Abstract
Context: Affinity chromatography is an efficient antibody, antigen and protein separation method based on the interaction between specific immobilized ligands and target antibody, antigen, and so on. Populations of available ligands can be used to separate antibodies or their Fab fragments. Similarly, antigens can be isolated by immunoaffinity chromatography (IAC) on immobilized antibodies of low affinity.
Objective: This review describes the advantages, the applications, as well as the drawbacks, of IAC in the separation and purification of antibodies and antigens.
Methods: The present review discussed all types of purification and isolation of antibodies and antigens by IAC, including purification of antibodies using immobilized and synthetic mimic proteins A, G and L; isolation of Fab fragments of antibodies; separation of antibodies against different antigen forms; isolation of antigens by immobilized antibodies and so on. These methods come from over 60 references compiled from all major databases.
Results: Purification of antigens with antibodies should choose low-affinity antibodies to avoid denaturation of most proteins. Concern for cost and safety, prompted research activities focused on novel synthetic ligands with improved properties such as lower cost, avoidance of the risk of contamination associated with natural ligands of human or animal origin to isolate antibodies and antigens.
Conclusion: It is anticipated that the improvements of IAC will have impact not only on large-scale production of antibodies but also on the generation of new affinity-based methods for the increasing number of proteins and antibody derivatives available by protein engineering and the proteomics revolution.
Introduction
Affinity chromatography, a highly selective separation technique, is a type of adsorption chromatography where the target molecule is reversibly adsorbed by a ligand immobilized onto an insoluble support. The ligand is selected with respect to its affinity for a biomolecule, such as the affinity of an antibody to its antigen or that of an antigen to its antibody. Immunoaffinity chromatography (IAC) is a process in which the binding affinity of an antigen to a parent antibody is used as a basis of separation. Firm and specific interactions between antigens and antibodies allow their versatile applications in IAC by using either immobilized antigens or antibodies (CitationSubramanian, 2002; CitationMartins et al., 2007). IAC was discovered in the 1930s. During the last 20–30 years, it has steadily become an invaluable tool in the life sciences with the development of chromatographic materials and immobilization methods.
Antibodies are isolated as an antibody class for immunodiagnostics and biopharmaceutics, and the higher levels of purification are beneficial for the diagnostic methods and for the therapeutic applications. Highly selective methods of antibodies and their Fab fragments purification are important for obtaining catalytic antibodies (CitationDubrovskaya et al., 2003; CitationPagetta et al., 2007), as well as revealing autoimmune catalytic antibodies (CitationNedonchelle et al., 2000). Pure antibodies are also necessary for biochips and related systems, a field which is growing rapidly (CitationKonovalova et al., 2007; CitationWarsinke, 2008). Monoclonal antibodies (MAbs) are useful for the treatment of a wide array of indications including autoimmune diseases, infectious diseases, cardiovascular diseases, transplant rejection and cancer. For instance, IgA, IgE, IgM and IgY increasingly find application in the cure and/or diagnosis of important diseases, especially for the IgG class, which plays most important role in the clinical application (CitationD’Agostino et al., 2008). Meanwhile, extremely purified-pooled polyclonal IgG, intravenous IgG and specific antibodies (hyperimmune IgG) have become the basis for standard therapies in a number of malignancies (CitationVerdoliva et al., 2002). With respect to the importance of purification of antibodies, more and more attention has been paid to IAC, which is an efficient protein separation method based on the interaction between target proteins and specific immobilized antibodies (CitationGrønborg et al., 2002; CitationSteen et al., 2002). In this review, we will describe advantages, applications as well as drawbacks of IAC in the separation and purification of antibodies and antigens.
Isolation of antibodies with affinity chromatography
Antibodies can be isolated from sera of immunized animals (polyclonal antibodies) or from the ascites or culture supernatant (MAbs). To isolate specific polyclonal antibodies from a serum, affinity chromatography on immobilized antigens is often used (CitationMuronetz & Korpela, 2003).
