Abstract
Context: Cnestis ferruginea (CF) Vahl ex DC (Connaraceae) is a shrub abundant in West Africa. Root preparations are used in traditional medicine to treat diverse conditions.
Objective: To evaluate the sub-chronic toxicological effects of the methanol root extract of CF.
Materials and methods: Groups of adult rats of both sexes were treated daily with distilled water (DW) and CF at doses of 80 (sub-therapeutic dose), 400 (therapeutic dose), and 1000 (supra-therapeutic dose) mg/kg orally for 90 days. Animals were weighed weekly and observed for behavioral and morphological changes. At the end, rats were sacrificed and blood samples collected for hematological and biochemical analysis. Vital organs were harvested, weighed, and assessed for in vivo antioxidants and histopathological changes. Sperm analysis and reversibility study were done, and mortality was recorded.
Results: CF at the therapeutic dose did not produce any significant irreversible deleterious effects on the weight of animals and vital organs, in vivo antioxidants, histopathological presentation, hematological, biochemical, and sperm parameters. Platelet anomaly was elicited as a delayed effect. Effects at the sub- and supra-therapeutic doses were similar but with delayed anemia in females and weight reduction and sterility in males as possible side effects. CF generally showed a potential to induce in vivo antioxidants.
Discussion and conclusion: Findings suggest that CF given over an extended period possess the potential to cause induction of in vivo antioxidants especially in the ovary. Possible side effects identified with CF, which necessitate caution, include delayed platelet anomaly and anemia in females, weight reduction, and sterility in males.
Introduction
Cnestis ferruginea (CF) Vahl ex DC (Connaraceae) is a shrub commonly found in deciduous forests and secondary scrublands (CitationBurkill, 1985). It is abundant in West Africa and other tropical regions. Common names include “Apose” Akan-Asante (Ghana), “Alabalu” Adyukru (Ivory Coast), “Sanhanguin” Kruguere (Liberia), “Treventi-ito” Balanta (Guinea Bissau), “Diakanare dire” Banyun (Senegal), “Selialiwo” Kissi (Sierra Leone), “Fupeleen” Diola (Gambia), and “Tangolo sebe” Manding-Dyula (Burkinafaso). In Nigeria, the plant is locally called “Fura amarya,” “otito” (Hausa); “Okpu nkita,” “amunkita” (Igbo); and “Akara oje”, “Bonyin bonyin” (Yoruba) (CitationBurkill, 1985). Various preparations of the plant are used in traditional medicine to treat diverse conditions. Root decoctions are used to treat headache, migraine, toothache, dysmenorrhea, skin infections, gynecological troubles, dysentery, urethral discharge, and sinusitis (CitationBurkill, 1985). Decoctions from the root are also employed as an appetite stimulant, purgative, skin ointment, aphrodisiac, and as remedy for snake bite. The antistress, analgesic, and anti-inflammatory activities of CF were previously investigated in our laboratory (CitationIshola & Ashorobi, 2007; CitationIshola et al., 2011).
Based on the tendency for prolonged use by local people to achieve wellness within the context of traditional medicine, this study was conducted to investigate the sub-chronic toxicological effects of the methanol root extract of CF administered for 90 days.
Materials and methods
Plant material
CF dried roots were obtained from a local herb market in Mushin, Lagos State, Nigeria, in October 2009. Identification and authentication of the plant was done by Prof. J. D. Olowokudejo of the Department of Botany and Microbiology, Faculty of Science, University of Lagos, Lagos, Nigeria, and Mr. J. Ariwaodo of the Forestry Research Institute of Nigeria (FRIN), Ibadan, Nigeria. A voucher specimen of the plant (FHI 108219) was deposited in the herbarium of FRIN.
Extraction
The dried roots of CF were cut into small pieces and milled into fine powder. Soxhlet extraction was carried out on plant powder (100 g) using methanol (500 mL). A rotary evaporator was used to concentrate the extract to dryness and the yield was 9.87% based on the derived weight of dried extract and the weight of the starting material.
Laboratory animals
Albino rats of both sexes (100–200 g) used in this study were obtained from the Laboratory Animal Centre of the College of Medicine, University of Lagos, Lagos, Nigeria. The animals were kept under standard environmental conditions (23–25°C, 12 h/12 h light/dark cycle) and fed with standard rodent pellet (Livestock Feed PLC, Lagos, Nigeria) and water ad libitum.The experimental protocol adopted in this study was approved by the Experimentation Ethics Committee on Animal Use of the College of Medicine, University of Lagos, Lagos, Nigeria and was in accordance with the United States National Institutes of Health Guidelines for Care and Use of Laboratory Animals in Biomedical Research (CitationAnonymous, 1985).
Sub-chronic toxicity test
A total of 62 albino rats were randomly divided into 4 groups of 15 or 16 animals each (7 or 8 males and 8 females). The male and female rats within each group were separately caged. Animals in the different groups were daily treated with distilled water (DW) (Group I; control) and CF at doses of 80 mg/kg (Group II), 400 mg/kg (Group III), and 1000 mg/kg (Group IV) orally. The doses of CF used in this study represent the sub-therapeutic, therapeutic, and supra-therapeutic doses, respectively, based on the modification of the protocol of CitationYemitan and Adeyemi (2004), CitationAmida et al. (2007), and CitationAdeyemi et al. (2010). The therapeutic dose of 400 mg/kg was the most effective dose in a previous study in our laboratory which investigated the analgesic and anti-inflammatory activities of the methanol root extract of CF (CitationIshola et al., 2011).
