Abstract
Context: Carlinae radix is an herbal drug, commonly used by the locals in southeastern Serbia for the treatment of respiratory and urogenital diseases and, externally, for various skin conditions. There still seems to be no detailed studies correlating the chemical composition of this drug and its ethnopharmacological uses.
Objective: Chemical composition, antimicrobial activity and mode of action of C. radix essential oil, isolated from commercial samples (confirmation of whose true biological identity was also the aim of this work) were analyzed. Antimicrobial potential of decoctions (extracts prepared by boiling plant material in a given solvent), used in ethnomedicine preferentially to the pure essential oil, was also investigated.
Materials and methods: The essential oil obtained by hydrodistillation was screened for antimicrobial activity by disc diffusion and broth microdilution methods. Effects of the oil on the growth of Staphylococcus aureus cells were investigated using turbidimetric measurements and visualized using scanning electron microscopy. Analyses of the chemical composition of the oils were done using gas chromatography and gas chromatography/mass spectrometry.
Results and discussion: Both the essential oil and the decocts exhibited a very high antimicrobial activity against all tested strains, with S. aureus as the most sensitive one [e.g., for the oil sample the values for minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) were 0.02, 0.04 µL/mL, respectively]. Growth curves of S. aureus demonstrated a significant decrease in turbidity (for the MIC concentration this amounted to ca. 70%) showing a concentration-dependent lysis of the cells, confirmed by scanning electron microscopy. Chemical composition, anatomical and morphological features of the sample pointed to Carlina acanthifolia L. (Asteraceae) instead of Carlina acaulis L. (Asteraceae).
Conclusion: The results showed significant antimicrobial effect of the essential oil and the decoctions and support the use of this plant in ethnomedicine for the treatment of various human infections, especially those caused by S. aureus. Adulteration of the drug would not cause significant differences in its biological activity, since chemical composition of the sample showed high similarity with those containing C. acaulis roots.
Introduction
Carlinae radix is a very common and frequently used drug in Serbia, which, in most cases, consists of Carlina acaulis L. (Asteraceae) roots. C. acaulis (stemless carline thistle, dwarf carline thistle, or silver thistle) is a perennial dicotyledonous flowering plant, native to alpine regions of central and southern Europe (CitationTutin et al., 1976). The common names are descriptive of the manner that its flower head rests directly upon a basal leaf rosette. The herbal drug C. radix is well known by its numerous medical properties and its ethnopharmacological usage is common in many countries. The drug contains up to 2% of essential oil, tannins, resins, and inulin (CitationKojić et al., 1998). The most common use of this plant is for the treatment of urogenital diseases and it is attributed with diuretic, diaphoretic, spasmolytic, tonic and sedative properties (CitationWillfort, 1959). Extracts of the roots are also used (external application) to treat herpetic eruptions, suppurating rashes (pyodermias) and other skin conditions, as well as toothache (CitationĐorđević et al., 2005). Previous studies showed that C. acaulis root volatile oil is an antibacterial and antifungal agent (CitationĐorđević et al., 1998), composed of only few constituents (CitationChalchat et al., 1996). However, commercial non-standardized samples of C. radix drug contain varying amounts of the roots of Carlina acanthifolia L., which is considered to be an adulterant or sometimes a proper substitute of the roots of C. acaulis (CitationĐorđević et al., 2005). C. acanthifolia essential oil was studied both chemically and biologically/pharmacologically (CitationĐorđević et al., 2005, Citation2007). It was ascertained that this oil possesses antimicrobial, gastroprotective, anti-inflammatory and antioxidant activity with carlina oxide as its main antioxidant. A striking similarity in the chemical composition of the oils of the two Carlina species could be noticed (CitationChalchat et al., 1996; CitationĐorđević et al., 2005).