Purification of antibodies on immobilized proteins A, G and L
The rapid detection and separation of Staphylococcus aureus and group G Streptococcus were based on the affinity chromatography interactions between Fc fragment of human IgG and protein A/G (located on the cell wall of S. aureus and group G Streptococcus) () (CitationXiao et al., 2007). Immobilized protein A, G and L possessing affinity for type 1–4 of IgGs can be used for the isolation of MAbs from ascites or culture supernatant () (CitationRoque et al., 2005a, 2007; CitationHahn et al., 2006; CitationCarter-Franklin et al., 2007; CitationPavlovic et al., 2007). Protein A is a cell wall protein of S. aureus, which binds selectively to Fc region of IgG but cannot form a complex with human IgG3. Protein A also binds to IgM and IgE (CitationJaoko et al., 2001; CitationCheng et al., 2006). Protein G, a surface IgG-binding protein of Streptococci groups C and G, has a special affinity for the Fc region of IgG. It binds to IgG such as human IgG of all four subclasses and also to mouse monoclonal IgG, as well as to IgM (CitationOmtvedt et al., 2006). It has been reported that the affinity constant is higher for protein G than for protein A for IgG. Protein L from Peptostreptococcus magnus (PpL) interacts with two-thirds of mouse and almost half of the human Ig repertoires. IgG-binding domains from PpL have been shown to bind specifically to κ-light chains (κ1, κ3 and κ4, but not κ2) and λ light chains of antibodies (CitationCossins et al., 2007).
Figure 1. (a) Schematic representation of an IgG molecule. IgG has a basic four-chain monomeric structure consisting of two identical heavy chains which contains one variable domain (VH) and three constant domains (CH1, CH2, and CH3), and two identical light chains. Between the CH1 and CH2 is the hinge region. An IgG molecule can be divided into two parts functionally: Fragment antigen-binding (Fab) fragment, which is the antigen-binding site, and Fragment crystallizable (Fc) fragment, which is the protein A-binding site (CitationYang et al., 2003). (b) The Staphylococcal protein A shown here is comprised with five homologous IgG-binding domains (E, D, A–C), which have high affnity with the side chains of His435 and Tyr436 of Fc1, and a cell-wall attaching structure (XM) (CitationHober et al., 2007).
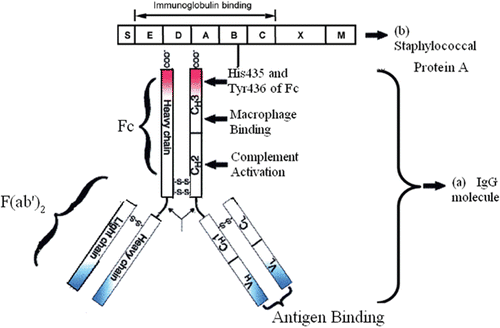
Table 1. Examples of purification of antibodies using synthetic mimic ligands of proteins A and L.
For example, protein A chromatography can separate several IgG1s, IgG2s, antibody fragments and Fc-fusion proteins using eluent with different pH value (CitationGhose et al., 2005). Protein G affinity chromatography can isolate free subunits of Fab (CitationPagetta et al., 2007).
Purification of antibodies using synthetic mimic ligands of proteins A and L
Compared to conventional protein A and L ligands, synthetic mimic ligands (mimic ligands of protein A and L) with pseudobiospecific properties can alternate them for purification of antibodies and surmount some drawbacks such as high cost, low binding capacity, limited life cycles and so on. This kind of ligands includes biological (peptides and engineered protein domains) and completely synthetic (designed dyes and de novo designed) molecules () (CitationPalombo et al., 1998; CitationRoque et al., 2005b).
Nilsson et al. firstly introduced combinatorial engineering of immunoglobulin-binding protein domains (domain Z, an SpA-analogue domain) and corresponding research has been focused on until now. Lead PpL mimetics (a 169-membered solid-phase ligand library) were synthesized by means of rational design and combinatorial chemistry for the purification of antibodies and small fragments, such as Fab and scFv, and as potential diagnostic or therapeutic agents. The results show that the most promising lead, ligand 8/7, behaves in a similar fashion to PpL in isolating Fab fragments from papain digests of human IgG to a final purity of 97%. Its properties of binding to IgG1 with K and λ isotypes (92% and 100% of loaded protein) and polyclonal IgG from sheep, cow, goat and chicken were also reflected in the efficient isolation of IgGs from crude samples (CitationRoque et al., 2005a) ().
Table 2. Properties of the Ig-binding bacterial proteins A, G and L.
