Abstract
Context: The search for newer compounds against pathogenic species continues unabated due to drug resistance. Traditionally, Tagetes erecta Linn. (Compositae) has been used for the treatment of various parasitic and microbial diseases.
Objective: To evaluate the antioxidant activity of the ethanol extract of Tagetes erecta roots and its cytotoxicity against prostate and HeLa cancer cell lines followed by activity-guided isolation.
Materials and Methods: The antioxidant screening was carried out using diphenylpicrylhydrazyl (DPPH) radical scavenging assay with serial concentrations ranging from 2 to 100 µg/mL, and cytotoxicity was evaluated against prostate (PC-3) and HeLa cell lines using microculture tetrazolium test (MTT) assay with concentrations ranging from 500 to 1.89 µg/mL. Isolation of the ethanol extract was carried out using column chromatography whereby 21 isolates were obtained (T1-T21), and the most active isolate was subjected for characterization using ultraviolet (UV), infrared (IR), nuclear magnetic resonance (NMR), and mass spectroscopic techniques.
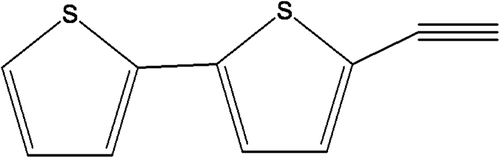
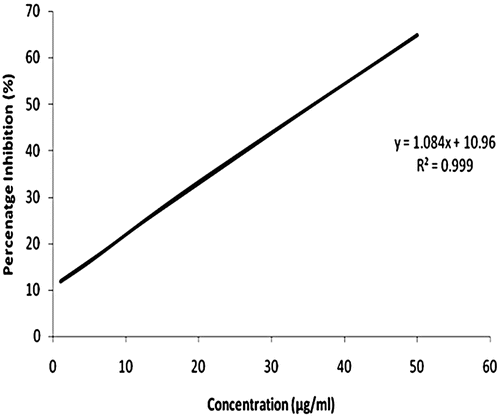
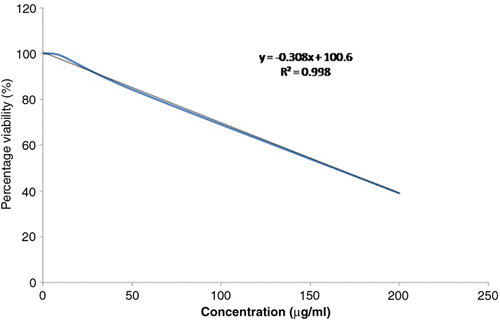
Results: The ethanol extract scavenged DPPH free radicals thereby exhibiting antioxidant activity with an IC50 of 35.9 µg/mL. In addition, the extract conferred noticeable cytotoxicity against the HeLa (LD50 of 164.28 µg/mL) and PC-3 cell lines (LD50 of 407.3 µg/mL). Among all the isolates, T3 showed antioxidant activity with IC50 of 11.56 µg/mL and cytotoxicity with LD50 of 12.5 µg/mL against HeLa and 30.25 µg/mL against PC-3 cell lines and was characterized as 2-ethynyl-5-(thiophen-2-yl) thiophene.
Discussion: The new thienyl compound (T3) exhibited profound antioxidant activity and cytotoxicity at relatively lower concentrations than the extract.
Conclusion: The observations provide support for the ethnobotanical use of the plant.