Rats were weighed on days 0, 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, 77, 84, and 90. The animals were generally observed for behavioral and morphological changes. At 90 days, 9 or 10 rats from each group (4 or 5 males and 5 females) were anaesthetized by administration of 1% chloralose in 25% urethane (w/v) (5 mL/kg, i.p.; CitationAdeyemi et al., 2010) and blood samples were collected through the retro-orbital plexus vein of rat eye for hematological and biochemical analysis. Rats were sacrificed by cervical dislocation.
Semen obtained from male rats was assessed for sperm motility, count, and morphology. Mortality in each treatment group was recorded in the course of the experiment. Six rats from each group (three per sex) were reserved for reversibility study in which treatment was discontinued and samples collected from animals after 30 days for analysis.
Effect on vital organs
After sacrificing the experimental animals at 90 days, vital organs including the brain, heart, liver, kidney, testis, and ovary were harvested. The organs were carefully examined for gross lesions and weighed (using Mettler-Toledo GmbH digital weighing balance [Type BD202, SNR 06653]). Standardization of the weight of organs to 100 g body weight of each animal was done. After weight determination, samples were taken from the vital organs for determination of in vivo antioxidants and malondialdehyde (MDA). The remaining parts of the organs were preserved in 10% formol-saline for histopathological assessment.
Measurement of in vivo antioxidants and MDA levels
The determination of catalase (CAT), superoxide dismutase (SOD), reduced glutathione (GSH), and MDA was done according to the protocol of CitationSoon and Tan (2002).
Histopathological assessment
Tissues fixed in 10% formol-saline were dehydrated in graded alcohol, embedded in paraffin, and cut into 4–5 μm thick sections. The sections were stained with hematoxylin-eosin for photomicroscopic assessment using a Model N-400ME photomicroscope (CEL-TECH Diagnostics, Hamburg, Germany) (CitationGaligher & Kozloff, 1971; CitationHabbu et al., 2008).
Hematological assessment
Blood samples from experimental animals were collected into EDTA (ethylenediamine-tetraacetate) bottles and analysed using standard procedures. Erythrocyte (RBC), hemoglobin (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count, mean platelet volume (MPV), platelet distribution width (PDW), red cell distribution width (RDW), and total and differential leukocyte (WBC), were determined using automated hematology analyzer.
Biochemical assessment
Blood samples collected into plain bottles were allowed to coagulate for 30 min, and serum was separated by centrifugation. Serum samples were analyzed for aspartate aminotransaminase (AST), alanine aminotransaminase (ALT), alkaline phosphatase (ALP), total bilirubin, total protein, albumin, total cholesterol, triglyceride, creatinine, urea, and uric acid using Roche and Cobas commercial kits and Roche/Hitachi 904 automated analyzer.
Sperm analysis
Seminal fluid obtained from male animals across the different treatment groups was analysed to determine sperm motility, count, and morphology using the methods of CitationCheesbrough (2000) and CitationOgli et al. (2009). Seminal fluid was collected according to the method of CitationOgli et al. (2009). After sacrifice, male rats were in turn strapped astride on their back on a dissecting board. A sterile surgical blade was used to make incision on the right scrotum and the right testis was removed with its ipsilateral epididymis into a beaker. The semen was expelled out of the epididymis into another beaker placed in water bath at 36°C.
Sperm motility
A drop (10–15 µL) of semen was placed on a slide such that the spermatozoa were evenly distributed and covered with a glass. The specimen was properly focused, and several fields were assessed for motility on examination using the 40× objective of the microscope. A total of 100 spermatozoa were counted and the number of motile cells was noted.
Sperm count
A 1 in 20 dilution of semen was carried out with sodium bicarbonate-formalin diluting fluid and thorough mixing was done. Using a Pasteur pipette, an improved Neubauer ruled chamber was filled with well-mixed diluted semen. After 3–5 min, the number of spermatozoa in an area of 2 sq mm was counted using the 10× objective of the microscope. The number of spermatozoa in 1 mL of fluid was calculated by multiplication of the number counted by 100,000.
Sperm morphology
A thin smear of the liquefied well-mixed semen was made on a slide. The smear was fixed with 95% v/v ethanol, while still wet, for 5–10 min and allowed to air-dry. The smear was washed with sodium bicarbonate-formalin solution to remove possibly present mucus. The smear was then rinsed severally with water. The smear was covered with dilute (1 in 20) carbon fuchsin and allowed to stain for 3 min. The stain was then washed off with water. Counterstaining was done by covering the smear with dilute (1 in 20) Loeffler’s methylene blue for 2 min. The stain was washed off with water, drained, and air-dried. The preparation was examined for normal and abnormal spermatozoa using the 40× objective of the microscope. A hundred spermatozoa were counted, and the percentages showing normal and abnormal morphology were estimated.
