Abstract
Context: Lichens have been used for various purposes such as dyes, perfumes and remedies in folk medicine indicating the pharmaceutical potential of lichens.
Objective: Lichen growth in nature is very slow. To overcome this major drawback, we standardized the culture media to culture the lichen Usnea complanata (Müll.Arg.) Motyka (Parmeliaceae) for (1) in vitro synthesis of natural lichen substances, and (2) determination of antioxidative and cardiovascular-protective activity of usnic acid and psoromic acid.
Materials and methods: Lichen U. complanata has been cultured in fermentor under submerged condition. Antioxidative and cardiovascular-protective activity of the extract and the purified lichen substances usnic and psoromic acid have been determined.
Results: Except methanol, all other extracts exhibited antioxidative action in terms of free radical scavenging activity (FRSA) with a half-inhibiting concentration (IC50) value of 22.86 to 25.0 µg/mL, nitric oxide radical scavenging activity (NORSA) 141.3 to 149.1 µg/mL and for lipid peroxidation inhibition (LPI) 125 to 157.9 µg/mL. Usnic acid or psoromic acid showed antioxidative action with IC50 values ranging from 0.174 to 0.271 mg/mL. Methanol and ethyl acetate extract showed hydroxy-3-methyl-glutaryl-CoA reductase (HMGR) inhibition of 65.18 to 74.81%. Only 43.47% inhibition of angiotensin converting enzyme (ACE) was shown by methanol extract. Usnic acid showed noncompetitive type of HMGR inhibition and uncompetitive type of ACE inhibition. Psoromic acid exhibited competitive type of HMGR inhibition and mixed type of ACE inhibition.
Discussion: U. complanata showed both cardiovascular-protective and antioxidant properties.
The lichen species U. complanata may be a natural bioresource for possible pharmaceutical applications.
Introduction
Free radicals in relation to cardiovascular diseases and oxidative modification of circulating lipoproteins are important for the development of atherosclerosis. Particularly low-density lipoproteins, which are known to be a risk factor for cardiovascular diseases, may promote atherogenesis for several reasons. This eventually causes disruption of cell membranes, leading to the release of cell contents and death. Antioxidant therapy may inhibit atherosclerosis and thereby prevent the clinical complications of the disease such as coronary artery disease, including hypertension, hypercholesterolemia, postmenopausal state, thrombotic tendency and the risk of developing myocardial infraction. In healthy individuals, antioxidants protect components of the body against free radical damage (CitationHalliwell, 1997).
Uncontrolled platelet aggregation is critical in arterial thrombosis and may cause life-threatening disorders such as heart attacks, unstable angina and reocclusion after angioplasty (CitationDavies & Thomas, 1985). Hence, in the treatment and prevention of these cardiovascular diseases, the inhibition of platelet aggregation is of fundamental importance (CitationAntiplatelet Trial lists Collaboration, 1994). Although it is well established that aspirin still provides an effective secondary prevention of cardiovascular disorders, this drug is reported to have side effects like hemorrhagic events and upper gastrointestinal bleeding (CitationRoderick et al., 1993).
β-Hydroxy-β-methylglutaryl coenzyme A (HMG-CoA) reductase (EC 1.1.1.34), a rate-limiting enzyme in endogenous cholesterol synthesis, is a 97-kDa glycoprotein and catalyzes the reductive deacylation of HMG-CoA to mevalonate (CitationFrimpong & Rodwell, 1994; CitationBochar et al., 1999). Hyperlipidemia or coronary heart diseases are caused by increased blood cholesterol levels. Therefore, lowering total cholesterol through the action of a HMG-CoA reductase inhibitor is very important for the prevention of hyperlipidemia. However, commercial antihyperlipidemia drugs, including mevastatin, have some disadvantages, such as high cost for low yield, ineffectiveness in vivo, and some side effects (CitationKim et al., 2005). These considerations have led to an effort in the search for novel natural compounds or sources to inhibit HMG-CoA reductase for lowering cholesterol levels.
Lichen-forming fungi are a large and diverse group of organisms that includes more than 13,000 described species. Lichens occur in all ecosystems on all continents and are the dominating organisms in extreme environments, including polar or alpine vegetations. They are able to grow on different substrates, including bare soil, rocks, barks of trees, wood, shells of barnacles and leaf surfaces. Past and current studies on biological activities of some lichen secondary metabolites of natural thallus exert a wide variety of biological actions including antibiotic, antimycobacterial, antiviral, anti-inflammatory, analgesic, antipyretic, plant growth inhibitory, antiherbivore, enzyme inhibitory, antiproliferative and cytotoxic effects, which are best supported by the available evidence (CitationMüller, 2001; CitationEsimone et al., 2009; CitationMolnár & Farkas, 2010; CitationShukla et al., 2010). The results of screening experiments indicate the potential of lichen compounds for possible pharmaceutical applications. However, the slow growth of lichens in nature and also in axenic culture (either of isolated or resynthesized partners in the controlled conditions in the laboratory) could be regarded as a major draw back to obtain large quantity of useful bioactive metabolites for the screening studies. Due to these hurdles, very few lichens and their secondary metabolites have been screened for limited biological activities so far.
If the lichen metabolites having potential biological activities are to be used for modern pharmaceutical/nutraceutical applications, then the suitable in vitro culture conditions of the source organisms should be optimized (CitationYamamoto et al., 1993; CitationHuneck & Yoshimura, 1996; Stocker-Worgotter & Elix, Citation2004; CitationBoustie & Grube, 2005).
Recently, we have tested the antimicrobial, antioxidative and cardiovascular-protective activity of the natural thallus extract of a lichen Usnea complanata (Müll. Arg.) Motyka (Parmeliaceae) in our laboratory. The extract showed moderate-to-strong action on the above biological activities (CitationMahadik et al., 2011).