The construction of peptide libraries also plays an important role in the synthesis of peptidic ligands. Protein A mimetic ligands such as a synthetic peptide (TG19318) or PAM and its inverse derivative (D-PAM), as well as ligand 22/8 were researched (CitationKabir, 2002; CitationVerdoliva et al., 2002; CitationD’Agostino et al., 2008). TG 19318, comprising four identical tripeptide chains linked to a central polylysine core, has been used to isolate polyclonal and MAbs of different classes (IgG, IgM, IgA and IgE) from different sources (serum, ascites and cell supernatants) and species. D-PAM, which is from the PAM peptide by replacing the natural amino acids with the corresponding D isomers, was able to achieve monoclonal IgG isolation from ascetic fluids and cellular supernatants and to obtain purification of polyclonal antibodies from serum. Ligand 22/8, consisting of two organic aromatic amines (3-aminophenol and 4-amino-1-napthol) linked to a scaffold of cyanuric chloride (triazine), displayed wider specificity than protein A, as it isolated IgG from a number of species, the order of adsorption being human > chicken > cow > rabbit > pig > horse > rat > goat > sheep > mouse.
Another approach has used a combination of molecular modeling and synthetic chemistry to design small molecule ligands that can mimic the Fc–protein A interaction. This method originates from the use of dyes as biomimetic ligands for protein binding (CitationGhose et al., 2006). Based on the principle that the dipeptide motif Phe-132:Tyr-133 plays an important role in the interaction of protein A with Fc portion of IgGs, corresponding synthesis of mimetic peptides has been paid attention to. For example, CitationLi et al. (1998) have designed and synthesized several molecules to mimic the Phe-132:Tyr-133 dipeptide by using 1,3,5-trichloro-triazine as the scaffold. Two synthetic, nonpeptidyl protein A mimetic ligands – Mab sorbent A1P and A2P were investigated to be used in the initial purification step for capturing of polyclonal antibodies (CitationGhose et al., 2006). They have small molecules mimicking the amino acids that are responsible for the specific binding interaction in protein A and the results showed that the initially achieved purity was approximately 80%.
Isolation of Fab fragments of antibodies
Advances in affinity chromatography, immobilized proteolytic enzymes (CitationJosic & Clifton, 2007), immobilized protein A (CitationBloom et al., 1989) and protein L (CitationRoque et al., 2005b) for separation of Fc fragments have been described. For example, CitationNing et al. (2003) reported a yield of anti-HBs Fab fragment by affinity chromatography with a purity of 95%.
Fab fragments can be obtained by using nonstoinchiometric polyelectrolyte complexes with attached antigens (CitationDainiak et al., 1998). The main problem for the production of Fab fragments from antibodies is the design of the proteolytic degradation so that splitting of antibodies into Fab and Fc fragments is nearly complete without damaging the Fab moiety. A 1.8-fold purification of antibodies was achieved without a chromatographic step yielding a preparation with more than 95% purity. Rather small damage of the antibodies not only protects binding sites of MAbs from proteolytic damage but also facilitates the proteolysis probably by exposing the antibody molecules in a way convenient for proteolytic attack by the enzyme. The described method is gentler toward antibodies because their binding sites are protected by binding to immobilized antigens.
Separation of antibodies against different antigen forms
Antibodies capable of interaction with a certain antigen form are necessary for research purposes and practical applications. In the case of MAbs, it is sufficient to select critically the required hybridomas. Although polyclonal antibodies against a protein still prove to be powerful tools to study proteins and their functions, CitationHata and Nakayama (2007) obtained specific antibodies from polyclonal antisera with the new HaloTag-based procedure in just two short steps: (1) simultaneous purification and covalent coupling of the antigen to Sepharose resin via the HaloTag and HaloLink reaction, and (2) affinity column purification of the polyclonal serum. This method is quite rapid and simple; potential epitopes can be assessed with relatively little effort for their ability to elicit the production of highly specific antibodies.
Recently, systematic studies for obtaining antibodies recognizing only nonnative molecular forms of a protein were carried out (CitationO’Nuallain et al., 2007). MAbs of two clones (antibodies of clone 6C5 and clone 6C7) reacting with the nonnative forms of denatured glyceraldehyde-3-phosphate dehydrogenase (dGAPDH), EC 1.2.1.12, were obtained. The investigation of the selected clones by IAC on immobilized oligomeric forms (varying from nonnative monomers to native tetramers) of antigen, including unfolded protein subunits, indicated that both clones of the antibodies effectively bound to active and inactive monomers and dimmers, as well as to the unfolded polypeptide, but not to the native tetrameric protein (CitationGoldberg, 1991; CitationGuijarro et al., 1995).
Polyclonal sera inherently contain a set of different forms of antibodies against an antigen. Different fractions of antibodies are achieved by IAC on immobilized native and denatured forms of antigens. Based on this method, two types of polyclonal antibodies specific to holoenzyme and dGAPDH respectively were separated from the same antiserum of chinchilla rabbits using the standard procedure of affinity chromatography (CitationMuronetz & Korpela, 2003; CitationArutyunova et al., 2004). In general, a great many of antibodies can be separated from polyclonal sera for their homogeneous properties against some antigens.