Acute toxicity study
The acute toxicity study of the methanol root extract of CF had previously been done in our laboratory and reported (CitationIshola et al., 2011). In the study, groups of 5 mice each fasted for 12 h prior to the experiment were administered CF orally at doses of 1, 2, 4, 8, and 10 g/kg. Mice were observed for 2 h posttreatment for behavioral changes and signs of toxicity. Mortality observed in each group within 24 h was recorded, and surviving mice were observed for signs of delayed toxicity for 7 more days. The LD50 was estimated by the log dose Probit analysis method.
Statistical analysis
Results obtained in this study are expressed as mean ± SEM. Data analysis was done using one-way ANOVA followed by Tukey’s multiple comparison test using GraphPad Prism 5 (GraphPad Software Inc., CA, USA). Results were considered significant when p < 0.05.
Results
Mortality in the sub-chronic toxicity test
No deaths were recorded in the control group throughout the study. However, 2/15, 4/16, and 4/15 deaths were recorded in the 80, 400, and 1000 mg/kg CF treatment groups, corresponding to 13.33, 25.00, and 26.67% mortality, respectively, in the main study.
Body weight
At doses of 80 and 400 mg/kg, CF did not produce any significant change in the weight of male and female rats compared with control. However, at the highest dose of 1000 mg/kg, CF elicited significant (p < 0.05, 0.01, 0.001) reductions in weight of male rats from the 21st day up to the 90th day, but there were no significant changes in the weight of female animals across the treatment intervals. Considering the mean change in weight by the 90th day, CF produced a significant (p < 0.05) reduction in the weight of male rats at 1000 mg/kg (16.00 ± 2.80 g) compared with control (40.00 ± 5.40 g). The extract did not produce any significant effect on weight in female rats at all doses used compared with control (). However, there was a significant (p < 0.001) difference in the weight of male (16.00 ± 2.80 g) and female rats (47.77 ± 6.26 g).
Table 1. Effects of C. ferruginea on sperm parameters and change in body weight of rats over 90 days.
Sperm parameters
As shown in , CF did not produce any significant effect on sperm motility and morphology (% abnormality). However, at the dose of 1000 mg/kg, CF elicited significant (p < 0.01) reduction in sperm count (10.00 ± 3.10 × 106) compared with control (22.00 ± 3.00 × 106). In the reversibility study, (), there was a significant (p < 0.05) reduction in sperm count in the group that received CF at the dose of 80 mg/kg (11.33 ± 1.64 × 106 relative to a value of 24.90 ± 0.67 × 106 for control).
Effect on vital organs
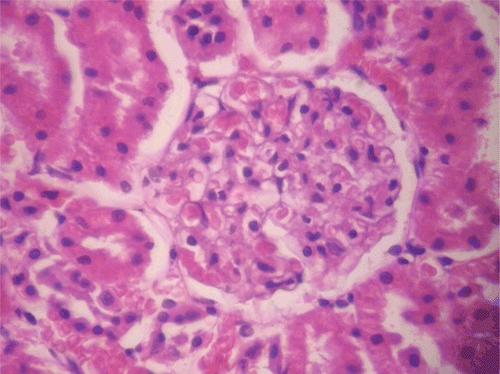
In male rats, CF did not produce any significant effect on the weight of the brain, heart, liver, and kidney at all doses used. However, at the highest dose of 1000 mg/kg, the extract produced significant (p < 0.05) reduction in the weight of the testes (0.38 ± 0.05 g) compared with control (0.76 ± 0.07 g). In female rats, CF elicited significant (p < 0.01, 0.001) effects only on the brain (increase; 1.10 ± 0.06 g at 80 mg/kg vs. 0.77 ± 0.04 g for control), liver (decrease; 3.70 ± 0.09 g at 80 mg/kg vs. 5.00 ± 0.18 g for control), and ovary (increase; 0.10 ± 0.00 at 400 mg/kg vs. 0.04 ± 0.00 for control) (). The effects of CF on the brain, liver, and ovary were reversed.
Table 2. Effects of C. ferruginea on weight of vital organs.
In vivo antioxidant enzymes and MDA
In male rats, CF did not produce any significant effect on organ levels of GSH, SOD, CAT, and MDA in respect of the kidney and brain. However, there was significant increase (p < 0.001) in the level of GSH in the liver (337.40 ± 89.91 U/mg protein compared to 62.32 ± 6.23 U/mg protein for control) and reduction (p < 0.01) in the testes (71.50 ± 17.81 U/mg protein compared with 262.60 ± 23.02 U/mg protein for control) at 1000 mg/kg. At 80 mg/kg, there was a significant increase in testes MDA (25.25 ± 1.69 U/mg protein) compared with control (9.82 ± 1.40 U/mg protein). In female rats, pronounced effects were only produced in respect of the liver and ovaries. For the liver, CF significantly (p < 0.01) increased the level of GSH at 1000 mg/kg (259.70 ± 30.98 U/mg protein) compared with control (58.86 ± 15.52 U/mg protein), and significantly (p < 0.05) reduced the level of SOD at 80 and 400 mg/kg (72.43 ± 14.33, 67.05 ± 14.65 U/mg protein respectively) relative to control (206.80 ± 37.17 U/mg protein). In respect of CAT, there was a significant (p < 0.05) reduction at 400 mg/kg (276.40 ± 56.67 U/mg protein) compared with control (831.30 ± 149.40 U/mg protein). There was no significant effect on liver MDA level. For the ovary, CF produced significant (p < 0.05, 0.01) increase in GSH level at 400 and 1000 mg/kg (374.20 ± 10.01, 326.10 ± 10.59 U/mg protein respectively) relative to control (110.50 ± 54.20 U/mg protein). For SOD, there was a significant (p < 0.05) increase at 80 mg/kg (217.70 ± 36.64 U/mg protein) compared with control (58.04 ± 26.16 U/mg protein). In respect of CAT, there was also a significant (p < 0.05) increase at 80 mg/kg and 400 mg/kg (953.80 ± 150.60, 980.00 ± 17.50 U/mg protein, respectively) compared with control (243.30 ± 98.55 U/mg protein). CF did not produce any significant effect on ovary MDA level.