In this paper, the antimicrobial activity of the essential oil hydrodistilled from a commercial sample of C. radix drug was investigated against a panel of the most common human pathogens. Since herbal samples of C. radix very often contain C. acanthifolia instead of C. acaulis roots, the analyses of the chemical composition of the sample’s hydrodistilled oil, together with morphological and anatomical features of the sample were performed in order to reveal the true biological source of the herbal drug in question. In addition, since the ethnopharmacological usage employs crude extracts rather than the pure essential oil, antimicrobial activities of C. radix decocts (water, wine, and vinegar) were screened as well. In order to potentially reveal the mechanism of the essential oil’s antimicrobial action, effects of the oil on the growth (and lysis) of Staphylococcus aureus cells were spectrophotometrically monitored and visualized using scanning electron microscopy (SEM).
Materials and methods
Plant material
The identity of the commercially acquired drug of C. radix purchased from a local herb shop (Jeligor, Svrljig) was determined according to morphological and anatomical features of the roots, as pointed out in the paper of CitationĐorđević et al. (2004) and other available literature (CitationTutin et al., 1976). Representative specimens of the plant material were deposited in the Herbarium of the Faculty of Science and Mathematics, University of Niš, under the voucher number 5492.
Extraction of the essential oil
Three batches of 100–150 g of the commercial sample of C. radix drug were air-dried to constant weight and subjected to hydrodistillation with ca. 1 L of distilled water for 3.5 h using the original Clevenger-type apparatus (CitationClevenger, 1928). The essential oils (1.05–1.60 g per batch) were obtained in the mean yield of 1.05% (w/w). The obtained oils were separated by extraction with freshly distilled diethyl ether and dried over anhydrous magnesium sulphate. The solvent was evaporated under a gentle stream of nitrogen at room temperature in order to exclude any loss of the essential oil and immediately analyzed. When the oil yields were determined, after the bulk of ether was removed under a stream of N2, the residue was exposed to vacuum at room temperature for a short period to eliminate the solvent completely. The pure oil was then measured on an analytical balance and multiple gravimetric measurements were taken during 24 h to ensure that all of the solvent had evaporated.
Preparation of decoctions
C. radix decoctions (water, apple vinegar, and white wine, the last two of commercial origin) were prepared as described previously (CitationWillfort, 1959). The roots (2 g), cut into small pieces, were extracted with 100 mL of boiling water, vinegar or wine for 10 min. After the plant material was filtered off, the obtained extracts (decocts) were made up with the appropriate extragents to 100 mL and further used as such in the antimicrobial tests.
GC and GC/MS analyses
The gas chromatography/mass spectrometry (GC/MS) analyses were repeated three times for each sample using a Hewlett-Packard 6890N gas chromatograph. The gas chromatograph was equipped with a fused silica capillary column DB-5MS (5% phenylmethylsiloxane, 30 m × 0.25 mm, film thickness 0.25 µm, Agilent Technologies, Santa Clara, USA) and coupled with a 5975B mass selective detector from the same company. The injector and interface were operated at 250 and 300°C, respectively. The oven temperature was raised from 70 to 290°C at a heating rate of 5°C/min and then isothermally held for 10 min. As a carrier gas helium at 1.0 mL/min was used. The samples, 1 μL of the oil solutions in diethyl ether (1:100), were injected in a pulsed split mode (the flow was 1.5 mL/min for the first 0.5 min and then set to 1.0 mL/min throughout the remainder of the analysis; split ratio 40:1). Мass selective detector was operated at the ionization energy of 70 eV, in the 35–500 amu range with a scanning speed of 0.34 s. GC (flame ionization detector) analyses were carried out under the same experimental conditions using the same column as described for the GC/MS. The percentage composition was computed from the GC peak areas without the use of correction factors. Qualitative analyses of the essential oil constituents were based on several factors. Firstly, the comparison of the essential oils linear retention indices relative to the retention times of C9-C23 n-alkanes on the DB-5MS column (CitationVandendool & Kratz, 1963) with those reported in the literature (CitationAdams, 2007). Secondly, by comparison of their mass spectra with those of authentic standards, as well as those from Wiley 6, NIST02, MassFinder 2.3. In addition, a homemade MS library with the spectra corresponding to pure substances and components of known essential oils was used, and finally, wherever possible, the identification was achieved by GC co-injection with an authentic sample (see , column identification). Relative standard deviation of repeated measurements (independent sample preparations and GC-MS) was for all substances below 1%.