Isolation of antigens by immobilized antibodies
The use of immobilized antigens for the isolation of antibodies seems to be an optimal method in the field of the affinity chromatography, which is based on the extremely specific interactions. Similarly, because of its high affinity and selectivity, IAC could be in principle of great value in the rapid purification of antigens proteins to high degrees of purity. The antibodies could either be used to purify natural proteins or be used for recombinant proteins purification in conjunction with other methods (CitationBlank et al., 2002). However, there are several main factors limiting the use of antibodies for affinity chromatography: (1) it needs the large investment of time and effort and thus cost to generate MAbs and to produce them at the scale required; (2) high specificities of the antigen–antibody interactions require isolation of specific antibodies for the isolation of antigens from a certain source; (3) immobilization of antibodies on a support often results in a decrease or complete loss of their antigen-binding properties and (4) high-affinity antibody–antigen complexes are difficult to dissociate, often leading to inactivation of the protein product during elution from the immobilized antibody (CitationThompson & Burgess, 2001).
Selection of low-affinity antibodies applicable for antigen purification
IAC has not been generally useful for the purification of proteins in their native state, because the relatively harsh conditions required for the dissociation of antigens from antibodies will cause denaturation of most proteins. However, it has been possible to obtain MAbs that bind to their antigens with low affinity. The antigens may be dissociated from these low-affinity antibodies under relatively mild conditions. Immunoaffinity columns constructed with such low-affinity MAbs have been very effective in the purification of proteins (CitationKellogg & Alberts, 1992).
Therefore, it is preferable to use antibodies of low affinity to purify antigens. Antibodies fragments (mono- and bivalent Fab fragments), as well as mini-antibodies (fragments of the hypervariable sites) usually exhibit a lower affinity to antigens (CitationEngström et al., 2005). The scheme of immunoaffinity isolation of surface antigen of hepatitis B virus was developed. The yield of purified HBs-antigen obtained with MAbs exceeded 90% (CitationNikolaenko et al., 2007). Immunodiagnostically useful Mycobacterium tuberculosis H37Ra protein antigens ES-31, ES-43 and EST-6 were isolated from detergent soluble sonicate (DSS) antigen using monospecific antibodies by affinity chromatography. ES-31, ES-43 and EST-6 antigens purified from both culture filtrate and DSS antigen showed similar seroreactivity with overall sensitivity 85, 80 and 75%, respectively (CitationUpadhye et al., 2007).
Isolation of proteins and other compounds with antibodies
Immobilized antibodies are used for purification or separation of various substrates, containing enzymes, peptides (CitationLiu et al., 2007), antibodies and cells (CitationPappas & Wang, 2007). Compared to standard purification methods, affinity chromatography offers high selectivity, hence high resolution, and usually high capacity for proteins of interest. Purification can be in the order of several thousand-fold. Purification that would otherwise be time-consuming, lower purity, difficult or even impossible using other techniques can be easily achieved with affinity chromatography. For example, β-lactoglobulin, prolactin and human protein C were isolated with antibodies immobilized by different methods. Alkaline phosphatase was purified with a 95% yield and pyruvate kinase was purified 2400-fold from an erythrocyte lysate with an 83% yield (CitationKuckelkorn & Jacobasch, 1990) in one step from a crude extract. Successful isolation of recombinant α-amylase (CitationBibi, 1989) and β-lactamase has also been described. Besides, the large variety of antibodies or other ligands immobilized on solid supports have been used to solve several problems like the selection of phosphorylated, glycosylated proteins. Antibodies specific for phosphorylated tyrosine residues were used for the selection of phosphorylated proteins (CitationPandey et al., 2000; CitationSteen et al., 2002).