Generally, there were no significant differences between corresponding values in male and female rats ().
Table 3. Effects of C. ferruginea on organ in vivo antioxidant enzymes and MDA and GSH levels.
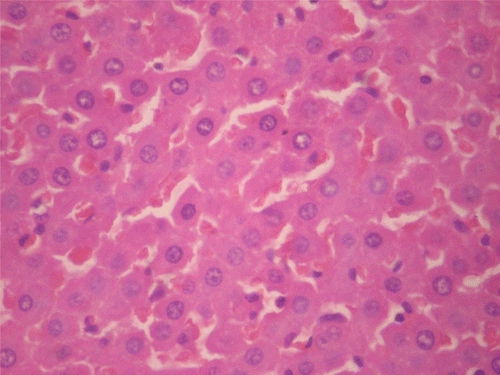
Histopathological assessment
A summary of findings from representative sections of vital organs from the various groups is presented below:
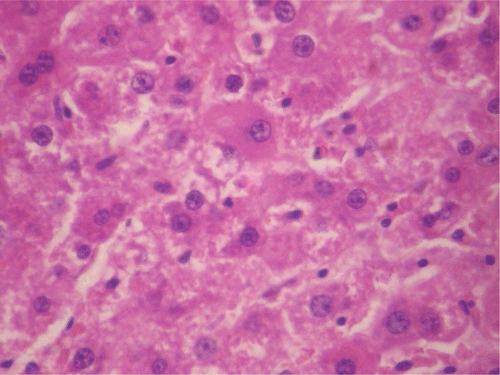
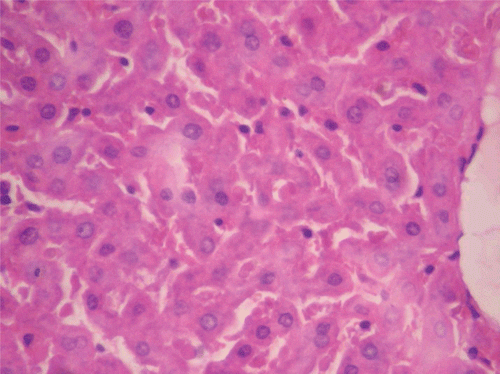
Figure 7. Histopathologic presentation of male rat liver of CF 400 mg/kg group (Intracellular accumulations/Fatty change; 400×).
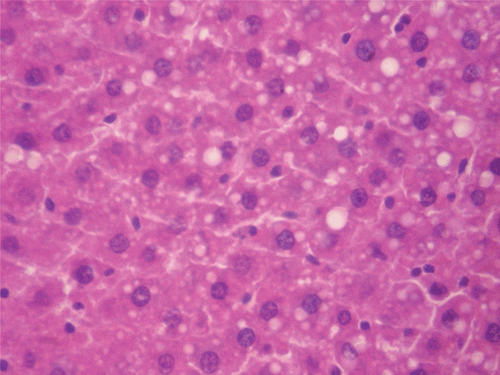
Figure 8. Histopathologic presentation of male rat liver of CF 1000 mg/kg group (Intracellular accumulations/Fatty change; 400×).
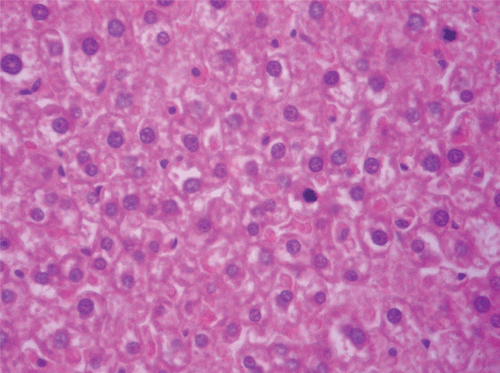
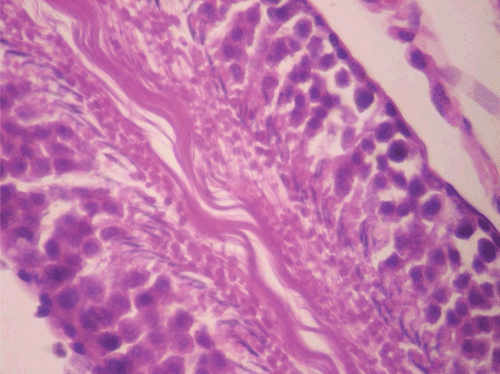
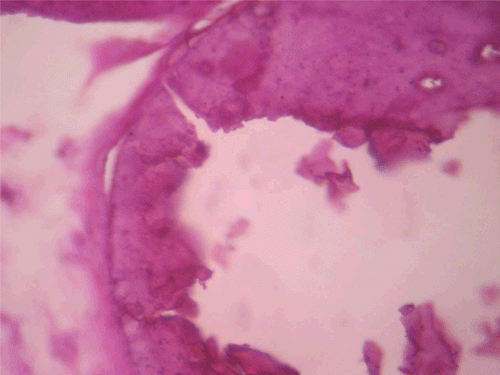
Figure 9. Histopathologic presentation of rat testes of CF 1000 mg/kg group (Inflammation/necrosis; 400×).