Table 1. Chemical composition (%) of Carlinae radix, Carlina acaulis and Carlina acanthifolia root essential oils [present study and the literature data (CitationChalchat et al., 1996; CitationĐorđević et al., 2005)].
Microorganisms and culture conditions
Antimicrobial activity assays were performed against seven American Type Culture Collection (ATCC) strains: Gram positive S. aureus 6538, Gram negative Escherichia coli 8739, E. coli 25922, Proteus vulgaris ATCC 8427, Pseudomonas aeruginosa ATCC 9027, Klebsiella pneumoniae 10031 and the yeast Candida albicans ATCC 10231.
Bacterial strains were maintained on Nutrient agar (NA), while C. albicans was maintained on Sabouraud Dextrose Agar (SDA) in the culture collection of the Microbiology Laboratory, Department of Biology and Ecology, Faculty of Science and Mathematics, University of Niš.
Determination of MIC and MBC/MFC
Antimicrobial activity determination was performed by a microdilution method as described previously (CitationStojanović-Radić et al., 2010). Briefly, overnight cultures of microorganisms were used for the preparation of suspension. Final size of the bacterial inoculum was 5 × 105 CFU mL−1. Stock solutions of the essential oil were prepared in 70% aqueous ethanol, whose final concentration never exceeded 5% (v/v) in a well. Dilution series were prepared in 96-well microtitre plates in the concentration ranges 0.001–50 µL/mL and 0.04–50% (v/v), respectively for the essential oil and the decocts. After attaining the right dilutions, the inoculums were added to all wells. The plates were incubated for 24 h at 37°C (bacteria) and 48 h at 30°C (yeast). Bacterial growth was determined by adding 20 µL of 0.5% triphenyl tetrazolium chloride (TTC) aqueous solution (CitationSartoratto et al., 2004). One inoculated well was included to allow control of the broth suitability for organism growth. One non-inoculated well, free of antimicrobial agents, was also included to ensure medium sterility. Positive controls were tetracycline and nystatin, whereas the solvents/extragents for the oil dilution (ethanol) or extraction liquids (water, vinegar and wine) were used as the negative controls. Minimal inhibitory concentration (MIC) was defined as the lowest concentration of the oil inhibiting visible growth (red colored pellet on the bottom of wells after the addition of TTC), while the minimal microbicidal (bactericidal and fungicidal) concentration (MBC/MFC) was defined as the lowest oil concentration killing 99.9% of bacterial/fungal cells. To determine MBC/MFC, the broth was taken from each well without visible growth and inoculated in Mueller Hinton Agar (MHA) for 24 h at 37°C and in SDA for 48 h at 30°C in the case of the tested yeast. Experiments were done in quintuplicate.
Disc diffusion assay
Disc diffusion method was performed as described previously (CitationRadulović et al., 2010b). The essential oil was diluted with ethanol to attain different test concentrations (undiluted oil, essential oil:ethanol-1:5, 1:10 and 1:20, v/v), while the decoctions were used as the prepared above, undiluted solutions. The discs (6 mm in diameter) were then impregnated with 15 µL of the oil solution or test decoctions and placed onto the inoculated agar (100 µL of 0.5 Mc Farland suspensions of S. aureus per plate). Negative controls were prepared using the solvents/extragents for the oil dilution (ethanol) or extraction liquids (water, vinegar, and wine). A tetracycline disc (30 µg) within each Petri dish was used as the positive control. The inoculated plates were incubated for 24 h at 37°C and the inhibition zones were measured. The experiment was done in triplicate. It should be stressed that this test was not intended for the meticulous comparison of the size of inhibition halos of different extracts, since a more diffusible but less active extract could give a bigger diameter than a non-diffusible but more active extract.