Lichen growth in nature is very slow and for screening of the biological activities of a particular species huge amount of natural thallus is required for the extraction, which is not feasible in most of the cases (CitationBrunauer & Stocker-Worgotter, 2005). In this condition, the alternative method is to culture them in the laboratory with suitable media and optimization for the production of large quantity of lichen metabolites.
With this background, the present work is an extension to our previous work. In this work we have standardized the culture media to culture the lichen U. complanata and their growth optimization for in vitro synthesis of lichen substances usnic acid and psoromic acid, and determined the antioxidative and cardiovascular-protective activity of total extract of cultured lichen tissue and purified compound alone.
Materials and methods
Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2-tert-butyl-4-methoxyphenol (BHA) and pyrocatechol were purchased from HiMedia, Merck. 3-Hydroxy-3-methylglutaryl-CoA reductase (HMGR), HMG- CoA, pravastatin, angiotensin converting enzyme (ACE), hippuryl-l-histidyl-l-leucine (HHL), captopril and plasmin were purchased from Sigma-Aldrich Chemical Co., USA. All other routine chemicals used were of AR grade obtained from one of the following suppliers SRL, HiMedia, Merck.
Natural thallus of lichen species U. complanata was collected from Silver Oak trees in Mahabaleshwar (Satara-District, Maharashtra, India) in July 2008 and authenticated by the lichen taxonomist Dr. U.V. Makhija at Mycology Group, Agharakar Research Institute, Pune. A part of the specimen (Accession no: 70.31) has been deposited at Ajrekar Mycological Herbarium (AMH), Agharkar Research Institute, Pune, India ().
Figure 1. A: Natural thallus of lichen Usnea complanata. B: 90-day-old culture cell derived from natural thallus fragments. C: Cells composed of blue colored fungal hyphae and dark green algal cells (micropreparation from the same culture). D: Culture in the conical flask containing liquid MY medium. E: Fermentation of cells derived from thallus.
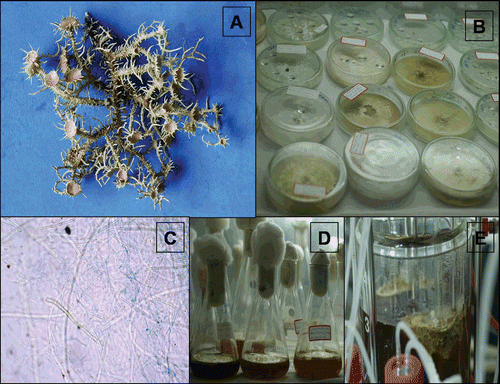
Culture of lichen U. complanata
Natural thallus of lichen species U. complanata was cultured following the methodology described by Yamamoto et al. (Citation1985). Briefly, the lichen natural thallus was washed with slow running tap water over night and subsequently with sterilized water (1 h) and finally homogenized with 5 mL of sterilized water using mortar and pestle under sterile conditions. The suspension was passed through a sterilized nylon filter with a 150 µm mesh. Retained fragments were inoculated in Petri dishes containing medium malt yeast (MY) extract (CitationAhmadjian, 1967), Lilly-Barnett (LB) (CitationLilly & Barnett, 1951), Bold’s basal medium (BBM) (CitationDeason & Bold, 1960), Murashige and Skoog (MS) (Citation1962), CitationBischoff and Bold (1963) and incubated in the culture room under 18–20°C temperature with alternating photoperiod of 10 h light/14 h dark with 50 to 80% humidity (). After 60 days of inoculation, very poor growth of the inoculum was observed in the LB, BBM, MS, Bischoff and Bold medium. However, inoculum on MY agar slants supported the growth of lichen tissue. A portion of growing cultured tissue was taken from the MY medium and stained with cotton blue, then examined under a microscope (Olympus CX21 Model, Japan). In order to know the production of lichen substances by the cultured tissue, the tissue was extracted with acetone and analyzed using solvent system tolune; 1,4 dioxane; acetic acid 180:45:5 (TDA) and tolune; ethyl acetate; formic acid 139:83:8 (TEF) with thin layer chromatography (TLC) (CitationCulberson, 1972) and high-performance liquid chromatography (HPLC) (CitationMahadik et al., 2011) (). After confirmation of the production of lichen substances by TLC spot visualization and HPLC, some part of the culture from the MY medium slants were transferred onto 250 mL capacity of Erlenmeyer flask containing 100 mL previously prepared liquid medium of MY kept in the culture room as stationary phase and incubated under the same conditions described above for a period of 90 days ().
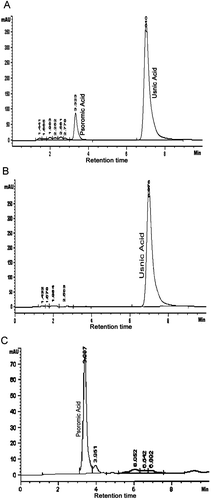
Figure 2. A: HPLC chromatogram showing production of usnic acid and psoromic acid in the three month old symbiont culture of U. complanata. B: HPLC chromatogram of purified usnic acid from in vitro cultured lichen U. complanata. C: HPLC chromatogram of purified psoromic acid from in vitro cultured lichen U. complanata.
Fermentation of lichen
After 30 days, a small portion of fresh cultured lichen material was collected from the Erlenmeyer flask and was then inoculated in the 5-L fermentor (BIOSTAT B plus-5L CC, Sartorius Stedim Biotech, Germany) containing 3 L of liquid medium of Malt-Yeast extract with pH 6.0 at stirrer 200 rpm (). Temperature was controlled between 18 and 20°C with an alternating photoperiod of 10 h light/14 h dark. The batch was run for a period of 30 days and then the lichen culture biomass was harvested. The lichen biomass was then weighed and further processed for extraction of biomass with different organic solvents and then processed for subsequent studies, i.e., quantification, isolation of lichen substances and to study their biological activities.
