Abstract
Context: Quercetin, a dietary-derived flavonoid, is ubiquitous in fruits and vegetables and plays important roles in human health by virtue of its antioxidant activity.
Objective: This study was conducted to investigate the possible modulatory effect of quercetin against hepatic lipemic-oxidative injury in rats fed with a high cholesterol diet (HCD), and to highlight the underlying mechanisms of such effect.
Materials and methods: Different groups of male Sprague–Dawley rats were used; one group was treated by gavage with HCD cocktail (1 mL/100 g) whereas another group was orally administered HCD-enriched with quercetin (15 mg/kg). Corresponding control animals were also used.
Results: Quercetin administration significantly decreased liver triglycerides (24%), liver total cholesterol (TC) (22%), serum TC (20%), serum low-density lipoprotein cholesterol (31%), and duplicated serum high-density lipoprotein cholesterol (HDL-C). This study also revealed that quercetin administration significantly reduced the activity of serum alanine aminotransferase (41%), aspartate aminotransferase (51%), and γ-glutamyl transpeptidase (G-GT) (35%). Significant inhibition of thiobarbituric acid-reacting substances (40%), together with a valuable enhancement of reduced glutathione (GSH) content (53%) in the liver homogenates, was observed. In addition, quercetin-treated hypercholesterolemic animals exhibited a reasonable improvement of hepatic antioxidant enzymes. Moreover, serum and liver content of nitric oxide (NO) were markedly decreased in this model (26 and 25%, respectively), and were almost normalized following quercetin administration.
Discussion and conclusion: These data revealed that quercetin has the ability to ameliorate HCD-induced lipemic-oxidative injury in rat liver possibly through its antioxidant potential and/or increased NO bioavailability.
Introduction
The diet-induced hypercholesterolemia has often been used to modify the normal lipid profile in order to study hypercholesterolemia-related injuries in different body organs. Liver plays a central role in the balance and metabolism of cholesterol, which is derived from endogenous biosynthesis, chylomicron remnants, and lipoprotein fractions, and so liver is the primary organ to be affected from ingested excessive cholesterol and subsequent complications (CitationKumar et al., 2006; CitationAmin & Abd El-Twab, 2009).
Hypercholesterolemia is usually associated with an increased risk for the development and progression of coronary artery disease and consequently of ischemic heart disease (CitationPrasad & Kalra, 1993). Hypercholesterolemia is known to impair endothelial functions that may include reduction of endothelial nitric oxide (NO) production (CitationZou et al., 2003; CitationKim et al., 2011). It has been reported that hypercholesterolemia increased generation of oxygen free radicals, which contributed to the deleterious effects on the organ tissues, including blood vessels, liver, and kidney (CitationScheuer et al., 2000; CitationZou et al., 2003).
Flavonoids are plant-based phenolic compounds, and are reported to exhibit a wide variety of biological and pharmacological activities. Quercetin, the most abundant dietary member of this family, is present in substantial amounts in fruits and vegetables and plays an important role in human health by virtue of its antioxidant activity and its ability to modify NO levels (CitationLopez-Revuelta et al., 2006; CitationMorales et al., 2006; CitationLee et al., 2011).
In the present study, we have conducted a model of experimental hypercholesterolemia, in order to investigate the ability of quercetin to restore the deteriorated serum lipid profile, liver function tests, and lipid contents. The antioxidant effect of quercetin on liver tissues was characterized by estimating lipid peroxides, glutathione content, and status of the endogenous antioxidant enzymes. Furthermore, serum and liver tissue NO were investigated. We, thus, aimed to evaluate the proposed protective role of quercetin against high cholesterol diet (HCD)-induced liver damage in a rat model and to highlight the underlying mechanisms of such protection.
Materials and methods
Chemicals
Quercetin (3,5,7,3,4-penthydroxy flavone) was provided as yellow powder from Sigma-Aldrich Corp (St. Louis, MO). It was dissolved in a mixture of dimethyl sulfoxide and distilled water (1:9 v/v). The HCD cocktail contained 100 g cholesterol, 30 g propylthiouracil, and 100 g cholic acid in 1 L of peanut oil (CitationFillios et al., 1956; CitationArafa, 2005). All other chemicals used were of the highest available commercial grade.