Effect of essential oil on growth of S. aureus
The effect of C. radix essential oil on the growth of S. aureus (exponential-phase cultures) was monitored via the measurement of turbidity changes in the bacterial culture, exposed to the oil during different time periods. Bacterial suspensions of S. aureus were prepared from overnight broth culture by adjusting its turbidity using a spectrophotometer (UV-VIS, Shimadzu 1650, Japan) so that the final bacterial concentration in medium was ~1 × 106 (determined by viable counting of the same suspension). Stock solutions of the oil were prepared in aqueous ethanol (70%). The essential oil was added to the bacterial suspensions in concentrations that corresponded to previously determined MIC and MBC values (0.02 and 0.04 µL mL−1, respectively) for S. aureus. Cultures were incubated at 37°C and at regular time intervals during 24 h of the treatment, samples were taken for turbidity measurements at wavelength of 600 nm. Untreated suspension, containing only the broth with the same solvent concentration without the volatile oil, was used as a control. Experiments were done in triplicate.
SEM analysis
The cells of S. aureus, after being exposed to the C. radix essential oil (ethanol solution; MBC concentration) for 24 h, were harvested by centrifugation for 15 min at 3500g. Cells were fixed with 2.5% glutaraldehyde, dehydrated using an ethanol gradient and then subjected to critical-point drying. The dried cells were then sputter-coated with gold under vacuum (JFC 1100 E, JEOL, Japan) and examined under a scanning electron microscope (JSM 5300, JEOL, Japan) (CitationCarson et al., 2002).
Results
Analyses (GC and GC/MS) of the essential oil (three batches) obtained from a commercial C. radix drug sample (and claimed by the seller to consist solely of C. acaulis) enabled the identification of 11 components, representing ca. 99% of the total oil. Results of the present and previous studies on C. acanthifolia and C. acaulis essential oils are given in (CitationChalchat et al., 1996; CitationĐorđević et al., 2005). The most dominant constituent of the herein analyzed oil was carlina oxide, 2-(3-phenylprop-1-ynyl) furan (98.9 ± 0.9%). Sum of the relative amounts of all other detected components was less than 1%. Since the chemical composition of the herein studied oil is to a significant level similar to both of the previously studied C. acanthifolia and C. acaulis oils, a closer determination of the commercial C. radix biological source was done. This was performed according to the morphological and anatomical features of the roots described in CitationĐorđević et al. (2004). The observed features undoubtedly showed that the roots in the sample belonged to C. acanthifolia.
Broth microdilution method was used to ascertain the antimicrobial effect of the C. radix drug essential oil against common human pathogens. As presented in , the results demonstrated a significant activity of the oil against all tested strains. The observed inhibitory activity was in the concentration range from 0.02 to 0.78 µL mL−1. On the other hand, slightly higher concentrations of the oil were necessary to obtain bactericidal activity against the tested strains, and were in the range 0.04–6.25 µL mL−1. The most prominent activity was the one exhibited against S. aureus, with inhibitory activity MIC = 0.02 µL mL−1, and minimal bactericidal concentration MBC = 0.04 µL mL−1, followed by the activity against P. vulgaris and P. aeruginosa (). Contrary to that, K. pneumoniae showed the highest resistance to the essential oil with MIC = 0.78 µL mL−1 and MBC = 6.25 µL mL−1.
Table 2. Minimal inhibitory and minimal bactericidal/fungicidal activity of the essential oil of commercial Carlinae radix (µL/mL).
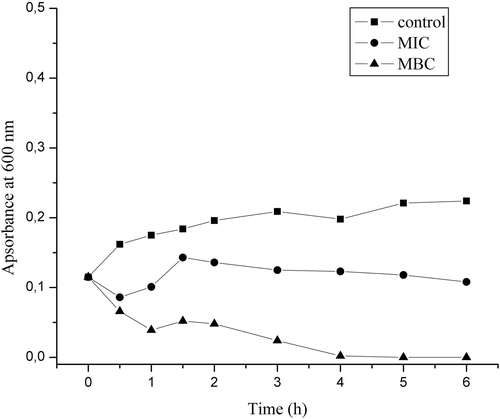
Investigation of the essential oil effect on bacterial growth and cell membrane was conducted against one of the most common human pathogens, S. aureus, which showed the highest sensitivity towards the investigated essential oil. The untreated control showed increase of turbidity demonstrating the regular exponential growth curve of the culture (). Treatment with the MIC concentration of the oil resulted in the inhibition of growth during the first 6 h, after which some very slight recovery was observed. After 24 h, MIC oil solution treatment resulted in the growth inhibition of 71.3% (when compared to the control). When the bacterial inoculum was treated with the solution of the oil having the MBC concentration, the absorbance significantly decreased 4 h after the addition of the oil (), indicating severe lysis of the cells. After this time period (4 h), no growth was observed at MBC concentration.