Extraction of lichen substances developed in vitro culture
Cultured tissue of lichen species U. complanata was air-dried at room temperature and then extracted by various organic solvents such as ethanol, methanol, ethyl acetate and acetone using a Soxhlet extractor. The extract obtained was filtered using Whatman No. 1 filter paper and then evaporated in a water bath at 40°C until a solid powder was produced. This powder was weighed and further dissolved in respective solvents.
Isolation and purification of usnic acid and psoromic acid by preparative TLC
In order to obtain pure lichen substances, usnic acid and psoromic acid from the total extract collected from cultured tissue in bioreactor and stationary flask culture were vacuum freeze dried for 12 h. Dried extract (5–10 g) was further dissolved in acetone for 4 h at room temperature and then preparative TLC was performed with solvent system TDA. For usnic acid, Rf class was found to be 6.0 cm and for psoromic acid it was 3.0 cm, which was similar to the respective standards used. The two separated spots of usnic acid and psoromic acid on silica plate were scraped carefully and redissolved separately in acetone. The supernatant obtained after centrifugation at 8000 rpm containing the pure lichen compound was carefully decanted to other screw cap bottle and then acetone was evaporated in a water bath at temperature 40°C. Finally, the purity of the substance was further checked by HPLC. Lichen substances were identified by their peak symmetry and their retention time (retention time for usnic acid was 6.9 min and for psoromic acid was 3.3 min), by comparison with authentic substances made to the standard concentration ( and ).
Detection of antioxidant compound present in the extract by TLC-DPPH analysis
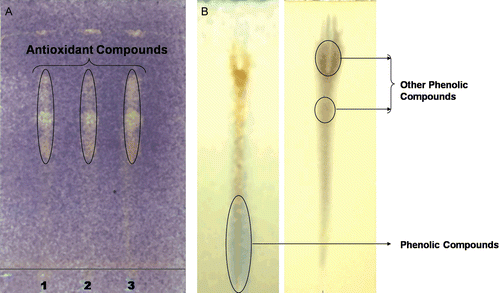
In order to determine the presence of antioxidant compounds in the crude lichen extracts, the extracts were analyzed by TLC using aluminium-backed TLC plates (Silica gel 60 F254) following the methodology of CitationOndrejovic et al. (2009). The TLC plates were developed with four mobile phases; methanol:formic acid (10:1) (developing to one-fourth of TLC plate length), chloroform:methanol (9:1) (developing to one-half of TLC plate length), toluene:acetone (7:3) (developing to three-fourth of TLC plate length) and hexane:ethyl acetate (5:1) (complete developing of TLC plate), respectively. The TLC plates were dried in the oven at 50°C for 5 min. To detect antioxidant compounds, chromatograms were sprayed with DPPH in methanol, as an indicator. The presence of antioxidant compounds was detected as yellow spots against a purple background ().
Figure 3. A: Yellow colored spot developed after spraying DPPH solution indicates presence of antioxidant compound in the extract of cultured lichen U. complanata. Spot 1: Ethanol extract, 2: acetone extract, 3: ethyl acetate extract. B: Identification type of phenolic group present in the cultured acetone extract of U. complanata symbiont.
Phenolic group detection TLC
Lichens mainly produce secondary metabolites are phenolic compounds along with other accessory pigments (CitationNash, 1996). In some cases, the purified compound alone is bioactive, and in other cases the total extract was reported to be bioactive, suggesting a synergistic effect with other compounds in the extract. Analysis of phenolic compounds present in the extract was done by the methodology described by us previously (CitationVerma et al., 2008) ().
Determination of polysaccharide and polyphenol content in the extract
Like other higher plants, lichens also produce polysaccharides and polyphenolic compounds. Thus, the polysaccharide and polyphenolic content in the culture extract were determined using the phenol-sulphuric acid method described by CitationDubois et al. (1956) and Folin-Ciocalteu reagent method proposed by CitationSlinkard and Singleton (1977) using pyrocatechol as a standard.
Antioxidative assay
Antioxidant activity of the cultured lichen extract and the purified lichen compound usnic acid and psoromic acid was measured in terms of free radical scavenging activity (FRSA), nitric oxide radical scavenging activity (NORSA) and inhibition of lipid peroxidation. Details of the antioxidant assay procedure with slight modification have been described by us earlier (CitationBehera et al., 2005).
HMGR inhibitory activity
HMGR inhibitory activity was determined following the method described by CitationKleemann and Kooistra (2005) with slight modification. The assay is based on the spectrophotometric measurement of the decrease in absorbance at 340 nm, which represents the oxidation of NADPH by the catalytic subunit of HMGR in the presence of the substrate HMG Co-A. The standard assay mixture contained 0.5 mL assay buffer, 0.1 mL NADPH and HMG Co-A (0.1 mL). The total reaction volume was 1 mL. The reaction was initiated by the addition of HMGR (5 μL) and mixture was incubated at room temperature for 5 min. Optical density was measured at 340 nm and HMGR activity was calculated. Pravastatin, standard inhibitor of HMGR, was used as a positive control.
ACE inhibition and fibrinolytic assay
The ACE inhibitory activity of the lichen purified compound usnic acid and psoromic acid was determined using the method of CitationCushman and Cheung (1971). Captopril (Sigma), a standard ACE inhibitor, was used as a positive control for comparison. Fibrinolytic activity of usnic acid and psoromic acid was estimated using the modified plate assay of CitationAstrup and Mullertz (1952) with slight modification using plasmin as standard. Fibrin plate clearance was monitored by measuring the clear zone and expressed in cm2. These two assays were carried out with slight modification. Procedures in detail are reported with modification in our previous article (CitationMahadik et al., 2011).