For proteomic projects and genomics projects, protein separation from plasma, serum, CSF, urine and other body fluid or cellular sources is an important step (CitationGuijarro et al., 1995; CitationKitao & Takata, 2006). Among various separation technologies, IAC is one of the most effective methods when the selected antibodies have the following properties: strong avidity, high specificity, low nonspecific binding or accumulative production. For instance, plasma proteins were isolated by chicken IgY antibodies (CitationHuang & Fang, 2008). Annexin V was purified from human placenta on column with appropriate monospecific antibodies. The yield of the protein is about 5 mg per 100 g of wet tissue through a two-stage procedure (CitationMikaelyan et al., 2008). With the development of purification methods for recombinant green fluorescent protein (rGFP) or recombinant proteins fused with GFP tag, the purity of rGFP (more than 97% homogeneity) using this method, which is based on high specificity and affinity of MAb against GFP, is superior to other methods (ZCitationHuang et al., 2008). Additionally, IAC with antibody fragments, which can be obtained based on the protein fragment complementation assay and the availability of antibody libraries, would be particularly attractive for the parallel purification of proteins for proteomics projects. For instance, generic, parallel and scalable protein purification was achieved with the method of the directed immobilization of recombinant antibody fragments as ligands (CitationBlank et al., 2002).
IAC is also the important and effective technology which can remove nonnative and denatured protein forms. For the increasing usage of recombinant proteins, it is especially necessary to separate functional proteins from their nonnative forms. Nonnative proteins can also be removed with antibodies immobilized on soluble polyelectrolytes (CitationMuronetz & Korpela, 2003). Based on this principle, the synthesis of the conjugate of poly(methacrylic acid) with MAbs was used to remove dGAPDH (CitationMuronetz et al., 2000).
The separation of proteins with the technology of IAC is also exemplified by various investigations. CitationTaylor et al. (2003) have reported two kinds of methods for immunoaffinity purification of human neutrophil flavocytochrome b (Cyt b) successively to analyze its structure and catalytic mechanism. One is that Cyt b was purified through using the p22phox-specific mAb 44.1, with gentle elution of Cyt b carried out by the addition of epitope-mimicking peptide. Although this method has some advantages such as good recoveries and higher purification of Cyt b, the limit is that purification by mAb 44.1 is only achieved using the low critical micelle concentration detergent dodecylmaltoside (CitationTaylor et al., 2003). Therefore, another improving method was investigated. The epitope-mapped mAb CS9 (p22phox subunit of Cyt b) was coupled to Sepharose beads to be used as an affinity matrix for single-step immunoaffinity purification of Cyt b from both human neutrophil and PLB-985 membrane fractions. The high efficiency of this method overcame the drawback of the previous one mentioned above (CitationLord et al., 2008). IAC using single-domain antibodies from Camelidae yielded a completely pure and functional ice structuring protein from Lolium perenne, which modifies ice crystal growth and therefore has potential application in medicine, biotechnology, agriculture and (frozen) foods. The results display that highly pure proteins can be recovered from biological material in a single-step process (CitationVerheesen et al., 2003).
Conclusions and perspectives
In this review, purification and isolation of antibodies and antigens by IAC have been discussed. Because of the specific binding between antibodies and antigens or proteins, antigens or proteins A, G and L are used to separate various antibodies (MAbs or polyclonal antibodies) such as IgGs, IgM, IgE, IgA, IgY, and so on, as well as Fab fragments of antibodies and so on, while antibodies are commonly used in affinity chromatography as versatile and specific means for isolating target molecules from complex mixtures. However, purification of antigens with antibodies should choose low-affinity antibodies to avoid denaturation of most proteins. With respect to the cost and safety, prompted research activities have being focused on novel synthetic ligands (synthetic mimic ligands of proteins A and L) with improved properties such as lower cost, avoidance of the risk of contamination associated with natural ligands of human or animal origin to isolate antibodies (CitationD’Agostino et al., 2008). What’s more, affinity chromatography (one of the protein separation approaches) on immobilized antibodies with specific binding to proteins plays an important role in the proteomic (phosphoprotemics or glycoproteomics) and genomic field.
The specific binding between antigen and antibody is the principle for their isolation by IAC but is also the limitations for the high affinity, leading to inactivation of the protein product during elution from the immobilized antibody. Therefore, approaches of selection of low-affinity antibodies or using polyol-responsive MAbs have been investigated to solve this problem (CitationThompson & Burgess, 2001). It is anticipated that the improvements of IAC will have impact not only on large-scale production of antibodies but also on the generation of new affinity-based methods for the increasing number of proteins and antibody derivatives available by protein engineering and the proteomics revolution.
Acknowledgment
We thank Miss Trista Talbot (Department of Chemistry, Brandeis University) for language editing of the manuscript.
Declaration of interest
The authors report no declarations of interest.
References
- Arutyunova EI, Arutyunov DY, Pleten’ AP, Nagradova NK, Muronetz VI. (2004). Antibodies specific to modified glyceraldehyde-3-phosphate dehydrogenase induce inactivation of the native enzyme and change its conformation. Biochim Biophys Acta, 1700, 35–41.