Figure 1. Growth of a Staphylococcus aureus culture during the first 24 h of treatment with the essential oil of commercial Carlinae radix.
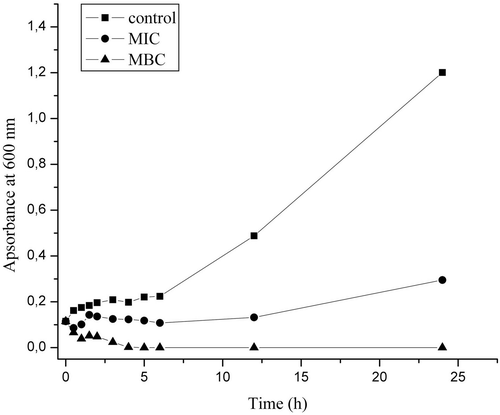
Figure 2. Growth of a Staphylococcus aureus culture during the first 6 h of treatment with the essential oil of commercial Carlinae radix.
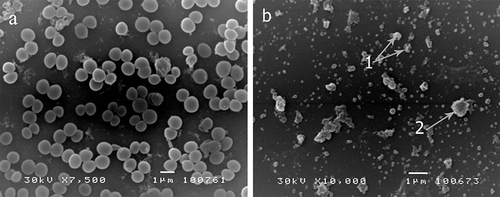
Study of SEM was carried out to visualize the effects of the tested essential oil on the morphology of S. aureus ATCC 6538. Control cells (untreated cells) showed a regular, smooth surface and were uniform in size after 24 h. The cells treated with the MBC concentration for 24 h showed very marked effects of the oil against this bacterium. After the treatment, all cells within the treated culture were totally collapsed and one could note only the cell debris remaining after disintegration of the cells, indicating lysis as the mechanism of the cell destruction ().
Figure 3. Scanning electronic micrographs of Staphylococcus aureus cells after the first 24 h of treatment with the essential oil of commercial Carlinae radix (a) control (untreated cells), (b) treated cells with the MBC concentration of the essential oil. Collapsed cells forming amorphous debris. Arrows indicate shrunk cell remains after lysis.
In order to study the antimicrobial activity of C. radix decoctions (water, wine, and vinegar), disc diffusion and broth microdilution methods were used. A disc diffusion method, used for the preliminary screening, showed that the only C. radix decoct that had any activity (inhibition zone 12.4 mm), was the one made with vinegar. On the other hand, growth inhibition zones of the C. radix essential oil, determined using the same methodology, were significantly larger (13.0–22.0 mm). The highest activity exhibited by the oil (inhibition zone of 22.0 mm) was comparable with the reference antibiotic, which had an inhibition zone of 23.1 mm. Noteworthy is the fact that the most active oil solution (in ethanol) was of the lowest concentration (1:20), while the applied pure oil induced the lowest inhibition zones. Contrary to the disc diffusion, the broth microdilution method demonstrated significant activities of all three decoctions against S. aureus. Once again, the most active one was the vinegar decoct whereas the lowest activity showed the C. radix water extract. MIC and MBC values of the oil were significantly lower than that of the decoctions ().
Table 3. Antimicrobial activity of Carlinae radix different extracts (%, v/v) against Staphylococcus aureus determined by the broth microdilution method.