Introduction
Cultivating ornamental plants, once considered to be gardener’s activity, has now become a commercial venture throughout the world. The cultivation of such plants even as a hobby may yield a lucrative business if done with a little care. The plants for decoration, termed as ornamentals, constitute a major component of the floriculture industry, and although India is very rich in ornamental flora, yet there arise a need for further exploration of the untapped wild potential ornamental plants. Marigold is a potential ornamental plant grown commercially in different parts of the world. Two widely cultivated marigolds from the Compositae family, the African (Tagetes erecta Linn.) and the French (Tagetes patula Linn.) are native to Mexico and South America (CitationKaplan, 1960). In India, these were introduced by the Portuguese. The marigolds spread quickly because of the ease in cultivation, longer blooming period, and beautiful flowers with excellent shelf life. They are extensively used for making garlands, religious offerings, and exhibitions (CitationRaghava, 1998). The name Tagetes was given after Tages, a demigod, known for his beauty. There are about 33 species of the genus Tagetes (CitationRydberg, 1915). Five species have been introduced into the Indian gardens viz. Tagetes erecta Linn. (Aztec or African marigold), Tagetes minuta Linn. (Tagetes glandulifera Schrank), Tagetes patula Linn. (French marigold), Tagetes lucida Cav. (sweet-scented marigold), and Tagetes tenuifolia Cav. (striped marigold).
Thiophenyl moieties have been an integral part of the genus Tagetes, and T. erecta has been previously reported for several thiophenes such as 5-(but-1-ol-3-ynyl)-2,2-bithienyl (CitationTripathi et al., 1992), and the “hairy root” cultures of T. erecta have also shown the presence of thienyl moieties such as α-terthienyl (CitationFlores, 1992). The roots of T. erecta have been previously reported for their insecticidal and nematocidal activities (CitationRasoanaivo et al., 1992; CitationVasudevan et al., 1997). Previous studies from our laboratory evaluated the antiplasmodial and antimicrobial potential of the crude successive extracts viz. petroleum ether, chloroform, ethyl acetate, methanol, and aqueous extracts of the roots of T. erecta (CitationGupta & Vasudeva, 2010). In the present study, we undertook an evaluation of the root extract for antioxidant activity using diphenylpicrylhydrazyl (DPPH) radical scavenging assay and its cytotoxic potential against prostate and HeLa cancer cell lines. Henceforth, the ethanol extract was subjected for activity-guided isolation.
Materials and methods
Plant material and extraction
The roots (4 kg) of T. erecta (Compositae) were collected in April, 2005 from Hisar district, Haryana, India. The plant was authenticated by Dr. H. B. Singh, taxonomist at National Institute of Science Communication and Information Resources (NISCAIR), New Delhi, and a voucher specimen (no. GJU/HS-509) has been retained at the Department of Pharmacognosy, Faculty of Pharmaceutical Sciences, Guru Jambheshwar University of Science and Technology, Hisar, Haryana. The roots were washed with water, air-dried at room temperature (30–40°C), and then ground to pass through a sieve of 1 mm. The air-dried and coarsely powdered roots (750 g) were exhaustively extracted with 5 L ethanol (95%) in a Soxhlet apparatus for three times repeatedly, and the combined extracts were evaporated to dryness in a rotary evaporator.
Column chromatography
Isolation was carried out using column chromatography as per the reported procedure (CitationVasudeva et al., 2007). Briefly, the dark brown extract (60 g) was shaken with 20% ethanol and subsequently refluxed with ethyl acetate and methanol. The ethyl acetate fraction was further concentrated in a rotary evaporator to yield a 20 g fraction which was mixed with 30 g silica gel (100–200 mesh); the resulting mixture was chromatographed over prepacked column consisting of 400 g silica gel (100–200 mesh) in 1.3 L of petroleum ether. The column was subjected to successive elutions starting with 100% petroleum ether followed by varying ratios of petroleum ether and ethyl acetate (95:5, 90:10, 85:15, 80:20, 75:25, 70:30, 65:35, 60:40, 55:45, 50:50, 45:55, 40:60, 35:65, 30:70, 25:75, 20:80, 15:85, 10:90, 5:95), and finally 100% ethyl acetate and these were labeled T1 to T21. The fractions of column eluents collected were subjected to thin layer chromatography (TLC) to check uniformity and homogeneity. Chromatographically identical fractions of column eluents were combined, concentrated, and labeled as T1 to T21. The spots on TLC were either visualized under ultraviolet (UV) light or with spray reagent, ceric ammonium sulfate in sulphuric acid, followed by heating at 120°C for 15 min.