ACE and HMG-CoA reductase inhibitory kinetics by usnic acid and psoromic acid
In order to study the ACE inhibitory kinetics, the substrate HHL concentrations 2.5, 5.0, 10, 15, 20 and 25 mM and inhibitor usnic acid and psoromic acid at concentrations of 0, 50 and 250 µg were used. Similarly for HMG-CoA reductase inhibitory kinetics, the substrate HMG-CoA concentrations 50, 100, 200, 250 and 300 mM and inhibitor usnic acid and psoromic acid at concentrations of 0, 50 and 250 µg were used. The mode of action of ACE and HMG-CoA reductase in the presence of inhibitor or without inhibitor was determined by using Lineweaver Burk plot analysis and their Km and Vmax values were recorded.
Stability and thermosensitivity of purified usnic acid and psoromic acid
The purified lichen metabolites usnic acid and psoromic acid were incubated at 4°C for 1 month in the refrigerator and residual activity in terms of lipid peroxidation inhibition (LPI) was estimated. Further, in order to know the thermosensitivity of the purified lichen metabolites, usnic acid and psoromic acid were incubated at 40°C for 2 h and LPI activity was estimated.
Results
In the present study, we have investigated the antioxidant and cardiovascular-protective activities of total extract and isolated compound usnic acid and psoromic acid alone of the lichen species U. complanata grown in stationary flask culture and in the fermentor.
Antioxidant activity of extract and purified compounds
Antioxidant activity in terms of FRSA, NORSA and LPI of the total extract and the purified compound usnic acid and psoromic acid alone were measured, and the results are presented in . All the individual organic solvent extract of lichen tissue showed concentration-dependent FRSA, NORSA and LPI activity. The increasing ethanol extract concentration from 25 to 100 µg/mL in the assay mixture showed 55 to 71% FRSA comparable to other organic solvent extract for this activity. In the case of NORSA, methanol extract concentration from 25 to 100 µg/mL showed 25 to 61% activity, whereas other solvent extract with same concentration showed 29 to 35% activity. Furthermore, methanol extract concentration of 25 to 100 µg/mL inhibited 27 to 60% lipid peroxidation, other solvent extract showed 18.5 to 40% LPI. All the solvent extracts that showed antioxidant activity were found to be less than the positive control used as BHA a synthetic antioxidant at a concentration of 50 µg/mL that showed 78.9% FRSA, 62.3% NORSA and 67.3% LPI ().
Table 1. Antioxidant activity in terms of FRSA, NORSA and LPI with various concentrations of extracts of cultured lichen.
As far as IC50 values in µg required for 50% FRSA, NORSA and LPI is concerned, it was found that only ethanol, acetone and ethyl acetate extract could scavenge 50% free radicals with an IC50 value ranging from 22.86 to 25 µg/mL, which is lower than the BHA (31.68 µg). However, to obtain 50% NORSA and LPI, the extract concentration required was 72.52 to 157.97 µg, which was found to be much higher than the BHA concentration ().
Table 2. IC50 values in μg of the cultured lichen U. complanata extract for FRSA, NORSA and LPI.
In many reports, it has been found that in some cases the purified compound alone is bioactive, and in other cases extract is bioactive, suggesting a synergistic effect with other compounds in the extract (CitationKowalski et al., 2011). Like other higher plant, lichen also produce secondary metabolites along with other accessory pigments and are reported to have many biological activities. The lichen species U. complanata identified chemotaxonomically purely on the basis of the production of lichen substances usnic acid and psoromic acid. Therefore, we thought to isolate and purify these two lichen compounds in order to know whether they are potentially bioactive.
Antioxidant activity of purified usnic acid and psoromic acid was ascertained in terms of FRSA, NORSA and LPI. The results are presented in . The purified compound usnic acid and psoromic acid showed concentration-dependent FRSA, NORSA and LPI activity. Usnic acid concentration from 0.005 to 0.2 mg/mL showed FRSA 4.85 to 51.2%, NORSA 23.5 to 53.2% and LPI 24.4 to 46.6%, respectively. Psoromic acid at the same concentration showed FRSA 2.8 to 36.8%, NORSA 19.3 to 47.5% and LPI 27.8 to 57.3%, respectively. The positive control BHA a synthetic antioxidant at a concentration of 0.05 mg/mL showed FRSA 78.9%, NORSA 62.3% and LPI 67.3%, respectively. These results showed 50% of antioxidant activity by usnic acid with an IC50 value ranging from 0.188 to 0.214 mg/mL and by psoromic acid with an IC50 value ranging from 0.174 to 0.271 mg/mL (). In general, antioxidant activity shown by the organic solvent extract or the purified usnic acid or psoromic acid showed moderate-to-strong antioxidant activity.
Table 3. Antioxidant activity in terms of FRSA, NORSA and LPI activities of lichen metabolites usnic acid and psoromic acid developed from lichen U. complanata in vitro culture.
Table 4. IC50 value in mg/mL of purified lichen metabolites usnic acid and psoromic acid from in vitro cultured lichen U. complanata for their antioxidant activity in terms of FRSA, NORSA and LPI.
Cardiovascular-protective activity of extract and purified compound
Cardiovascular-protective activity of the solvent extract and the purified compound usnic acid and psoromic acid obtained from the cells of lichen U. complanata grown under submerged fermentation condition is presented in the . This activity was measured in terms of HMGR and ACE inhibition and fibrinolytic activity. At the concentration of 60 µg/mL, methanol and ethyl acetate solvent extract showed 65.18 to 74.81% HMGR inhibition and was found higher than the ethanol and acetone extract which inhibited HMGR from 2.22 to 21.48% at the same concentration. However, HMGR inhibition showed by the extracts was found to be lower than the positive standard HMGR inhibitor pravastatin (95.55%) at concentration of 50 µg/mL ().
Table 5. HMGR inhibition by the solvent extract of in vitro cultured lichen U. complanata in the presence of 200 μM concentration of HMG-COA substrate.