- Bibi E. (1989). Purification of TEM-1 beta-lactamase by immunoaffinity chromatography. Biochem J, 263, 309–311.
- Blank K, Lindner P, Diefenbach B, Plückthun A. (2002). Self-immobilizing recombinant antibody fragments for immunoaffinity chromatography: Generic, parallel, and scalable protein purification. Protein Expr Purif, 24, 313–322.
- Bloom JW, Wong MF, Mitra G. (1989). Detection and reduction of protein A contamination in immobilized protein A purified monoclonal antibody preparations. J Immunol Methods, 117, 83–89.
- Carter-Franklin JN, Victa C, McDonald P, Fahrner R. (2007). Fragments of protein A eluted during protein A affinity chromatography. J Chromatogr A, 1163, 105–111.
- Cheng CA, John JA, Wu MS, Lee CY, Lin CH, Lin CH, Chang CY. (2006). Characterization of serum immunoglobulin M of grouper and cDNA cloning of its heavy chain. Vet Immunol Immunopathol, 109, 255–265.
- Cossins AJ, Harrison S, Popplewell AG, Gore MG. (2007). Recombinant production of a VL single domain antibody in Escherichia coli and analysis of its interaction with peptostreptococcal protein L. Protein Expr Purif, 51, 253–259.
- Dainiak MB, Izumrudov VA, Muronetz VI, Galaev IY, Mattiasson B. (1998). Conjugates of monoclonal antibodies with polyelectrolyte complexes – an attempt to make an artificial chaperone. Biochim Biophys Acta, 1381, 279–285.
- D’Agostino B, Bellofiore P, De Martino T, Punzo C, Rivieccio V, Verdoliva A. (2008). Affinity purification of IgG monoclonal antibodies using the D-PAM synthetic ligand: Chromatographic comparison with protein A and thermodynamic investigation of the D-PAM/IgG interaction. J Immunol Methods, 333, 126–138.
- Dubrovskaya VV, Andryushkova AS, Kuznetsova IA, Toporkova LB, Buneva VN, Orlovskaya IA, Nevinsky GA. (2003). DNA-hydrolyzing antibodies from sera of autoimmune-prone MRL/MpJ-lpr mice. Biochemistry Mosc, 68, 1081–1088.
- Engström HA, Andersson PO, Ohlson S. (2005). Analysis of the specificity and thermodynamics of the interaction between low affinity antibodies and carbohydrate antigens using fluorescence spectroscopy. J Immunol Methods, 297, 203–211.
- Ghose S, Allen M, Hubbard B, Brooks C, Cramer SM. (2005). Antibody variable region interactions with protein A: implications for the development of generic purification processes. Biotechnol Bioeng, 92, 665–673.
- Ghose S, Hubbard B, Cramer SM. (2006). Evaluation and comparison of alternatives to protein A chromatography Mimetic and hydrophobic charge induction chromatographic stationary phases. J Chromatogr A, 1122, 144–152.
- Goldberg ME. (1991). Investigating protein conformation, dynamics and folding with monoclonal antibodies. Trends Biochem Sci, 16, 358–362.
- Grønborg M, Kristiansen TZ, Stensballe A, Andersen JS, Ohara O, Mann M, Jensen ON, Pandey A. (2002). A mass spectrometry-based proteomic approach for identification of serine/threonine-phosphorylated proteins by enrichment with phospho-specific antibodies: identification of a novel protein, Frigg, as a protein kinase A substrate. Mol Cell Proteomics, 1, 517–527.
- Guijarro JI, Jackson M, Chaffotte AF, Delepierre M, Mantsch HH, Goldberg ME. (1995). Protein folding intermediates with rapidly exchangeable amide protons contain authentic hydrogen-bonded secondary structures. Biochemistry, 34, 2998–3008.
- Hahn R, Shimahara K, Steindl F, Jungbauer A. (2006). Comparison of protein A affinity sorbents III. Life time study. J Chromatogr A, 1102, 224–231.
- Hata T, Nakayama M. (2007). Rapid single-tube method for small-scale affinity purification of polyclonal antibodies using HaloTag Technology. J Biochem Biophys Methods, 70, 679–682.
- Hober S, Nord K, Linhult M. (2007). Protein A chromatography for antibody purification. J Chromatogr B Analyt Technol Biomed Life Sci, 848, 40–47.
- Huang L, Fang X. (2008). Immunoaffinity fractionation of plasma proteins by chicken IgY antibodies. Methods Mol Biol, 425, 41–51.