All the isolates were screened for antioxidant activity and cytotoxicity against cancer cell lines. Among all, the most active isolate was selected for characterization using various techniques, viz., UV, infrared (IR), nuclear magnetic resonance (NMR), and gas chromatography coupled with mass spectroscopy (GC-MS). The melting point was determined in open capillary tube and the UV spectrum was obtained on Simatzu spectrometer. IR spectrum (KBr cm−1) was recorded on a Perkin Elmer spectrophotometer, using KBr pellets. The 1H NMR and 13C NMR spectra were recorded on a Bruker Avance II 400 NMR spectrometer (chemical shift in δ ppm). The electrospray ionization (EI) positive mode mass spectra was obtained in Agilent 5975C GC-MS using Agilent 19091S-433 (30 m × 250 µm × 0.25 µm) column.
DPPH radical scavenging assay
In order to measure antioxidant activity, the DPPH free radical scavenging assay was carried out according to the procedure described by CitationBlois (1958). The ethanol extract and isolates at various concentrations (2–100 µg/mL) were added to a 0.1 mM solution of DPPH in methanol, and the reaction mixture was shaken vigorously. The amount of DPPH remaining was calculated by the decrease in absorbance at 520 nm after 30 min, and the radical scavenging activity was obtained from the following equation:
The antioxidant activity of the ethanol extract and isolates was expressed as IC50. The IC50 value is defined as the concentration (µg/mL) of the extract required for inhibiting the formation of DPPH radical by 50%. Sodium ascorbate was used as a positive control.
MTT assay
Prostate (PC-t3) and HeLa cancer cell lines were purchased from the National Centre for Cell Science (NCCS), Pune, India. The (microculture tetrazolium test) MTT assay was performed by a modification of the method described by CitationMosmann (1983). Briefly for the assay, both the cancer cells were plated in 96-well falcon plates at a density of 104 cells/well in 100 µL DMEM (Dubelco modified eagle medium). Before treatment, the cells were allowed to adhere to the plate for 24 h. Afterward, the medium was replaced by the same volume of test medium containing the specific substance. Each concentration ranging from 500 to 1.89 µg/mL for the ethanol extract and isolates, including the control, which contained vehicle alone (1% dimethyl sulfoxide [DMSO]), was applied to 3 wells. The cells were then incubated for 24 h at 37°C. The tetrazolium salt is cleaved to purple formazan crystals by metabolically active cells. MTT labeling reagent (10 µL) was added to each well after 24 h of treatment, respectively, and the plates were incubated for 4 h at 37°C. Solubilization solution (100 µL) was then added and incubated overnight at 37°C. Spectrophotometric absorbance of each well was measured at 570 nm using a Benchmark Microplate Reader (BioRad Laboratories, Hercules, California, Unites States). The percentage cell viability was calculated for each tested concentration, and the result expressed as LD50. The assay was performed in triplicates and repeated in three independent experiments, and paclitaxel (Admac, India) was used as a positive control.
Results
Characterization of active compound
Among all the isolates, T3 conferred profound antioxidant activity and noticeable cytotoxicity against both the cancer cell lines and was therefore selected for characterization in order to get a profound idea of the putative active principle responsible for the observed effect. T3 was subjected for characterization using various spectroscopical techniques, viz., UV, IR, NMR, and mass and was identified as compound 1 (). The compound was further purified by crystallization from methanol. The compound was isolated as a light brown crystalline adduct, and the following parameters were obtained: melting point. 213°C; UV (CHCl3) λmax 241, 305 nm. IR (KBr) γmax 2363, 2189 cm−1 spectrum indicated the presence of acetylenic group. 1H NMR (CDCl3, 300 MHz) spectrum of the compound 1 demonstrated the presence of five well-resolved signals in the aromatic region which were assigned to thiophene moieties: 7.15 (1H, dd, J = 5.08, 1.08 Hz, H-5′); 7.12 (1H, dd, J = 2.7, 1.08 Hz, H-3′); 7.02 (1H, d, J = 3.8 Hz, H-4′); 6.98 (1H, d, J = 3.6 Hz, H-3′); 6.96 (m, 1H, J = 3.4 Hz, H-4′). The GC-MS scanning was carried out using EI positive mode with m/z 191 showing the molecular ion peak. On the basis of these evidences, the probable structure of the isolated compound () has been elucidated as 2-ethynyl-5-(thiophen-2-yl) thiophene.