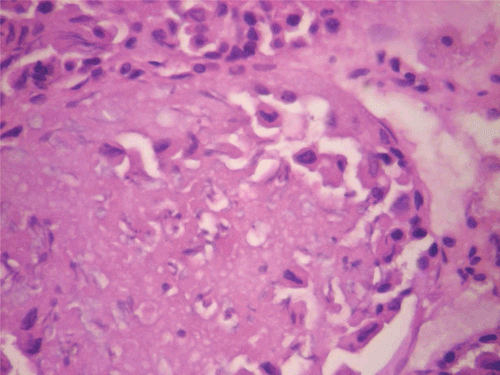
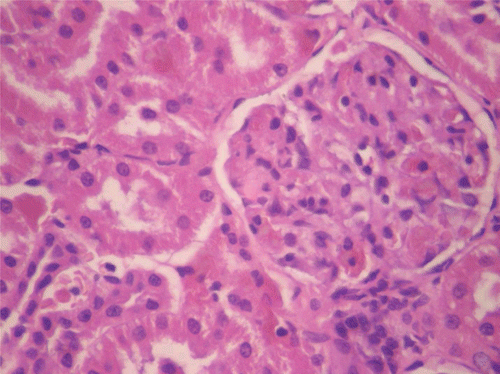
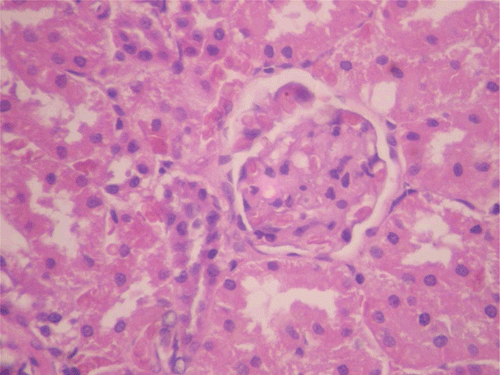
In respect of the reversibility study, histopathological assessment of the vital organs revealed normal presentations in the heart, kidneys (), and ovaries at all doses of CF. However, in the liver for CF at the dose of 1000 mg/kg, congestion (histologic section of liver showed prominent hepatic sinusoids which were engorged with red blood cells) was observed (M; ). Calcification (areas of necrosis and deposition of calcium salts and amorphous debris within some seminiferous tubules, with loss of lining germ cell series as well) was observed in the testes for CF at the dose of 80 mg/kg ().
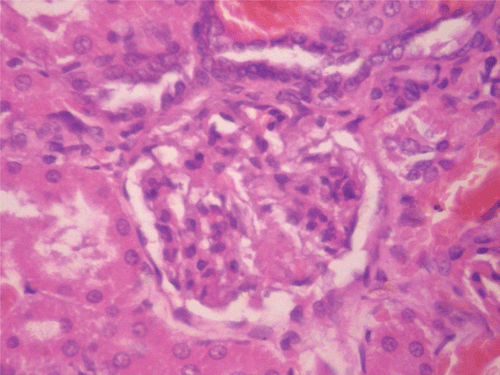
Figure 10. Histopathologic presentation of male rat kidney of CF 80 mg/kg group, reversibility study (Normal; 400×).
Hematological parameters
As shown in in respect to male rats, CF did not produce any significant effect on all parameters evaluated except on RDW at doses of 400 and 1000 mg/kg in which there were significant (p < 0.05) reductions (32.73 ± 1.15%, 32.85 ± 0.88%, respectively) compared with control (37.46 ± 1.32%). In female rats, CF did not elicit any significant effect on all parameters relative to control. In the reversibility study (), the effect of CF on RDW was reversed, but there were delayed significant (p < 0.05, 0.01) effects on MPV (decrease; 7.60 ± 0.10 fL at 400 mg/kg vs. 8.27 ± 0.07 fL in control) in male rats and on Hb (decrease; 12.43 ± 0.30 g/dL at 80 mg/kg vs. 14.67 ± 0.37 in control), PCV (decrease; 41.23 ± 0.49% at 80 mg/kg vs. 48.20 ± 1.37% in control), and MPV (increase; 8.50 ± 0.20 fL at 400 mg/kg vs. 7.80 ± 0.21 fL in control) in female rats.
Table 4. Effects of C. ferruginea on hematological parameters (main study).
Table 5. Effects of C. ferruginea on hematological parameters (reversibility study).
Biochemical parameters
As shown in , CF did not produce any significant effect on all parameters assessed in both sexes except in the case of total bilirubin in female rats in which there was a significant (p < 0.05) increase (6.45 ± 0.35 mmol/L) compared with control (4.94 ± 0.17 mmol/L). This effect was, however, reversed in the reversibility study (). In both sexes, there were no significant delayed effects in respect of all the parameters evaluated.