Animals
Thirty male Sprague–Dawely rats, weighing 160–180 g, were obtained from the animal facility of the Faculty of Pharmacy at Al-Azhar University, Cairo, Egypt. The animals were fed a standard chow (El-Nasr Company, Abou-Zaabal, Cairo, Egypt) with free access to water, and kept in wire-floored cages under standard laboratory conditions at room temperature (25 ± 2°C), and a 12 h light/dark cycle. The animal experiments were conducted according to the guidelines for the care and use of laboratory animals stated by College of Pharmacy, Al-Azhar University, Cairo, Egypt.
Experimental design
Animals were divided equally into three groups, 10 animals each. The first group of animals received peanut oil orally for 7 consecutive days prior to feeding with high cholesterol diet cocktail (1 mL/100 g), for another 14 successive days (CitationFillios et al., 1956; CitationArafa, 2005). The second group of rats was administered quercetin alone for 7 consecutive days in a dose of 15 mg/kg (CitationVessal et al., 2003; CitationCoskun et al., 2005), then continued for the following 14 days concomitantly with the high cholesterol diet cocktail. Animals of the third group served as controls and received peanut oil orally as a vehicle (1 mL/100 g), adapting the same route of administration and schedules of treatments as in the first and second groups.
After the end of the specified period of the experiment, fasting blood samples were obtained from abdominal aorta under light ether anesthesia and used for determination of lipid profile, alanine aminotransferase (ALT), aspartate aminotransferase (AST), G-GT, and NO. Finally, the animals were sacrificed and the liver of each animal was quickly dissected and rinsed in ice-cooled physiological saline, then plotted between two filter papers and transferred into pre-weighed vials to determine the wet weight. Homogenization of each liver was carried out in a suitable volume of ice-cold 0.15 M potassium chloride using a Potter-Elvehjem homogenizer. Different aliquots of the liver homogenate were used for the determination of triglycerides (TG), total cholesterol (TC), lipid peroxidation, as thiobarbituric acid-reacting substances (TBARS), reduced glutathione (GSH), and enzyme activities of catalase and superoxide dismutase (SOD). The level of NO was also determined in liver tissue homogenates.
Biochemical analysis
The method of CitationFossati and Prencipe (1982) was used to determine TG. TC was assayed according to the method of CitationRoeschlau et al. (1974). HDL-C was determined following the method of CitationWarnick et al. (1982). Low-density lipoprotein cholesterol (LDL-C) was then calculated according to the equation of CitationFriedewald et al. (1972): LDL-C = TC–HDL-C–TG/5. The transaminase activity (AST and ALT) was determined according to the method of CitationReitman and Frankel (1957). The activity of G-GT was determined following the method described by CitationTeitz (1987). TG and TC of liver homogenate were determined following the same methods as for serum determination after lipid extraction. To extract lipids, aliquots of the liver homogenate were extracted with chloroform-methanol followed by separation and evaporation of the chloroform-methanol layer (CitationHalim et al., 1997). Protein content of the collected serum and tissue samples was measured by the method of CitationLowry et al. (1951). Liver homogenate was used for determination of lipid peroxides expressed as TBARS (CitationMihara & Uchiyama, 1978), and GSH (CitationEllman, 1959). The enzyme activity of SOD (CitationMcCord & Fridovich, 1969) and catalase (CitationAebi, 1974) was also carried out in the liver homogenates. Serum and tissue concentrations of NO were measured as its stable metabolites, nitrate, and nitrite. Nitrate was first reduced by nitrate reductase to nitrite and then nitrite was determined spectrophotometrically by the Griess reaction (CitationGreen et al., 1982).
Statistical analysis
Data are expressed as mean ± standard deviation (SD) for the groups. Data analysis was evaluated by one-way ANOVA (analysis of variance) followed by Tukey–Kramer test for multiple comparisons. A 0.05 level of probability was used as the criterion for significance.