- Jaoko WG, Lund M, Michael E, Simonsen PE. (2001). A simple and quick method for enhanced detection of specific IgE in serum from lymphatic filariasis patients. Acta Trop, 80, 51–57.
- Josic D, Clifton JG. (2007). Use of monolithic supports in proteomics technology. J Chromatogr A, 1144, 2–13.
- Kabir S. (2002). Immunoglobulin purification by affinity chromatography using protein A mimetic ligands prepared by combinatorial chemical synthesis. Immunol Invest, 31, 263–278.
- Kellogg DR, Alberts BM. (1992). Purification of a multiprotein complex containing centrosomal proteins from the Drosophila embryo by chromatography with low-affinity polyclonal antibodies. Mol Biol Cell, 3, 1–11.
- Kitao H, Takata M. (2006). Purification of TAP-tagged proteins by two-step pull down from DT40 cells. Subcell Biochem, 40, 409–413.
- Konovalova EV, Savvateeva EN, Dement’eva EI, Filippova MA, Turygin AIu, Osipova TV, Riabykh TP, Rubina AIu, Zasedatelev AS. (2007). [Development of a biochip for quantitative determination of two forms of prostate specific antigen using internal calibration curve]. Mol Biol (Mosk), 41, 734–738.
- Kuckelkorn U, Jacobasch G. (1990). Purification of pyruvate kinase from human erythrocytes by immunoaffinity chromatography. Biomed Biochim Acta, 49, S313–S316.
- Li R, Dowd V, Stewart DJ, Burton SJ, Lowe CR. (1998). Design, synthesis, and application of a protein A mimetic. Nat Biotechnol, 16, 190–195.
- Liu FF, Dong XY, Wang T, Sun Y. (2007). Rational design of peptide ligand for affinity chromatography of tissue-type plasminogen activator by the combination of docking and molecular dynamics simulations. J Chromatogr A, 1175, 249–258.
- Lord CI, Riesselman MH, Gripentrog JM, Burritt JB, Jesaitis AJ, Taylor RM. (2008). Single-step immunoaffinity purification and functional reconstitution of human phagocyte flavocytochrome b. J Immunol Methods, 329, 201–207.
- Martins S, Lourenço S, Karmali A, Serralheiro ML. (2007). Monoclonal antibodies recognize conformational epitopes on wild-type and recombinant mutant amidases from pseudomonas aeruginosa. Mol Biotechnol, 37, 136–145.
- Mikaelyan MV, Poghosyan GG, Gasparyan VK. (2008). Rapid purification of annexin V from human placenta by affinity chromatography. Prep Biochem Biotechnol, 38, 152–157.
- Muronetz VI, Kazakov SV, Dainiak MB, Izumrudov VA, Galaev IY, Mattiasson B. (2000). Interaction of antibodies and antigens conjugated with synthetic polyanions: On the way of creating an artificial chaperone. Biochim Biophys Acta, 1475, 141–150.
- Muronetz VI, Korpela T. (2003). Isolation of antigens and antibodies by affinity chromatography. J Chromatogr B Analyt Technol Biomed Life Sci, 790, 53–66.
- Nedonchelle E, Pitiot O, Vijayalakshmi MA. (2000). A preliminary study for isolation of catalytic antibodies by histidine ligand affinity chromatography as an alternative to conventional protein A/G methods. Appl Biochem Biotechnol, 83, 287–294.; discussion 294
- Nikolaenko IV, Goncharenko VS, Shimko NN, Galkin AIu. (2007). [Isolation of surface antigen of hepatites B virus]. Ukr Biokhim Zh, 79, 114–122.
- Ning D, Junjian X, Xunzhang W, Wenyin C, Qing Z, Kuanyuan S, Guirong R, Xiangrong R, Qingxin L, Zhouyao Y. (2003). Expression, purification, and characterization of humanized anti-HBs Fab fragment. J Biochem, 134, 813–817.
- Omtvedt LA, Royle L, Husby G, Sletten K, Radcliffe CM, Harvey DJ, Dwek RA, Rudd PM. (2006). Glycan analysis of monoclonal antibodies secreted in deposition disorders indicates that subsets of plasma cells differentially process IgG glycans. Arthritis Rheum, 54, 3433–3440.
- O’Nuallain B, Allen A, Kennel SJ, Weiss DT, Solomon A, Wall JS. (2007). Localization of a conformational epitope common to non-native and fibrillar immunoglobulin light chains. Biochemistry, 46, 1240–1247.