Discussion
The results obtained by GC and GC/MS analysis of the C. radix commercial sample essential oil, showing 11 identified constituents with carlina oxide as the dominant one, are in agreement with the previous studies of the volatile secondary metabolites of Carlina taxa (CitationChalchat et al., 1996; CitationĐorđević et al., 2005). The current commercial sample, although points to C. acanthifolia has 7.4% more carlina oxide than C. acanthifolia (CitationĐorđević et al., 2005) and 1.7% more than C. acaulis (CitationChalchat et al., 1996), this being the major difference in the findings of this paper. Generally speaking, for the majority of the aromatic plant species (oil yield >0.1% (w/w)) of the family Asteraceae, the production of volatile metabolites is much more complex in terms of their end products and the corresponding essential oils were reported to consist of up to 200 identified different compounds (CitationRadulović et al., 2010a, CitationRadulović & Blagojević, 2010a). Nevertheless, some other Asteraceae taxa (e.g., the roots of Telekia speciosa (Schreb.) Baumg.) are also characterized with a quite simple essential oil profile, dominated by a single compound (CitationChalchat et al., 2004). As previously mentioned, the commercial drug C. radix sometimes contains roots of C. acanthifolia, which is considered both as an adulterant and a substitute of C. acaulis roots (CitationĐorđević et al., 2005). It has been shown that in some cases, the essential oil profile can be used to confirm the botanical identification of plant taxa (CitationRadulović & Blagojević, 2010b). For that reason, we compared the chemical composition of the herein studied C. radix essential oil with the composition of the previously investigated C. acanthifolia and C. acaulis oils (). Unfortunately, the volatile profile of C. radix oil could neither be used to confirm, nor to disapprove the claims (given by seller) that the drug in question consisted solely of C. acaulis. A closer determination of the commercial C. radix biological source, done according to the morphological and anatomical features of the roots, showed that the roots in the sample belonged to C. acanthifolia. This is in accordance with the results of CitationĐorđević et al. (2004), whose examination of C. radix drug samples has shown that most of them did not come from C. acaulis, but consisted largerly of C. acanthifolia roots.
The results obtained by antimicrobial activity testing surprisingly showed that the herein determined activity against all strains (MIC = 0.02–0.78 µL mL−1 and MBC = 0.04–6.25 µL mL−1) was much higher (13-fold) in comparison to the results of previous studies (CitationĐorđević et al., 2007) that reported the activity of this oil with MIC 2.5, 5 and 10 µL mL−1. As the antimicrobial properties of carlina oxide were already confirmed (CitationKernoczi et al., 1987; CitationWichtl, 2002), the authors of the mentioned study suggested that the active principle of the oil was its main constituent (carlina oxide, ca. 90%). The relative amount of carlina oxide in the presently investigated C. radix oil (99.1 ± 0.7%) was higher than in the one obtained from C. acanthifolia (ca. 90%) (). However, one should be very careful when assuming that the mentioned difference in percentage of the main component of the oils might be the main cause of such a pronounced increase of activity. It seems that the antimicrobial nature of essential oils can be also attributed to the synergistic/antagonistic interactions of the compounds constituting the oils rather than to the presence of a single inhibitory agent (CitationSavelev et al., 2003; CitationRadulović et al., 2007). The principal oil component(s) may be neither the sole nor even the principal carrier(s) of the corresponding antimicrobial activity, and, although they can contribute, the presence of which can not usually fully explain the results of activity assays (CitationStojanović-Radić et al., 2010; CitationRadulović et al., 2010b). Bearing this in mind, one could speculate that the significantly lower activity of C. acanthifolia oil (CitationĐorđević et al., 2007) is an effect of an antagonistic interactions of other (minor) oil constituents [e.g., β-sesquiphellandrene (2.8%), ar-curcumene (1.6%), α-zingiberene (2.4%)] with carlina oxide, and that these were either not present or had relative amounts less than 0.5% in the presently studied oil ().
Turbidity measurements of the growing S. aureus exponential-phase cultures confirmed that the oil (tested in the MIC concentration) induced a substantial inhibition of bacterial growth. The observed changes of the culture turbidity indicated a very fast effect of the essential oil on bacterial cells, targeting probably the cell membrane. This assumption is supported by the fact that after only 30 min, a significant decrease in turbidity occurred at both tested oil concentrations, demonstrating the concentration dependent lysis. Scanning electronic micrographs confirmed these results, showing very marked effects toward S. aureus cells. Shrinkage of the cells, visualized in , suggests the possibility of a weakened cell envelope (CitationAnam et al., 2010) of S. aureus and marks the cell membrane as the most possible and primary target site of the investigated essential oil (CitationChatterjee et al., 2006; CitationHenriques et al., 2010).