Results
Hypercholesterolemia-induced liver injury
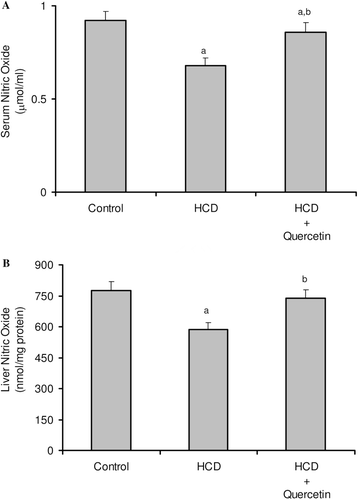
The consequences of feeding male rats with HCD resulted in significant amplification of both liver TG and TC (). HCD induced an observable increase in serum TC whereas its effect on serum TG was not significant when compared to the control rats (). Serum HDL-C was significantly reduced, accordingly most of serum TC increase was carried on the atherogenic lipoprotein particle, as LDL-C (). HCD-fed animals also revealed an elevation of serum ALT, AST, and G-GT activities when compared to the control group (). Liver tissues of HCD-fed rats showed significant increase of TBARS levels (261%) () and significant decrease of reduced glutathione content (42%) () compared to the liver of vehicle-receiving animals. Besides, hypercholesterolemic diet resulted in a significant inhibition of the activities of antioxidant enzymes, catalase, and superoxide dismutase (SOD), in liver homogenate as compared to the control group (). Moreover, HCD feeding significantly decreased both serum level of nitrite (26%) (), and nitrite concentration of liver homogenate (25%) () when compared to the control levels.
Table 1. Effect of quercetin on liver triglycerides and cholesterol in rats fed with HCD.
Table 2. Effect of quercetin on the activities of liver antioxidant enzymes in rats fed with HCD.
Figure 1. Effect of quercetin on serum lipid profile in rats fed with HCD. Data are presented as mean ± SD, n = 10. Multiple comparisons were achieved using one-way ANOVA followed by Tukey–Kramer as post-ANOVA test. a,b: indicate significant change from control and HCD groups, respectively, at p < 0.05.
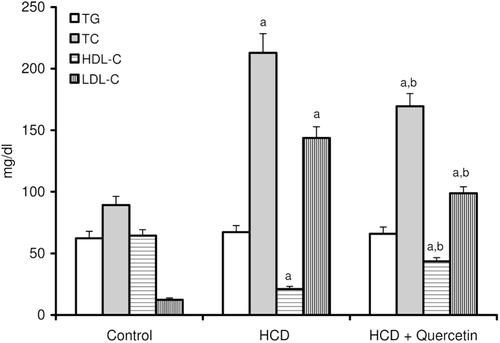
Figure 2. Effect of quercetin on the activities of serum ALT, AST and G-GT in rats fed with HCD. Data are presented as mean ± SD, n = 10. Multiple comparisons were achieved using one way ANOVA followed by Tukey–Kramer as post-ANOVA test. a,b: indicate significant change from control and HCD groups, respectively, at p < 0.05.
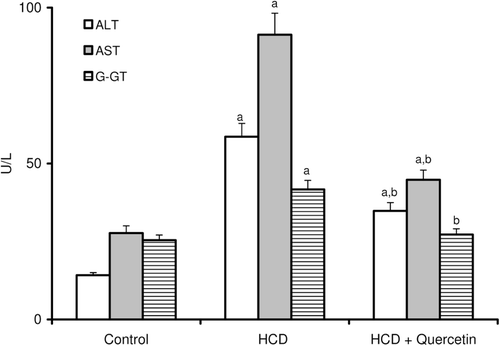
Figure 3. Effect of quercetin on the level of lipid peroxides (TBARS) in the liver of rats fed with HCD. Data are presented as mean ± SD, n = 10. Multiple comparisons were achieved using one way ANOVA followed by Tukey–Kramer as post-ANOVA test. a,b: indicate significant change from control and HCD groups respectively, at p < 0.05.