Table 6. Effects of C. ferruginea on serum biochemical parameters (main study).
Table 7. Effects of C. ferruginea on serum biochemical parameters (reversibility study).
Discussion
Herbal remedies are generally considered and promoted as safe relative to orthodox medicines mainly based on history of use by indigenous people whose belief and confidence in traditional medicine is unequivocal. It has, however, been reported that many African plants used medicinally and widely assumed to be safe are potentially toxic due to the presence of toxic and potentially lethal constituents (CitationFennell et al., 2004). The continuous surge in the popularity and patronage of herbal remedies, and the fact that reports of efficacy far outnumber those of toxicity, necessitate concern. This is mainly due to the adverse effects of potentially toxic constituents in plants (e.g., aristolochic acids, pyrrolizidine alkaloids, benzophenanthrine alkaloids, lectins, viscotoxins, saponins, diterpenes, cyanogenetic glycosides, and furonocoumarins (CitationFennell et al., 2004) which can be fatal. Based on these facts, it has been said that pharmacological and toxicological evaluations of medicinal plants are essential for drug development (CitationAhmed et al., 2005; CitationIbarrola et al., 2000).
Due to multiplicity of usage and tendency for prolonged use of the root decoction in traditional medicine, as aphrodisiac and for treatment of conditions like gynecological diseases, skin infections, urethral discharge, and sinusitis (CitationBurkill, 1985), CF methanol root extract was investigated in this study for possible sub-chronic toxicity effects using a 90-day administration schedule at determined sub-therapeutic (80 mg/kg), therapeutic (400 mg/kg), and supra-therapeutic (1000 mg/kg) doses.
Administered for 90 days at the therapeutic dose, the methanol root extract of CF did not produce any significant effect on the weight of rats over the entire duration of administration. According to CitationRaza et al. (2002) and CitationTan et al. (2008), reductions in body weight gain and internal organ weights are simple and sensitive indices of toxicity after exposure to toxic substances. In general terms, there was also no significant change in the weight of vital organs except in respect of the liver (decrease) and ovary (increase) in female rats. These changes were reversed after cessation of therapy. In vivo antioxidants and MDA assays showed reductions in liver SOD and CAT but no change in MDA levels in female rats. Mammalian cells contain endogenous antioxidant enzymes, including SOD, glutathione peroxidase, and catalase which are able to detoxify free radicals by converting them to more stable molecules within the cell (CitationSaeed et al., 2005). MDA, a major breakdown product of lipid peroxides, is an index of lipid peroxidation. The overwhelming of antioxidant enzymes by free radicals results in the depletion of the antioxidant defenses and induction of lipid peroxidation evident in elevation of MDA level (CitationAkindele et al., 2010). In respect of the ovary, CF not only caused significant increase in GSH and CAT but also produced no change in MDA level. Histopathological assessment of the ovary and liver of female animals showed normal presentations. CF at the therapeutic dose did not elicit any significant change in biochemical and sperm parameters assayed. In respect of hematological parameters, CF did not generally produce any significant effect except in respect of RDW which was increased in male rats. Red blood cell distribution width is a measure of variation of RBC width and it is often used with MCV to determine the cause of anemia. A low MCV in combination with low RDW may denote iron deficiency but in this case the MCV value was not significantly different from control. Also the observed effect on RDW was reversed on cessation of treatment. An observation that was made in the reversibility study is the reduction in MPV value for male rats and increase in female rats. These may suggest delayed effects. MPV is a measure of the average size of platelets. The MPV value gives an idea of platelet production in the bone marrow, and it is interpreted with the platelet count. Extremely low MPV value is associated with thrombocytopenia as is the case with aplastic anemia and high MPV value correlates with increased peripheral destruction observed in conditions such as immune thrombocytopenic purpura and myeloproliferative diseases. However, in this study, the delayed effect observed on MPV was not accompanied by a change in platelet count. Findings in this study suggest that the methanol root extract of CF administered at the therapeutic dose is relatively safe given over an extended period with the potential to enhance antioxidant activity in the ovary. However, results showed that CF had a tendency to produce delayed platelet anomaly at this dose.