The results obtained by the disc diffusion method showed that only the vinegar decoct exhibited antimicrobial activity (data not shown). This could be explained by its higher acidity (S. aureus growth is optimal at neutral pH (CitationValero et al., 2009), combined with the most probably somewhat different chemical composition compared to the other two extracts in water or wine (). However, vinegar itself had no activity on bacterial growth in these tests. A synergistic action of the combined higher acidity and additional constituents present in the vinegar extract can not be either excluded. On the other hand, essential oil inhibition zones obtained by disc diffusion method were significantly larger. This implies that C. radix metabolites extractable with water, wine or vinegar have much lower antimicrobial activities/or are present in much lower quantity than those constituents that are volatile under hydrodistillation conditions (and thus insoluble in water). It must be stressed that the hydrosoluble compounds (that could be extracted from the plant material with water/wine/vinegar) can not be distilled with steam (i.e., hydrodistilled). Moreover, it is reasonable to expect that the methodology used for the preparation of decoctions would result in a loss of steam volatiles of the roots (plant material was immersed in boiling water/wine/vinegar and heated for 10 min in an open vessel). Thus, generally speaking, the chemical composition of the decoctions and the oil is expected to be completely different. All mentioned above corroborates the fact that (volatile) non-polar (poorly extractable with water/wine/vinegar) compounds are the main active antimicrobial principles of C. radix. In addition, the relation between the solvent concentration and obtained activity might be explained in terms of the differing solubility/diffusion capability of the tested oil (ethanol enabling better diffusion through the agar) (CitationRadulović et al., 2010b).
Conclusions
To summarize, the antimicrobial assays (and the chemical analyses), as well as the comparison of the herein obtained and previously published results on a related topic (CitationChalchat et al., 1996; CitationĐorđević et al., 1998,Citation2005,Citation2007) pointed to the essential oil of commercial C. radix as the potent antimicrobial agent, which most probably affects the cell envelope, resulting in the lysis of bacterial cells. It has been shown that the water/wine/vinegar soluble C. radix compounds also possess antimicrobial activity, but, in the tested concentration, significantly less pronounced than that of the oil. Moreover, the obtained results suggest that synergistic/antagonistic interactions of the oil/decoct constituents might exist. Finally, C. radix herbal medicines (e.g., non-polar solvent extracts) should be the subject of further toxicological studies, which would confirm their safety for human utilization in the treatment of infections caused by the tested set of pathogens. The high level of similarity in the chemical composition of the essential oils of C. acaulis and C. acanthifolia (previous studies) indicates that both roots seem to have similar ingredients and antimicrobial effects. All these results support the use of this commercial drug in ethnomedicine for the treatment of various human infections, especially those caused by S. aureus strain.
Declaration of interest
This study was financially supported by the Ministry of Education and Science of Serbia (project number 172061).
References
- Adams RP. (2007). Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Carol Stream, IL, USA: Allured Publishing Corporation.
- Anam K, Suganda AG, Sukandar EY, Kardono LBS. (2010). Antibacterial effect of component of Terminalia muelleri Benth against Staphylococcus aureus. Int J Pharm, 6, 407–412.
- Carson CF, Mee BJ, Riley TV. (2002). Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother, 46, 1914–1920.
- Chalchat JC, Đorđević S, Gorunović M. (1996). Composition of the essential oil from the root of Carlina acaulis L. Asteraceae. J Essent Oil Res, 8, 577–578.
- Chalchat JC, Maksimović Z, Petrović S. (2004). Isolantolactone, the principal constituent of the essential oil from underground parts of Telekia speciosa (Schreb.) Baumg. Asteraceae. Arh Farm, 54, 15–23.
- Chatterjee I, Somerville GA, Heilmann C, Sahl HG, Maurer HH, Herrmann M. (2006). Very low ethanol concentrations affect the viability and growth recovery in post-stationary-phase Staphylococcus aureus populations. Appl Environ Microbiol, 72, 2627–2636.
- Clevenger JP. (1928). Content of essential oil in plants. Am Perfum Essent Oil Rev, 23, 467–503.
- Ðorđević S, Lukić S, Mraović M, Zizić S. (1998). Antimicrobial activity of essential oil of Carlina acaulis L., Asteraceae. Pharmaceut Pharmacolog Lett, 4, 126–128.