As far as ACE inhibition by the solvent extract is concerned, the methanol extract at the concentration of 10 µg/mL inhibited ACE 43.47%, which is almost double than the ethanol and acetone extract 21.73% to 23.18% at the same concentration. No inhibition of ACE was found by the ethyl acetate extract at the same concentration ().
Table 6. ACE inhibition by different solvent extract of in vitro cultured lichen U. complanata in the presence of substrate HHL at a concentration 10 mM.
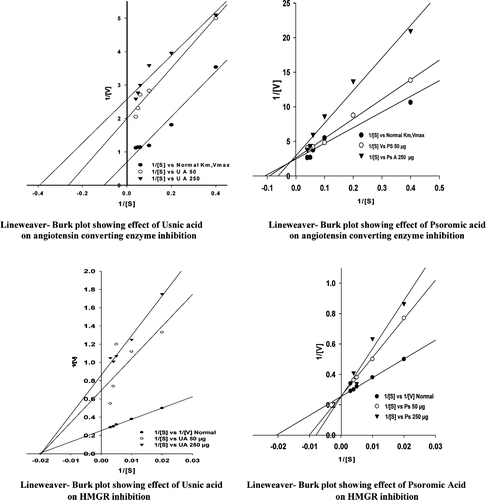
The mode of action of purified usnic acid and psoromic acid on the HMGR and ACE was studied by inhibition kinetics ( and ). In the case of HMGR inhibition, usnic acid used as inhibitor at the concentration of 50 and 250 µg with the substrate HMG-CoA concentration 50, 100, 200, 250 and 300 mM showed noncompetitive type of inhibition with a Vmax 1.4 and 1.16 U/mgP; Km 51.0 mM. Further, 50 and 250 µg psoromic acid used for the inhibition of HMGR showed competitive type of inhibition with Vmax 3.8 U/mgP and Km 100 to 125 mM. As far as inhibition kinetics of ACE is concerned by the inhibitor usnic acid and psoromic acid at the same concentration with the substrate concentration HHL 2.5, 5.0, 10.0, 15.0, 20.0 and 25.0 mM, usnic acid showed uncompetitive type of inhibition with Vmax 0.50 and 0.38 U/mL; Km 3.78 and 2.55 mM and psoromic acid showed mixed type of inhibition with Vmax 0.38 and 0.34 U/mL; Km 10.86 and 16.06 mM, respectively.
Table 7. Enzyme kinetics for inhibition of ACE and HMG-CoA reductase enzyme by the lichen acids developed in vitro and their Vmax and Km values.
Figure 4. Lineweaver-Burk plots were drawn from assays using a range of lichen metabolite (inhibitor i.e. usnic and psoromic acid) at concentrations (0, 50, 250 µg/ml) with various substrate HMG-CoA concentrations (50, 100, 200, 250 and 300 mM) for HMGR and substrate HHL at concentration of 2.5, 5.0, 10, 15, 20 and 25 mM for ACE inhibitition.
Fibrinolytic activity in terms of zone of hydrolysis of fibrinogen in the presence of purified lichen compound usnic acid and psoromic acid was measured and the results are presented in . At 100 µg concentration of usnic acid or psoromic acid shown zone of hydrolysis 0.8 to 1.4 cm2. Plasmin (standard positive control) at a concentration of 12.5 µg showed zone of hydrolysis 1.6 cm2. This result indicates that usnic acid or psoromic acid has very poor fibrinolytic activity potential.
Table 8. Fibrinolytic activity in terms of zone of hydrolysis of fibrinogen in the presence of lichen metabolites purified from the in vitro cultured lichen U. complanata.
Phytochemical content in the various solvent extract
Since solvent extract had shown from moderate-to-strong antioxidative and cardiovascular-protective activity, in order to know apart from the lichen substances (usnic acid and psoromic acid) what other compounds might play a role in the observed biological activities, we measured total polyphenol and polysaccharide content in the solvent extract. The results are presented in . A concentration of 100 µg of solvent extracts showed total soluble polyphenol ranging from 17.5 to 24.5 µg and polysaccharide 7.76 to 16.45 µg.
Table 9. Polyphenol and polysaccharide content in the 100 μg of in vitro cultured U. complanata extract.
Stability and thermosensitivity of usnic acid and psoromic acid
Stability and thermosensitivity of usnic acid and psoromic acid isolated from the cell grown from lichen U. complanata under submerged fermentation condition has been studied and the results are presented in . In this study, on day 1, we measured antioxidative activity in terms of LPI by adding usnic acid and psoromic acid individually at concentration of 100 µg/mL in the assay mixture and measured the LPI activity. Further, the usnic acid and psoromic acid that were preserved in the refrigerator for 20 days at 4°C were again tested for its potential for LPI activity. About 4.6% to 6.5% decrease in the LPI activity was found. Similarly, thermosensitivity of usnic acid and psoromic acid was also tested by keeping the bottle containing usnic acid and psoromic acid in a water bath at 40°C for 2 h. After 2 h of thermal incubation again LPI was measured. The concentrations of usnic acid and psoromic acid used in the assay were same as indicated above; 14.21% to 20.48% decrease in LPI activity was observed. This result suggests that the purified usnic acid and psoromic acid can retain the LPI potential for longer period, if they will be preserved at <4°C.
Table 10. Incubation stability of the lichen metabolites isolated and purified from in vitro cultured lichen U. complanata measured antioxidative activity in terms of LPI.
Discussion
Lichens are well-known for the diversity of secondary metabolites that they produce and display various biological activities. Throughout the ages, lichens have been used for various purposes as dyes, perfumes and remedies in folk medicines and also have potential healing/curing power. Even though the lichens, or the natural compounds they produce, have immense potential for the development of important therapeutic drugs, they have been long neglected by the modern pharmaceutical industries. The main reason for this is lichens grow very slow in nature and their axenic culture in laboratory is very difficult. For studying the biological activities of lichens, screening program has been the starting point in the drug research (CitationShukla et al., 2010). For screening of biological activities, a huge amount of natural lichen thallus is required for extraction of the metabolites, which is not feasible in most cases. To avoid this problem, the alternative is culturing of lichen in vitro and their optimization for the biosynthesis of desired natural lichen secondary compounds. With the above few lines of information, the experimental results of the present study have been discussed.