Antioxidant activity
The ethanol extract of the roots of T. erecta showed antioxidant activity () at relatively higher concentration in comparison with the standard (sodium ascorbate). The IC50 for the ethanol extract was found to be 35.9 µg/mL whereas the IC50 for sodium ascorbate was found to be 5.32 µg/mL. Among all the isolates, the new thienyl constitutent (T3) exhibited DPPH free radical scavenging activity with IC50 value of 11.56 µg/mL ().
Table 1. IC50 against DPPH free radical depicting antioxidant activity and LD50 against HeLa and prostate (PC-3) cancer cell lines obtained for the ethanol extract and isolates of the roots of Tagetes erecta Linn.
MTT assay
The ethanol extract and isolates were solublized in 1% DMSO and tested with varying concentrations ranging from 500 to 1.89 µg/mL, for evaluation of cytotoxicity against the prostate and HeLa cancer cell lines. The ethanol extract showed noticeable cytotoxicity against both the cells lines with LD50 of 164.28 µg/mL against HeLa cells () and 407.3 µg/mL against PC-3 cancer cell lines. Among all the isolates, compound 1 (T3) conferred cytotoxicity against the prostate and HeLa cancer cell lines with relatively lower LD50 concentrations of 12.5 µg/mL against HeLa and 30.25 µg/mL against PC-3 cell lines ().
Discussion
The last two decades have witnessed a clear global inclination for drugs and health foods from natural resources. With the realization of health hazards and toxicity associated with indiscriminate use of synthetic drugs and remedies, introduction of postmarketing surveillance and active adverse drug reaction monitoring, more than ever a need has been felt for an integrated system of medicine which would minister to the human body as a whole, safety being a major concern. The solution may perhaps be hidden into the traditional system of medicines such as “Ayurveda” or the “Science of life” (CitationKalia, 2005).
With time, there has been an ever increasing anticipation by researchers worldwide with their growing interest in antioxidants, particularly in those intended to prevent the presumed deleterious effects of free radicals in the human body (CitationMolyneux, 2004). The univalent reduction of molecular oxygen results in reactive oxygen species (ROS). In addition to its fatal effects on human body such as tissue degeneration, ROS may also join in generation as well as in malign transformation of cancer cells. If newer compounds from natural sources can enhance the level of antioxidation within the body and clear the ROS in cancer cells, they may inhibit the cell’s growth and may thereby play a vital role to treat cancer in combination with the anticancer drugs (CitationLee et al., 2003; CitationLeng et al., 2005). In our research, the ethanol extract of T. erecta was found to scavenge the DPPH free radical indicating that this property may contribute equally or enhance its cytotoxic potential. The observed antioxidant activity of the extract of T. erecta may be due to the presence of tannins and flavonoids found in preliminary phytochemical investigation (CitationGupta et al., 2009).