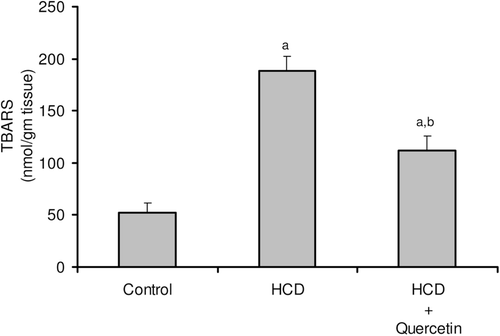
Figure 4. Effect of quercetin on reduced glutathione (GSH) content in the liver of rats fed with HCD. Data are presented as mean ± SD, n = 10. Multiple comparisons were achieved using one-way ANOVA followed by Tukey–Kramer as post-ANOVA test. a,b: indicate significant change from control and HCD groups, respectively, at p < 0.05.
Figure 5. Effect of quercetin on the level of nitric oxide in serum (A) and liver (B) in rats fed with HCD. Data are presented as mean ± SD, n = 10. Multiple comparisons were achieved using one-way ANOVA followed by Tukey–Kramer as post-ANOVA test. a,b: indicate significant change from control and HCD groups, respectively, at p < 0.05.
Effect of quercetin on hypercholesterolemia-induced liver injury
Concomitant administration of quercetin with the hypercholesterolemic diet significantly decreased the high liver TG and TC by about 24 and 22%, respectively (). Likewise, the high-serum TC and LDL-C were obviously decreased by about 20 and 31%, respectively, when compared to rats fed HCD alone (). Furthermore, serum HDL-C was almost duplicated with regard to animals fed HCD alone (). Significant reduction was obtained in the activity of serum ALT (41%), AST (51%), and G-GT (35%) in animals fed quercetin-admixed HCD when compared to animals fed HCD alone (). Quercetin treatment resulted in a realistic reversal of the decrease in liver SOD and catalase activity (). In addition, quercetin administration significantly reduced the level of liver TBARS while obviously increased liver total GSH content ( and ). Ultimately, HCD-induced decrease of serum level of nitrite and its liver tissue content was almost normalized following quercetin treatment ( and ).
Discussion
Diet-induced hypercholesterolemia is the principal contributor to the pathogenesis of myocardial and cerebral infarction. Elevated plasma concentration of cholesterol, especially of LDL, is recognized leading to the development of atherosclerosis. Hypercholesterolemia resulted from feeding HCD to the rats is brought about by the abnormal lipoprotein metabolism, and is always useful for the assessment of the protective effect of many presumed hypolipidemic agents (CitationMahley & Holcombe, 1977; CitationOhta et al., 2003).
Results of the present study showed that HCD induced a marked increase in the serum level of TC, which is consistent with previous findings of CitationBeynen et al. (1987) and CitationMonte and Jimenez (1993), whereas the serum level of TG was not significantly elevated, which is in contrast to CitationFungwe et al. (1992), who reported that feeding rats with high concentration of cholesterol-containing diet significantly increased plasma TG. However, the findings of CitationKamada et al. (2005) and CitationLee et al. (2011), who did not detect any change in serum TG in neither normal nor diabetic rats fed with high fat diet, coped with the current results. In the same time, the findings of CitationFungwe et al. (1992) was similar to our results in the context of increasing serum HDL-C and decreasing LDL-C ().
