba K, Abe S, Mizuno D. (1981). [Antitumor activity of hot water extract of dandelion, Taraxacum officinale-correlation between antitumor activity and timing of administration (author’s transl)]. Yakugaku Zasshi, 101, 538–543.
- Barreira JC, Ferreira IC, Oliveira MB, Pereira JA. (2008). Antioxidant activity and bioactive compounds of ten Portuguese regional and commercial almond cultivars. Food Chem Toxicol, 46, 2230–2235.
- Beal MF. (1996). Mitochondria, free radicals, and neurodegeneration. Curr Opin Neurobiol, 6, 661–666.
- Bolaños JP, Almeida A, Stewart V, Peuchen S, Land JM, Clark JB, Heales SJ. (1997). Nitric oxide-mediated mitochondrial damage in the brain: mechanisms and implications for neurodegenerative diseases. J Neurochem, 68, 2227–2240.
- Brown GC. (2007). Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans, 35, 1119–1121.
- Bucher JR, Tien M, Morehouse LA, Aust SD. (1983). Redox cycling and lipid peroxidation: The central role of iron chelates. Fundam Appl Toxicol, 3, 222–226.
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. (2007). Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat Rev Neurosci, 8, 766–775.
- Castillo J, Rama R, Dávalos A. (2000). Nitric oxide-related brain damage in acute ischemic stroke. Stroke, 31, 852–857.
- Cho SY, Park JY, Park EM, Choi MS, Lee MK, Jeon SM, Jang MK, Kim MJ, Park YB. (2002). Alternation of hepatic antioxidant enzyme activities and lipid profile in streptozotocin-induced diabetic rats by supplementation of dandelion water extract. Clin Chim Acta, 317, 109–117.
- Coyle JT, Puttfarcken P. (1993). Oxidative stress, glutamate, and neurodegenerative disorders. Science, 262, 689–695.
- Domitrovic R, Jakovac H, Romic Z, Rahelic D, Tadic Z. (2010). Antifibrotic activity of Taraxacum officinale root in carbon tetrachloride-induced liver damage in mice. J Ethnopharmacol, 130, 569–577.
- Fukushima T, Koide M, Ago Y, Baba A, Matsuda T. (2006). T-817MA, a novel neurotrophic agent, improves sodium nitroprusside-induced mitochondrial dysfunction in cortical neurons. Neurochem Int, 48, 124–130.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. (1982). Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem, 126, 131–138.
- Halliweel B. (1995). How to characterize an antioxidant: An update. Free radical and oxidative stress: Environments, Drugs and Food Additives. Portland Press, 72–102.
- Halliwell B, Gutteridge JM. (1981). Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: The role of superoxide and hydroxyl radicals. febs Lett, 128, 347–352.
- Hu C, Kitts DD. (2003). Antioxidant, prooxidant, and cytotoxic activities of solvent-fractionated dandelion (Taraxacum officinale) flower extracts in vitro. J. Agric Food Chem, 51, 301–310.
- Hu C, Kitts DD. (2005). Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomedicine, 12, 588–597.
- Jeon HJ, Kang HJ, Jung HJ, Kang YS, Lim CJ, Kim YM, Park EH. (2008). Anti-inflammatory activity of Taraxacum officinale. J Ethnopharmacol, 115, 82–88.
- Kaur G, Alam MS, Jabbar Z, Javed K, Athar M. (2006). Evaluation of antioxidant activity of Cassia siamea flowers. J. Ethnopharmacol, 108, 340–348.
- Kisiel W, Barszcz B. (2000). Further sesquiterpenoids and phenolics from Taraxacum officinale. Fitoterapia, 71, 269–273.
- Koh YJ, Cha DS, Ko JS, Park HJ, Choi HD. (2010). Anti-inflammatory effect of Taraxacum officinale leaves on lipopolysaccharide-induced inflammatory responses in RAW 264.7 cells. J. Med Food, 13, 870–878.
- Lopes GK, Schulman HM, Hermes-Lima M. (1999). Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim Biophys Acta, 1472, 142–152.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J. Biol Chem, 193, 265–275.
- Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. (1994). The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun, 201, 748–755.
- Mates JM, Sanchez-Jimenez FM. (2000). Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol, 32, 157–170.
- Minotti G, Aust SD. (1987). An investigation into the mechanism of citrate-Fe2+-dependent lipid peroxidation. Free Radic Biol Med, 3, 379–387.
- Moran JF, Klucas RV, Grayer RJ, Abian J, Becana M. (1997). Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: Prooxidant and antioxidant properties. Free Radic Biol Med, 22, 861–870.
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol Methods, 65, 55–63.
- Muralidhara GKS. (2008). Effect of Centella asiatica leaf powder on oxidative markers in brain regions of prepubertal mice in vivo and in vitro efficacy to ameliorate 3-NPA-induced oxidative stress in mitochondria. Phytomedicine, 15, 971–984.
- Nie BM, Yang LM, Fu SL, Jiang XY, Lu PH, Lu Y. (2006). Protective effect of panaxydol and panaxynol on sodium nitroprusside-induced apoptosis in cortical neurons. Chem Biol Interact, 160, 225–231.
- Niu XL, Ichimori K, Yang X, Hirota Y, Hoshiai K, Li M, Nakazawa H. (2000). Tanshinone II-A inhibits low density lipoprotein oxidation in vitro. Free Radic Res, 33, 305–312.
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 95, 351–358.
- Pazos M, Gallardo JM, Torres JL, Medina I. (1999). Activity of grape polyphenols as inhibitors of the oxidation of fish lipids and frozen fish muscle. Food Chem, 92, 547–557.
- Peschel W, Sánchez-Rabaneda F, Diekmann W, Plescher A, Gartzía I, Jiménez D. (2006). An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem, 97, 137–150.
- Puntel RL, Nogueira CW, Rocha JB. (2005). Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochem Res, 30, 225–235.
- Rauhala P, Khaldi A, Mohanakumar KP, Chiueh CC. (1998). Apparent role of hydroxyl radicals in oxidative brain injury induced by sodium nitroprusside. Free Radic Biol Med, 24, 1065–1073.
- Riobó NA, Clementi E, Melani M, Boveris A, Cadenas E, Moncada S, Poderoso JJ. (2001). Nitric oxide inhibits mitochondrial NADH:ubiquinone reductase activity through peroxynitrite formation. Biochem J, 359, 139–145.
- Romero C, Benedí J, Villar A, Martín-Aragón S. (2010). Involvement of Hsp70, a stress protein, in the resistance of long-term culture of PC12 cells against sodium nitroprusside (SNP)-induced cell death. Arch Toxicol, 84, 699–708.
- Sah NK, Kumar S, Subramanian M, Devasagayam TP. (1995). Variation in the modulation of superoxide-induced single-strand breaks in plasmid pBR322 DNA by biological antioxidants. Biochem Mol Biol Int, 35, 291–296.
- Sas K, Robotka H, Toldi J, Vécsei L. (2007). Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J. Neurol Sci, 257, 221–239.
- Singleton VL, Orthofer R, Lamuela-Raventos RM. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu Reagent. Methods Enzymol, 299, 152–178.
- Wang H, Gao, XD, Zhou GC, Cai L, Yao WB. (2008). In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias auxillaris fruit. Food Chem, 106, 888–895.