The ethanol extract of the flowers of T. erecta was recently evaluated for antioxidant activity using the DPPH radical scavenging assay, and it was found that the IC50 for the ethanol extract (3.4 µg/mL) was half than that of the IC50 reported for the standard ascorbic acid (7 µg/mL) indicating the tremendous antioxidant potential of the flowers which was presumably attributed to the presence of carotenoids in the flowers, such as lutein and zeaxanthin (CitationChivde et al., 2011). In contrast, the essential oil of the flowers of T. erecta also exhibited antioxidant activity but relatively lesser in comparison with the flower extract, whereby it was found to scavenge only 64.3% of the DPPH free radicals (CitationGutierrez et al., 2006). The antioxidant potential of the aerial parts of T. erecta find application in a wide range of therapeutics, for example, the hydroalcoholic extract of the leaves of T. erecta was recently reported for its wound-healing activity which may be duly attributed to the free radical scavenging action of the phytoconstituents such as flavonoids present in it (CitationKiranmai et al., 2011). The present study explored the untouched domain of the plant, that is, the roots of T. erecta and evaluated its antioxidant and cytotoxic potential for the first time. The results indicated the antioxidant activity of the ethanol extract of the roots of T. erecta whereby it showed noticeable free radical scavenging effect but at a IC50 that was seven fold higher than the standard drug (sodium ascorbate) thereby arising a need for the exploration of putative active principles present in the extract. The antioxidant activity of the new thienyl derivative was observed at relatively lesser concentration in comparison with the ethanol extract, and this observation strongly marks to the antioxidant potential of the thiophenyl moieties present in the roots of T. erecta that remained unexplored till date.
The MTT assay, which evaluates the mitochondrial enzyme succinate dehydrogenase activity, is a preliminary assay technique that have been used since time for the evaluation of cytotoxicity of several herbs against a wide range of tumor cell lines, for example, the butanol extract of Garcinia mangostana Linn. (Clusiaceae) at LD50 ≥85 µg showed noticeable cytotoxicity against HEp2 cells (CitationMaleeka Begum & Kavitha, 2011), the ethanol root extracts of Polyathia laui Merr., and Polyathia rumphii Merr. (Annonaceae) inhibited proliferation of four human cancer cell lines, viz., lung cancer, hepatocellular caricinoma, gastric cancer, and myelogenous leukemia cell lines with 50% inhibitory concentrations ranging between 6 and 100 µg/mL, demonstrating potential antitumor activities tested using MTT assay (CitationYuan et al., 2011). The ethanol and aqueous extracts of the aerial parts of Tagetes lucida Cav. exhibited cytotoxicity against human breast cancer T47D and cervix cancer HeLa cell lines with ethanol extract showing profound activity with LD50 of 1.82 µg/mL and ≥50 µg/mL against T47D and HeLa cancer cell lines whereas the aqueous extract was effective with LD50 of 13.2 and 18.94 µg/mL against HeLa and T47D cell lines (CitationVega-Avila et al., 2009). In the present study, the ethanol extract of the roots of T. erecta exhibited noticeable cytotoxicity against both the tested cells lines with LD50 of 164.28 µg/mL against HeLa and 407.3 µg/mL against PC-3 cancer cell lines. Among all the isolates, the new thienyl compound, T3, showed profound cytotoxicity against the prostate and HeLa cancer cell lines at concentrations that were 14-fold lower than the ethanol extract but 3-fold higher than the standard drug, paclitaxel. All the other isolates were found to exhibit LD50 in the concentration range of 25–70 µg/mL for HeLa and 40–90 µg/mL for PC-3 cancer cell lines, which were relatively higher than T3. These observations further signify the potential of T3 for its development as a compound of therapeutic intervention for the treatment of various types of cancers.
Conclusions
The ethanol extract conferred noticeable antioxidant activity and cytotoxicity against the prostate and HeLa cancer cell lines. Comparatively, the newly isolated thienyl constituent (compound 1) exhibited antioxidant efficacy and cytotoxicity at much lower concentrations than the extract, supporting toward its development as a potential therapeutic agent for the treatment of cancer with further insight toward the possible mechanisms of actions. The in vivo efficacy studies and further optimization of the active constituent (compound 1) are in progress. This is the first report on the antioxidant and cytotoxic potential of the roots of T. erecta, and the results obtained strongly provide support for the ethnobotanical use of the plant.