- Đorđević S, Lakušić B, Petrović S, Niketić M. (2004). Morpho-anatomical characteristics of Carlina acaulis subsp. caulescens and C. acanthifolia subsp. utzka (Asteraceae). Arh Farm, 54, 773–783.
- Đorđević S, Petrović S, Ristić M, Đoković D. (2005). Composition of Carlina acanthifolia root essential oil. Chem Nat Comp, 41, 410–412.
- Dordevic S, Petrovic S, Dobric S, Milenkovic M, Vucicevic D, Zizic S, Kukic J. (2007). Antimicrobial, anti-inflammatory, anti-ulcer and antioxidant activities of Carlina acanthifolia root essential oil. J Ethnopharmacol, 109, 458–463.
- Henriques AF, Jenkins RE, Burton NF, Cooper RA. (2010). The intracellular effects of manuka honey on Staphylococcus aureus. Eur J Clin Microbiol Infect Dis, 29, 45–50.
- Kernóczi Z, Héthelyi E, Dános B, Tétényi P. (1987). Presence of carlina oxide in plants of Hungary. Stabilization and antimicrobial effect. Acta Pharm Hung, 57, 171–181.
- Kojić M, Stamenković V, Jovanović D. (1998). Lekovite biljke jugoistočne Srbije. Zavod za udžbenike i nastavna sredstva, Beograd.
- Radulović N, Misić M, Aleksic J, Doković D, Palic R, Stojanović G. (2007). Antimicrobial synergism and antagonism of salicylaldehyde in Filipendula vulgaris essential oil. Fitoterapia, 78, 565–570.
- Radulović NS, Blagojević PD, Skropeta D, Zarubica AR, Zlatković BK, Palić RM. (2010a). Misidentification of tansy, Tanacetum macrophyllum, as yarrow, Achillea grandifolia: A health risk or benefit? Nat Prod Commun, 5, 121–127.
- Radulović NS, Blagojević PD. (2010a). Volatile profiles of Artemisia alba from contrasting serpentine and calcareous habitats. Nat Prod Commun, 5, 1117–1122.
- Radulović NS, Blagojević PD. (2010b). Plant volatiles providing additional evidences to the occurence of a wild-growing population of Calamintha vardarensis (Greuter et Burdet) Šilic outside of its natural habitat. Chem Biodivers, 7, 2856–2868.
- Radulović NS, Dekić MS, Stojanović-Radić ZZ, Zoranić SK. (2010b). Geranium macrorrhizum L. (Geraniaceae) essential oil: A potent agent against Bacillus subtilis. Chem Biodivers, 7, 2783–2800.
- Sartoratto A, Machado ALM, Delarmelina C, Figueira GM, Duarte MCT, Rehder VLG. (2004). Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz J Microbiol, 35, 275–280.
- Savelev S, Okello E, Perry NS, Wilkins RM, Perry EK. (2003). Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol Biochem Behav, 75, 661–668.
- Stojanović-Radić Z, Čomić Lj, Radulović N, Dekić M, Ranđelović V, Stefanović O. (2010). Chemical composition and antimicrobial activity of Erodium species: E. ciconium L., E. cicutarium L. and E. absinthioides Willd. (Geraniaceae). Chem Pap, 64, 368–377.
- Tutin TG, Heywood HV, Burges NA, Valentine DH, Walters SM, Webb DA. (1976). Flora Europaea, Vol. 4. Cambridge: University Press.
- Valero A, Pérez-Rodríguez F, Carrasco E, Fuentes-Alventosa JM, García-Gimeno RM, Zurera G. (2009). Modelling the growth boundaries of Staphylococcus aureus: Effect of temperature, pH and water activity. Int J Food Microbiol, 133, 186–194.
- Vandendool H, Kratz PD. (1963). A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr, 11, 463–471.
- Wichtl M. (2002). Teedrogen und Phytopharmaca. Stuttgart, Germany: Wissenschaftliche Verlagsgesellschaft.
- Willfort R. (1959). Gesunheit durch Heilkräuter. Linz, Austria: Rudolf Trauner Verlag.