Lichen culture
The culture of lichen species U. complanata has been tried with various media, i.e., MY, LB, BB, Bischoff and Bold, and MS medium. Although initially observed very slow growth of mycelia in LB, BB, Bischoff and Bold, and MS medium till the period of 60 days, thereafter there was no growth of mycelia. When microscopically observed, the grown tissue in LB, BB, Bischoff and Bold, and MS medium, we found that there was no algal cells development. Further, we have checked the production of lichen substances usnic acid and psoromic acid by TLC method but we could not find even trace of lichen substances production except few unidentified pigments spots. However, MY medium favored the growth of symbionts for the production of usnic acid and psoromic acid in this lichen under laboratory conditions as evident by microscopical, TLC and HPLC testing. In the fermentor, we observed both myco- and photobionts aggregatively grown (evident from slide prepared from wet lichen culture) and produced usnic and psoromic acids. When this species has been cultured under stationary flask containing liquid broth of MY medium extract (), after 90 days of incubation afforded 0.705 g dry biomass/flask in that batch culture from 30 conical flasks was collected, total biomass obtained was 21.1 g, whereas under fermentation 11.1 g dry biomass was obtained after 30 days. Quantity of purified compounds obtained was usnic acid 31 mg and psoromic acid 27 mg from 15 g dried cultured biomass. Our results are in agreement with those reported that the chemical profile of the bionts is influenced and modulated qualitatively and quantitatively by varying osmotic conditions, composition of the nutrient medium, the physiological state or culture age, culture conditions, or the presence of photobiont (CitationHamada, 1988, Citation1996; CitationHonegger & Kutasi, 1990; CitationLeuckert et al., 1990; CitationYoshimura et al., 1994; CitationHamada & Miyagawa, 1995; CitationKon et al., 1997; CitationMolina et al., 2003; CitationStocker-Worgotter et al., 2009).
Studies on antioxidative, cardiovascular-protective effects of extracts and purified compounds
The lichen substances are unique as they are unknown in other plant sources. Lichens contain many characteristic aromatic compounds with known antiviral, antimicrobial, antiproliferative, antimitotic and antioxidant activities (CitationDembitsky, 1992; CitationHuneck & Yoshimura, 1996; CitationHuneck, 1999; CitationMüller, 2001; CitationUpreti & Chatterjee, 2007; CitationHanus et al., 2008; CitationVerma et al., 2008). Lichens may be a good potential source of bioactive phytochemicals.
With this information, in the present study we have evaluated the antioxidative, cardiovascular-protective potential of the total extract and two lichen substances isolated and purified from a lichen species that grow in stationary flask culture and in the fermentor. Antioxidative activity was measured in terms of FRSA, NORSA and LPI. All the extract showed concentration-dependent antioxidant activity, in which ethanol extract had 71% FRSA, methanol extract showed NORSA 61% and LPI 60% at a higher concentration of 100 µg/mL were found to be lower than the BHA a synthetic antioxidant (positive control) at a concentration of 50 µg/mL. As far as half-inhibiting ethanol, acetone and ethyl acetate extract concentration for FRSA is concerned, IC50 values of 22.86 to 25.0 µg/mL; NORSA 141.3 to 149.1 µg/mL; and for LPI 125 to 157.9 µg/mL was obtained. Since the total extract obtained from the symbiotic tissue of lichen U. complanata contains usnic acid, psoromic acid (a Depsidones), phenols and polysaccharides as evident by our metabolite composition analysis, it is very difficult to specify which component of the individual solvent extract had major effect on the antioxidant activity. Further, we have tested the extract for the presence of phenolic groups by a TLC method (). The results indicated that the extract contains lichen substances along with unidentified other phenol hydroxyl compounds. Therefore, again purified usnic acid and psoromic acid alone have been tested for the antioxidative action, we found FRSA, NORSA and LPI with an IC50 value for usnic acid from 0.188 to 0.214 mg/mL and for psoromic acid 0.174 to 0.271 mg/mL. However, there are reports on many lichen species that contains depsides, depsidone and usnic acid classes of compounds possessing important physiological properties (CitationShukla et al., 2010). It has been found that depsidones are more efficient antioxidants that could be related to a larger incorporation into lipidic microdomains (CitationHidalgo et al., 1994). Bridging at the phenolic group in the p-position can increase the antioxidant activity of phenols due to more efficient overlap of the substitute orbital within the aromatic π system (CitationBurton et al., 1985).