Considering the sub-therapeutic dose, the methanol root extract of CF did not produce any significant effect on the body weight of male rats all through the 90-day treatment period. However, in respect of female rats, there was significant increase in body weight from the 7th to the 42nd day only. In respect of the mean change in weight over the 90-day period, there was no significant change in the body weight of this set of animals compared with control. For the weight of vital organs, there were generally no significant changes except in the case of the brain (increase) and liver (decrease) for female rats. The possible concern from this observation is addressed by the fact that there was no significant change in the level of antioxidant enzymes and MDA in respect of the brain, ruling out the involvement of lipid peroxidation, while the liver presented with concurrent reduction in SOD level but no change in MDA. In respect of histopathological assessment, the presentation of the female rat liver was normal while that of the male showed congestion. There was a significant increase in the MDA level in the testes of male rats while there was a significant increase in SOD and CAT levels, without a change in MDA, in the ovary of female animals. However, there was no significant change in the weight of these organs and histopathological assessment showed normal presentations. Assessment of hematological parameters showed no deleterious effect in the 90-day study but reversibility study results showed a reduction in Hb and PCV in female rats. These findings possibly suggest induction of anemia as a delayed effect in female rats. Biochemical and sperm parameters were not significantly affected in the main study at the sub-therapeutic dose of 80 mg/kg. However, reduction in sperm count was observed in the reversibility study. This may be a delayed effect as a consequence of significant lipid peroxidation (increased MDA) observed in the testes. Presentations in the histopathological report give credence to this assertion. There was calcification showing as areas of necrosis and deposition of calcium salts and amorphous debris within some seminiferous tubules, with loss of lining germ cell series as well. This calls for caution in the use of the root extract of CF as an aphrodisiac in traditional medicine, as reported by CitationBurkill (1985). Findings in this study suggest that the methanol root extract of CF administered at the sub-therapeutic dose is relatively safe administered over an extended period but with the potential to cause delayed anemia in females and infertility in males. CF in this study also showed a tendency to enhance antioxidant activity in the ovary.
In respect of the supra-therapeutic dose of the methanol root extract of CF administered for 90 days, there were significant reductions in weight change in male rats from the 21st to the 90th day and in the overall mean change in weight. In female rats, there were no significant reductions in weight during the period of treatment and in the overall mean change in weight. For vital organs, the weight of the testes in male and liver in female animals were significantly reduced, reversibly in respect of the later. CF administered at the supra-therapeutic dose increased liver and ovary GSH levels in both sexes with no change in MDA. However in the testes, the GSH level was not only reduced but also showed no change in MDA. Histopathology results showed normal female rat liver and ovary. The male rat liver revealed intracellular accumulation/fatty change while the testes showed inflammation/necrosis (dead cells and amorphous debris seen with aggregation of inflammatory cells). The finding in the testes is corroborated by the observed reduction in sperm count. As observed at the therapeutic dose, the supra-therapeutic dose also caused significant reduction in RDW in male rats, but there was no significant change in the MCV. For the biochemical parameters, there was a significant increase in total bilirubin in female rats. The destruction of hemoglobin leads to the generation of bilirubin which is conjugated in the liver and excreted in bile. Any overload or blockage of this system results in the elevation of the level of bilirubin which is indicative of possible liver disease or anemia (CitationCarter, 1989). CitationNavarro and Senior (2006) categorized the patterns of liver injury as hepatocellular (elevated ALT), cholestatic (elevated ALP and total bilirubin) and mixed (elevated ALP and ALT). However, results obtained in this study rule out cholestatic liver injury as there was no significant change in the level of ALP and also excludes anemia as there was no significant change in Hb, PCV, and other relevant hematological parameters. An important observation is the reversal of the elevated total bilirubin level in female rats upon cessation of treatment with CF. Findings in this study suggest that the methanol root extract of CF administered at the supra-therapeutic dose is also relatively safe given over an extended period with a capacity to enhance antioxidant activity in the liver and ovary. However, CF exhibited a potential to cause weight reduction and sterility in males at this dose. The tendency of the extract to cause sterility calls for caution in its use as aphrodisiac in traditional medicine especially in view of inherent issues concerning dosage in this system of healthcare.
The differences observed in this study in the effects of CF on body weight changes, organ weight and liver toxicity in male and female rats may be due to sex-related differences in body composition, hormones (CitationNies & Spielberg, 1995) and pharmacokinetic and pharmacodynamic properties (CitationWang & Huang, 2007).
Mortalities recorded in the course of this study may be a pointer to sub-chronic toxicity. However, in view of the manifestation of dyspnea by majority of the animals just before death, the mortalities are more likely to be administration related. The absence of adverse behavioral and morphological changes coupled with the results obtained with the parameters evaluated in surviving rats give credence to this assertion.
In respect of the acute toxicity test, the methanol root extract of CF administered orally caused no mortality at 1 and 2 g/kg but mortality was 20, 60, and 100%, respectively, at doses of 4, 8, and 10 g/kg, as previously reported by our research team (CitationIshola et al., 2011). The LD50 was estimated to be 5.22 g/kg. Mice exhibited dose-dependent calmness, hypoactivity, diarrhea, and increased breath frequency. In accordance with the assertion of CitationHayes (1989) that no dose-related toxicity should be considered above 5 g/kg body weight, the methanol root extract of CF can be considered safe administered acutely via the oral route.
Conclusions
The results obtained in this study suggest that the methanol root extract of CF administered over an extended period is relatively safe with the potential to cause induction of antioxidant enzymes especially in the ovary. Identified possible deleterious effects, which necessitate caution in the use of the extract for an extended period, include delayed platelet anomaly (therapeutic dose), delayed anemia in females and sterility in males (sub-therapeutic dose), and weight reduction and sterility in males (supra-therapeutic dose).
Acknowledgements
The authors are grateful to Mr. Micah Chijioke (Department of Pharmacology), Mr. Duncan Ota, Mr. Simon Dike (Department of Physiology), Mr. Sunday O. Adenekan (Department of Biochemistry), and Dr. Daniel Ike Awelimobor (Department of Morbid Anatomy), all of the Faculty of Basic Medical Sciences, College of Medicine, University of Lagos, Lagos, Nigeria, for the technical assistance given in the course of this research work. For this reason also, the authors express there appreciation to Mr. Anthony A. Ani of the AIDS Prevention Initiative Nigeria (APIN) Laboratory, Lagos University Teaching Hospital (LUTH), Lagos, Nigeria.