The quercetin-enriched diet exhibited a notable hypolipidemic effect as evidenced by its modulating impact on the serum lipid profile of rats. Quercetin lowered serum TC and serum LDL-C whereas it duplicates the level of serum HDL-C, which were significantly malformed, following the hypercholesterolemic diet. No significant effect was observed for serum TG concentrations following administration of quercetin-enriched diet (). The lipid lowering effects of quercetin have reported controversial data. Most studies have shown that quercetin has no beneficial effect on the plasma lipid profile (CitationYamamoto & Oue, 2006: CitationPerez-Vizcaino & Duarte, 2010; CitationEgert et al., 2010; CitationZhao et al., 2011). In contrast, other studies have reported that quercetin-rich supplementation reduces serum concentrations of TC and increases serum concentrations of HDL-cholesterol (CitationIgarashi & Ohmuma, 1995; CitationKamada et al., 2005; CitationGnoni et al., 2009; CitationLee et al., 2011), which cope with the results of the present study. Several explanations were suggested of how quercetin is able to influence serum lipid profile. CitationIgarashi and Ohmuma (1995) stated that quercetin decreases serum and liver TC level in rats through increasing its fecal excretion. It was also reported that quercetin reduces de novo synthesis of fatty acids and consequently cholesterol biosynthesis and lipoproteins formation (CitationGnoni et al., 2009).
Liver is one of the most common organs affected by hyperlipidemia, as it plays a central role in the synthesis and metabolism of lipids. Hyperlipidemia led to a rise in the generation of reactive oxygen species (ROS), leading to an increased oxidant stress. It has been reported that oxidants and oxidative modifications do play a major role in permanent tissue damage (CitationScheuer et al., 2000; CitationKajikawa et al., 2011). The activities of serum AST, ALT, and G-GT have been increased in animals maintained on HCD compared to control animals (), which may be attributed to hyperlipidemia that resulted in liver tissue injury (CitationAmin & Abd El-Twab, 2009). This study also revealed that HCD caused a marked increase in the liver TG and cholesterol contents (). The marked deposition of TG in the rat liver was explained by increasing the rate of hepatic lipogenesis, concomitantly with the ability of ROS to block its secretion into the plasma (CitationFerreira Ede et al., 2011). It has also been suggested that high contents of liver cholesterol is due to disturbed catabolism of cholesterol into bile acids (CitationHalim et al., 1997). Results of the present study revealed a significant increase in TBARS generation (), accompanied by a significant decrease in the level of GSH (), SOD, and catalase enzyme activities (), which indicate the ability of HCD to modulate liver oxidative status, and support the notion suggesting that hyperlipidemia is able to modulate liver functions through increasing oxidative stress (CitationKumar et al., 2006; CitationYang et al., 2008; CitationNepal et al., 2011). Administration of quercetin significantly alleviates HCD-induced oxidative damage. Quercetin is a strong oxygen radical scavenger and also a good metal chelator. The antioxidant and free radical scavenging activities of quercetin have been primarily attributed to its flavonoid fraction. Flavonoids prevent the oxidative injury and cell death through scavenging of oxygen radicals protecting against lipid peroxidation (CitationCoskun et al., 2005; CitationLopez-Revuelta et al., 2006; CitationSithisarn et al., 2006).
Moreover, the present study demonstrated that serum and hepatic NO concentration decreased significantly in HCD-fed rats ( and ), which have been interpreted that lower concentration of NO plays an important role in the pathogenesis of HCD-induced hepatic injury. Similar results were reported by CitationHu et al. (2008), CitationKorou et al. (2010), and CitationEccleston et al. (2011). The production of oxygen radicals may cause a progressive NO decline by increasing its degradation that will lead to deficient endothelium-dependent responses (CitationBarua et al., 2003; CitationAmin & Abd El-Twab, 2009). Besides, hypercholesterolemia is known to be associated with an impaired endothelial NO production and consequent alteration in endothelial nitric oxide synthase (eNOS) abundance and activity (CitationFeron et al., 1999). Our study revealed that quercetin significantly increased NO levels in both serum and liver tissues of HCD-fed rats. Quercetin administration was proved to induce the eNOS expression, increasing the NO level that is important to maintain hepatocyte proliferation (CitationVicente-Sánchez et al., 2008; CitationInukai et al., 2010). Furthermore, CitationRomero et al. (2010) observed that quercetin enhanced the formation of NO through inhibition of eNOS phosphorylation.