Cardiovascular-protective effects of extract and purified lichen compound usnic acid and psoromic acid were evaluated in terms of their HMGR inhibition, ACE inhibition and fibrinolytic potential. Like antioxidative potential, cardiovascular-protective effects were also found by lichen extract and the purified compound in a dose-dependent manner. Methanol and ethyl acetate extract were strong HMGR inhibitors (65.18–74.81%) at a concentration of 60 µg/mL but methanol extract alone showed moderate inhibition of ACE (43.47%) at 10 µg/mL. When the mode of action of purified lichen compound usnic acid and psoromic acid on the inhibition of the HMGR and ACE was studied by inhibition kinetics, usnic acid showed a noncompetitive type and psoromic acid competitive type HMGR inhibition; further, usnic acid showed uncompetitive type and psoromic acid had mixed type of ACE inhibition. As far as fibrinolytic activity is concerned, even at higher concentration (100 µg/mL) of usnic acid or psoromic acid, there was no or very poor zone of hydrolysis of fibrinogen. Further phytochemical composition analysis of the extract showed the presence of total soluble polyphenol ranging from 17.5 to 24.5 µg and polysaccharide 7.76 to 16.45 µg at the concentration of 100 µg of solvent extract. The variation in the mode of action by the extract or purified compound toward the cardiovascular-protective effects could be attributed to several reasons. Secondary substances produced by the “tissue” cultures in many cases chemistry are usually different from the chemosyndrome of the corresponding natural lichen thalli (Yamamoto et al., Citation1985, Citation1993). Frequently, phenol and polysaccharide production differs both qualitatively and quantitatively from that seen in intact thalli in nature (CitationLeuckert et al., 1990; CitationKon et al., 1997; CitationMahadik et al., 2011). These differences may arise due to osmotic conditions (CitationHamada & Miyagawa, 1995), nutrient supply (CitationHamada, 1996), the physiological state or culture age (CitationYoshimura et al., 1994), culture conditions (CitationHamada, 1996) or inadequate extraction methods that could also be an artifactual cause of these differences.
As far as stability and thermosensitivity of the purified usnic acid and psoromic acid for the observed biological activity antioxidative potential is concerned, the experimental results indicated that both the compounds had slightly decreased in LPI potential preserved at 4°C for 20 days, whereas 14.21 to 20.48% decrease in LPI potential at 40°C incubated for 2 h. This decrease in LPI activity could be attributed to the decrease of the concentration of active compound(s) or to the decomposition of active compound(s) at higher temperature (CitationHiguchi et al., 1993). The results suggested that the purified usnic acid and psoromic acid can be preserved for longer period ≤4°C to retain their antioxidative potential.
In conclusion, we were able to culture lichen U. complanata in stationary flask and in fermentor under submerged condition with the production of lichen substances usnic acid and psoromic acid. The cultured lichen extracts and purified usnic acid and psoromic acid showed moderate-to-strong antioxidative inhibition of HMGR and ACE activities. The properties of lichen substances make them possible pharmaceutical applications. However, applications derived from the activities described for usnic acid and psoromic acid have to be thoroughly studied, particularly antioxidative, antihyperlipidemia and antihypertensive domains. With current culturing techniques and rapid growth of this lichen, fungi can be screened industrially for potentially useful natural products.
Acknowledgments
We are very grateful to the Department of Biotechnology, Government of India, New Delhi, for financial support. We are thankful to Dr. U.V. Makhija, Mycology Group, for helping in taxonomic identification of lichen species. We are also thankful to Dr. D.G. Naik, Chemistry Group, Agharkar Research Institute, for providing the HPLC facility.
Declaration of interest
The Authors report no conflicts of interest.
References
- Ahmadjian V. (1967). Studies on lichenized fungi. Bryologist, 64, 168–179.
- Antiplatelet Trial lists Collaboration. (1994). Collaborative overview of randomised trials of antiplatelet therapy prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. British Med J, 308, 81–106.
- Astrup T, Mullertz S. (1952). The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys, 40, 346–351.
- Behera BC, Verma N, Sonone A, Makhija U. (2005). Evaluation of antioxidant potential of the cultured mycobiont of a lichen Usnea ghattensis. Phytother Res, 19, 58–64.
- Bischoff HW, Bold HC. (1963). Some soil algae from enchanted rock and related algal species. Phycological studies IV. Uni Texas Publ No. 6318, 1–95.
- Bochar DA, Stauffacher CV, Rodwell VW. (1999). Investigation of the conserved lysines of Syrian hamster 3-hydroxy-3-methylglutaryl coenzyme A reductase. Biochemistry, 38, 15848–15852.
- Boustie J, Grube M. (2005). Lichens-a promising source of bioactive secondary metabolites. Plant Genetic Res, 3, 273–287.
- Brunauer G, Stocker-Worgotter E. (2005). Culture of lichen fungi for future production of biologically active compounds. Symbiosis, 38, 187–201.
- Burton GW, Doba T, Gabe EJ, Hughes L, Lee FL, Prasad L, Ingold KU. (1985). Autoxidation of biological molecules. 4. Maximizing the antioxidant activity of phenols. J Am Chem Soc, 107, 7053–7065.
- Culberson CF. (1972). Improved conditions and new data for the identification of lichen products by a standardized thin-layer chromatographic method. J Chromato, 72, 113–125.
- Cushman DW, Cheung HS. (1971). Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol, 20, 1637–1648.
- Davies MJ, Thomas AC. (1985). Plaque fissuring–the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J, 53, 363–373.
- Deason TR, Bold HC. (1960). Phycological studies I. Exploratory studies of Texas soil algae. Univ of Texas Pub No. 60022.
- Dembitsky VM. (1992). Lipids of lichens. Prog Lipid Res, 31, 373–397.
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. (1956). Colorimetric method for determination of sugars and related substances. Anal Chem, 28, 350–356.
- Esimone CO, Grunwald T, Nworu CS, Kuate S, Proksch P, Uberla K. (2009). Broad spectrum antiviral fractions from the lichen Ramalina farinacea (L.) Ach. Chemotherapy, 55, 119–126.
- Frimpong K, Rodwell VW. (1994). Catalysis by Syrian hamster 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proposed roles of histidine 865, glutamate 558, and aspartate 766. J Biol Chem, 269, 11478–11483.
- Halliwell B. (1997). Antioxidants and human disease: A general introduction. Nutr Rev, 55, S44–9; discussion S49.
- Hamada N, Miyagawa H. (1995). Secondary metabolites from isolated lichen mycobionts cultured under different osmotic conditions. Lichenologist, 27, 201–205.
- Hamada N. (1988). Depside from isolated mycobionts II. Lichenologist, 20, 294–295.
- Hamada N. (1996). Induction of the production of lichen substances by non-metabolites. Bryologist, 99, 68–70.