- Pagetta A, Tramentozzi E, Corbetti L, Frasson M, Brunati AM, Finotti P. (2007). Characterization of immune complexes of idiotypic catalytic and anti-idiotypic inhibitory antibodies in plasma of type 1 diabetic subjects. Mol Immunol, 44, 2870–2883.
- Palombo G, Verdoliva A, Fassina G. (1998). Affinity purification of immunoglobulin M using a novel synthetic ligand. J Chromatogr B Biomed Sci Appl, 715, 137–145.
- Pandey A, Podtelejnikov AV, Blagoev B, Bustelo XR, Mann M, Lodish HF. (2000). Analysis of receptor signaling pathways by mass spectrometry: identification of vav-2 as a substrate of the epidermal and platelet-derived growth factor receptors. Proc Natl Acad Sci U S A, 97, 179–184.
- Pappas D, Wang K. (2007). Cellular separations: A review of new challenges in analytical chemistry. Anal Chim Acta, 601, 26–35.
- Pavlovic M, Chen R, Kats AM, Cavallo MF, Saccocio S, Keating P, Hartmann JX. (2007). Highly specific novel method for isolation and purification of lupus anti-DNA antibody via oligo-(dT) magnetic beads. Ann N Y Acad Sci, 1108, 203–217.
- Roque AC, Taipa MA, Lowe CR. (2005a). An artificial protein L for the purification of immunoglobulins and fab fragments by affinity chromatography. J Chromatogr A, 1064, 157–167.
- Roque AC, Taipa MA, Lowe CR. (2005b). Synthesis and screening of a rationally designed combinatorial library of affinity ligands mimicking protein L from Peptostreptococcus magnus. J Mol Recognit, 18, 213–224.
- Roque AC, Silva CS, Taipa MA. (2007). Affinity-based methodologies and ligands for antibody purification: Advances and perspectives. J Chromatogr A, 1160, 44–55.
- Steen H, Kuster B, Fernandez M, Pandey A, Mann M. (2002). Tyrosine phosphorylation mapping of the epidermal growth factor receptor signaling pathway. J Biol Chem, 277, 1031–1039.
- Subramanian A. (2002). Immunoaffinity chromatography. Mol Biotechnol, 20, 41–47.
- Verdoliva A, Pannone F, Rossi M, Catello S, Manfredi V. (2002). Affinity purification of polyclonal antibodies using a new all-D synthetic peptide ligand: comparison with protein A and protein G. J Immunol Methods, 271, 77–88.
- Taylor RM, Burritt JB, Foubert TR, Snodgrass MA, Stone KC, Baniulis D, Gripentrog JM, Lord C, Jesaitis AJ. (2003). Single-step immunoaffinity purification and characterization of dodecylmaltoside-solubilized human neutrophil flavocytochrome b. Biochim Biophys Acta, 1612, 65–75.
- Thompson NE, Burgess RR. (2001). Identification of polyol-responsive monoclonal antibodies for use in immunoaffinity chromatography. Curr Protoc Mol Biol, Chapter 11, Unit11.18.
- Upadhye V, Saha-Roy S, Shende N, Kumar S, Harinath BC. (2007). Isolation of Mycobacterium tuberculosis protein antigens ES-3 1, ES-43 and EST-6 of diagnostic interest from tubercle bacilli by affinity chromatography. Indian J Exp Biol, 45, 599–602.
- Verheesen P, ten Haaft MR, Lindner N, Verrips CT, de Haard JJ. (2003). Beneficial properties of single-domain antibody fragments for application in immunoaffinity purification and immuno-perfusion chromatography. Biochim Biophys Acta, 1624, 21–28.
- Warsinke A. (2008). Electrochemical biochips for protein analysis. Adv Biochem Eng Biotechnol, 109, 155–193.
- Xiao X, Yang X, Liu T, Chen Z, Chen L, Li H, Deng L. (2007). Preparing a highly specific inert immunomolecular-magnetic beads for rapid detection and separation of S. aureus and group G Streptococcus. Appl Microbiol Biotechnol, 75, 1209–1216.
- Yang L, Biswas ME, Chen P. (2003). Study of binding between protein A and immunoglobulin G using a surface tension probe. Biophys J, 84, 509–522.
- Zhuang R, Zhang Y, Zhang R, Song C, Yang K, Yang A, Jin B. (2008). Purification of GFP fusion proteins with high purity and yield by monoclonal antibody-coupled affinity column chromatography. Protein Expr Purif, 59, 138–143.