Acknowledgments
The authors are thankful to Central Instrumentation Facility, Punjab University, Chandigarh, India for providing the IR and NMR spectroscopical data and Dr. Uma Pathak and Subhankar Bhattacharya, Defence Research and Development Establishment, Gwalior for GC-MS analysis.
Declaration of interest
There are no conflicts of interest.
References
- Blois MS. (1958). Antioxidant determinations by the use of a stable free radical. Nature, 181, 1199–1200.
- Chivde BV, Biradar KV, Shiramana RS, Maoj KV. (2011). In vitro antioxidant activity studies on the flowers of Tagetes erecta L. (Compositae). Int J Pharm Biol Sci, 2, 223–229.
- Flores HE. (1992). Plant roots as chemical factories. Chem Ind, 10, 374–377.
- Gupta P, Vasudeva N. (2010). In vitro antiplasmodial and antimicrobial potential of Tagetes erecta roots. Pharm Biol, 48, 1218–1223.
- Gupta P, Vasudeva N, Sharma SK. (2009). Pharmacognostical study and preliminary phytochemical screening of the roots of Tagetes erecta Linn. Hamdard Med, 52, 153–160.
- Gutierrez RMP, Luna HH, Garrido SH. (2006). Antioxidant activity of Tagetes erecta essential oil. J Chil Chem Soc, 51, 883–886.
- Kalia AN. (2005). Textbook of Industrial Pharmacognosy, first edition, New Delhi, India: CBS publishers and distributor.
- Kaplan L. (1960). Historical and ethnobiological aspects of domestication in Tagetes. Econ Bot, 14, 200–202.
- Kiranmai M, Kazim SM, Ibrahim M. (2011). Combined wound healing activity of Gymnema sylvestre & Tagetes erecta Linn. Int J Pharmaceut Appl, 2, 135–140.
- Lee SE, Shin HT, Hwang HJ, Kim JH. (2003). Antioxidant activity of extracts from Alpinia katsumadai seed. Phytother Res, 17, 1041–1047.
- Leng B, Liu XD, Chen QX. (2005). Inhibitory effects of anticancer peptide from Mercenaria on the BGC-823 cells and several enzymes. FEBS Lett, 579, 1187–1190.
- Maleeka Begum SF, Kavitha.(2011). Antimicrobial, antioxidant and antitumor activity of the Garcinea mangostana. Int J Current Res, 2, 27–31.
- Molyneux P. (2004). The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol, 26, 211–219.
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods, 65, 55–63.
- Raghava SPS. (1998). Pusa Narangi Gainda and Pusa Basanti Gainda: New marigolds. Indian Horticulture, 43, 31.
- Rasoanaivo P, Petitjean A, Ratsimamanga-Urverg S, Rakoto-Ratsimamanga A. (1992). Medicinal plants used to treat malaria in Madagascar. J Ethnopharmacol, 37, 117–127.
- Rydberg, PA. (1915). Tagetes. North Am Flora, 34, 148–159.
- Tripathi AK, Khan SA, Vaishnava MM, Gupta KR. (1992). Compounds of taxonomic significance in Tagetes. Fitoteropia, 63, 85.
- Vasudeva N, Gupta P, Sharma SK. (2007). A new bithienyl constituent from Tagetes erecta Linn roots. Indian J Heterocy Ch, 16, 303–304.
- Vasudevan P, Kashyap S, Sharma S. (1997). Tagetes: A multipurpose plant. Biosci Technol, 62, 29–35.
- Vega-Avila E, Espejo-Serna A, Alarcón-Aguilar F, Velasco-Lezama R. (2009). Cytotoxic activity of four Mexican medicinal plants. Proc West Pharmacol Soc, 52, 78–82.
- Yuan Y, Huang G, Wang T, Chen G. (2011). In vitro screening of five Hainan plants of Polyathia (Annonaceae) against human cancer cell lines with MTT assay. J Med Plant Res, 5, 837–841.