- Hanus LO, Temina M, Dembitsky V. (2008). Biodiversity of the chemical constituents in the epiphytic lichenized ascomycete Ramalina lacera grown on difference substrates Crataegus sinaicus, Pinus halepensis, and Quercus calliprinos. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, 152, 203–208.
- Hidalgo ME, Fernández E, Quilhot W, Lissi E. (1994). Antioxidant activity of depsides and depsidones. Phytochemistry, 37, 1585–1587.
- Higuchi M, Miura Y, Boohene J, Kinoshita Y, Yamamoto Y, Yoshimura I, Yamada Y. (1993). Inhibition of tyrosine activity by cultured lichen tissues and bionts. Planta Med, 59, 253–255.
- Honegger R, Kutasi V. (1990). Anthraquinone production in three aposymbiotically cultured teloschistalean lichen mycobionts. In: Nardon P, Gianinazzi-Pearson V, Grenier AM, Margulis M, Smith DC, eds. Endocytobiology IV. Paris: Institute National de la Recherche Agronomique, 175–178.
- Huneck S. (1999). The significance of lichens and their metabolites. Naturwissenschaften, 86, 559–570.
- Huneck S, Yoshimura I. (1996). Identification of lichen substances. Berlin: Springer-Verlag.
- Kim HJ, Lee DH, Hwang YY, Lee KS, Lee JS. (2005). Characterization of beta-hydroxy-beta-methylglutaryl coenzyme A reductase inhibitor from Pueraria thunbergiana. J Agric Food Chem, 53, 5882–5888.
- Kleemann R, Kooistra T. (2005). HMG-CoA reductase inhibitors: effects on chronic subacute inflammation and onset of atherosclerosis induced by dietary cholesterol. Curr Drug Targets Cardiovasc Haematol Disord, 5, 441–453.
- Kon Y, Iwashina T, Kashiwadani H, Wardlaw JD, Elix JA. (1997). A new dibenzofuron, isostrepsilic acid, produced by cultured mycobiont of the lichenized ascomycete Usnea orientalis. J Jap Bot, 72, 67–71.
- Kowalski M, Hausner G, Piercey-Normore MD. (2011). Bioactivity of secondary metabolites and thallus extracts from lichen fungi. Mycosci, 52, 413–418.
- Leuckert C, Ahmadjian V, Culberson CF, Johnson A. (1990). Xanthones and depsidones of the lichen Lecanora dispersa in nature and of its mycobionts in culture. Mycologia, 82, 370–378.
- Lilly VG, Barnett HL. (1951). Physiology of Fungi. New York: McGraw-Hill.
- Mahadik ND, Morey MV, Behera BC, Makhija UV, Naik DG. (2011). Cardiovascular-protective, antioxidative, and antimicrobial properties of natural thallus of lichen Usnea complanata. Lat Am J Pharm, 30, 220–228.
- Molina MC, Crespo A, Vicente C, Elix JA. (2003). Differences in the composition of phenolics and fatty acids of cultured mycobiont thallus of Physconia distorta. Plant Phys Biochem, 41, 175–180.
- Molnár K, Farkas E. (2010). Current results on biological activities of lichen secondary metabolites: A review. Z Naturforsch C J Biosci, 65, 157–173.
- Müller K. (2001). Pharmaceutically relevant metabolites from lichens. Appl Microbiol Biotechnol, 56, 9–16.
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant, 15, 473–497.
- Nash TH. (1996). Lichen Biology. Cambridge: Cambridge University Press, 1–289.
- Ondrejovic M, Ondrigova Z, Kubincova J. (2009). Isolation of antioxidants from Alchemilla xanthochlora. Nova Biotechnologica, 9, 313.
- Roderick PJ, Wilkes HC, Meade TW. (1993). The gastrointestinal toxicity of aspirin: An overview of randomised controlled trials. Br J Clin Pharmacol, 35, 219–226.
- Shukla V, Joshi GP, Rawat MSM. (2010). Lichens as a potential natural source of bioactive compounds: A review. Phytochem Rev, 9, 303–314.
- Slinkard K, Singleton VL. (1977). Total phenol analysis: Automation and comparison with manual methods. Am J Enol Vitic, 28, 49–55.
- Stocker-Worgotter E, Elix JA. 2004. Experimental studies of lichenized fungi: Formation of rare depsides and dibenzofurans by the cultured mycobiont of Bunodophoron patatgonicum (Spaerophoraceae, lichenized Ascomycota). Bibliotheca Lichenologica, 88, 659–669.
- Stocker-Worgotter E, Hager A, Elix JA. (2009). Intraspecific chemical variation within the crustose lichen genus Haematomma: Anthraquinone production in selected cultured mycobionts as a response to stress and nutrient supply. Phytochem Rev, 8, 561–569.
- Upreti DK, Chatterjee S. (2007). Significance of lichens and their secondary metabolites: A review. In: Ganguli BN, Deshmukh SK, eds. Fungi Multifaceted Microbes. New Delhi: Anamaya, 169–188.
- Verma N, Behera BC, Makhija U. (2008). Antioxidant and hepatoprotective activity of a lichen Usnea ghattensis in vitro. Appl Biochem Biotechnol, 151, 167–181.
- Yamamoto Y, Mizuguchi R, Yamada Y. 1985. Tissue cultures of Usnea rubescens and Ramallina yasudae and production of usnic acid in their cultures. Agric Biol Chem, 49, 3347–3348.
- Yamamoto Y, Miura Y, Higuchi M, Kinoshita Y. (1993). Using lichen tissue cultures in modern biology. Bryologist, 96, 384–393.
- Yoshimura I, Kinoshita Y, Yamamoto Y, Huneck S, Yamada Y. (1994). Analysis of secondary metabolites from lichen by high performance liquid chromatography with a photodiode array detector. Phytochem Anal, 5, 195–205.