Declaration of interest
The authors report no conflicts of interest.
References
- Adeyemi OO, Akindele AJ, Nwumeh KI. (2010). Acute and subchronic toxicological assessment of Byrsocarpus coccineus Schum. and Thonn. (Connaraceae) aqueous leaf extract. Int J Appl Res Nat Prod, 3, 1–11.
- Ahmed M, Khanna D, Furst DE. (2005). Meloxicam in rheumatoid arthritis. Expert Opin Drug Metab Toxicol, 1, 739–751.
- Akindele AJ, Ezenwanebe KO, Anunobi CC, Adeyemi OO. (2010). Hepatoprotective and in vivo antioxidant effects of Byrsocarpus coccineus Schum. and Thonn. (Connaraceae). J Ethnopharmacol, 129, 46–52.
- Amida MB, Yemitan OK, Adeyemi OO. (2007). Toxicological assessment of the aqueous root extract of Sanseviera liberica Gerome and Labroy (Agavaceae). J Ethnopharmacol, 113, 171–175.
- Anonymous (1985). Guide for the use of laboratory animals DHHS, PHS, NIH Publication No. 85-23 (1985 Revised).
- Burkill HM. (1985). The useful plants of West Tropical Africa. Vol. 1, 2nd edition, Families A–D. Royal Botanic Gardens, Kew, UK.
- Carter WJ. (1989). The interpretation of common pathology tests. London, UK: Medicine Digest Limited. p. 6.
- Cheesbrough M. (2000). Examination of semen. In: District Laboratory Practice in Tropical Countries (Part 2). Cambridge, UK: Cambridge University Press, 130–132.
- Fennell CW, Lindsey KL, McGaw LJ, Sparg SG, Stafford GI, Elgorashi EE, Grace OM, van Staden J. (2004). Assessing African medicinal plants for efficacy and safety: pharmacological screening and toxicology. J Ethnopharmacol, 94, 205–217.
- Galigher AE, Kozloff EN. (1971). Essentials of practical microtechniques, 2nd ed. Philadelphia, PA: Lea and Febiger. p. 77.
- Habbu PV, Shastry RA, Mahadevan KM, Joshi H, Das SK. (2008). Hepatoprotective and antioxidant effects of Argyreia speciosa in rats. Afr J Tradit Complement Altern Med, 5, 158–164.
- Hayes AW. (1989). Guidelines for acute oral toxicity testings. Principles and Methods of Toxicity, 2nd ed. New York, NY: Raven Press Ltd. Table 4, p. 185.
- Ibarrola DA, Hellión-Ibarrola MC, Montalbetti Y, Heinichen O, Alvarenga N, Figueredo A, Ferro EA. (2000). Isolation of hypotensive compounds from Solanum sisymbriifolium. J Ethnopharmacol, 70, 301–307.
- Ishola IO, Akindele AJ, Adeyemi OO. (2011). Analgesic and anti-inflammatory activities of Cnestis ferruginea Vahl ex DC (Connaraceae) methanolic root extract. J Ethnopharmacol, 135, 55–62.
- Ishola IO, Ashorobi RB. (2007). Anti-stress potential of aqueous root extract of Cnestis ferruginea. Int J Pharmacol, 3, 295–298.
- Navarro VJ, Senior JR. (2006). Drug-related hepatotoxicity. N Engl J Med, 354, 731–739.
- Nies A, Spielberg S. (1995). Principles of therapeutics. In: The pharmacological basis of therapeutics, 9th edition, edited by J Hardman and L Limbird, 43–62. New York: McGraw-Hill.
- Ogli SA, Enyikwola O, Odeh SO. (2009). Evaluation of the efficacy of separate oral supplements compared with the combined oral supplements of vitamins C and E on sperm motility in Wistar rats. Niger J Physiol Sci, 24, 129–135.
- Raza M, Al-Shabanah OA, El-Hadiyah TM, Al-Majed AA. (2002). Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci Pharm, 70, 135–145.
- Saeed SA, Urfy MZS, Ali TM, Khimani FW, Gilani A. (2005). Antioxidants: Their role in health and disease. Int J Pharmacol, 1, 226–233.
- Soon YY, Tan BK. (2002). Evaluation of the hypoglycemic and anti-oxidant activities of Morinda officinalis in streptozotocin-induced diabetic rats. Singapore Med J, 43, 077–085.
- Tan PV, Mezui C, Enow-Orock G, Njikam N, Dimo T, Bitolog P. (2008). Teratogenic effects, acute and sub chronic toxicity of the leaf aqueous extract of Ocimum suave Wild (Lamiaceae) in rats. J Ethnopharmacol, 115, 232–237.
- Wang J, Huang Y. (2007). Pharmacogenomics of sex difference in chemotherapeutic toxicity. Curr Drug Discov Technol, 4, 59–68.
- Yemitan OK, Adeyemi OO. (2004). Toxicity studies of the aqueous root extract of Lecaniodiscus cupanioides. Nigerian J Health Biomed Sci, 3, 20–23.