In conclusion, results of the present study revealed that quercetin had obviously a hypocholesterolemic effect and indicate that administration of quercetin exerts a hepatoprotective effect against lipemic-oxidative injury induced by HCD in rats, possibly through prevention of oxidative damage to liver through its direct antioxidant effect. In addition, quercetin restored both serum and liver levels of NO in HCD-fed rats that could be through participation in the regulation of NO production. Additional study on human subjects is needed to shed some light on the possibility to make use of quercetin as a part of hepatoprotective strategies against hyperlipidemia associated with bad nutritional habits.
Declaration of interest
The authors report no conflicts of interest.
References
- Aebi H. (1974). Catalase. In: Bergmeyer, HU (Ed.). Methods of Enzymatic Analysis. New York: Academic Press. pp 674–684.
- Amin KA, Abd El-Twab TM. (2009). Oxidative markers, nitric oxide and homocysteine alteration in hypercholesterolimic rats: Role of atorvastatine and cinnamon. Int J Clin Exp Med, 2, 254–265.
- Arafa HM. (2005). Curcumin attenuates diet-induced hypercholesterolemia in rats. Med Sci Monit, 11, BR228–BR234.
- Barua RS, Ambrose JA, Srivastava S, DeVoe MC, Eales-Reynolds LJ. (2003). Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: An in vitro demonstration in human coronary artery endothelial cells. Circulation, 107, 2342–2347.
- Beynen AC, Lemmens AG, Katan MB, De Bruijne JJ, Van Zutphen LF. (1987). Cholesterol metabolism and esterases in four strains of rats with differential cholesterolemic responses to a high-cholesterol, high-cholate diet. Comp Biochem Physiol, B, 87, 41–48.
- Coskun O, Kanter M, Korkmaz A, Oter S. (2005). Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res, 51, 117–123.
- Eccleston HB, Andringa KK, Betancourt AM, King AL, Mantena SK, Swain TM, Tinsley HN, Nolte RN, Nagy TR, Abrams GA, Bailey SM. (2011). Chronic exposure to a high-fat diet induces hepatic steatosis, impairs nitric oxide bioavailability, and modifies the mitochondrial proteome in mice. Antioxid Redox Signal, 15, 447–459.
- Egert S, Boesch-Saadatmandi C, Wolffram S, Rimbach G, Müller MJ. (2010). Serum lipid and blood pressure responses to quercetin vary in overweight patients by apolipoprotein E genotype. J Nutr, 140, 278–284.
- Ellman GL. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys, 82, 70–77.
- Ferreira Ede S, Silva MA, Demonte A, Neves VA. (2011). Soy β-conglycinin (7S globulin) reduces plasma and liver cholesterol in rats fed hypercholesterolemic diet. J Med Food, 14, 94–100.
- Feron O, Dessy C, Moniotte S, Desager JP, Balligand JL. (1999). Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J Clin Invest, 103, 897–905.
- Fillios LC, Andrus SB, Mann GV, Stare FJ. (1956). Experimental production of gross atherosclerosis in the rat. J Exper Med 104, 539–552.
- Fossati P, Prencipe L. (1982). Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem, 28, 2077–2080.
- Friedewald WT, Levy RI, Fredrickson DS. (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 18, 499–502.
- Fungwe TV, Cagen L, Wilcox HG, Heimberg M. (1992). Regulation of hepatic secretion of very low density lipoprotein by dietary cholesterol. J Lipid Res, 33, 179–191.
- Gnoni GV, Paglialonga G, Siculella L. (2009). Quercetin inhibits fatty acid and triacylglycerol synthesis in rat-liver cells. Eur J Clin Invest, 39, 761–768.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. (1982). Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem, 126, 131–138.
- Halim AB, el-Ahmady O, Hassab-Allah S, Abdel-Galil F, Hafez Y, Darwish A. (1997). Biochemical effect of antioxidants on lipids and liver function in experimentally-induced liver damage. Ann Clin Biochem, 34 (Pt 6), 656–663.
- Hu MY, Li YL, Jiang CH, Liu ZQ, Qu SL, Huang YM. (2008). Comparison of lycopene and fluvastatin effects on atherosclerosis induced by a high-fat diet in rabbits. Nutrition, 24, 1030–1038.
- Igarashi K, Ohmuma M. (1995). Effects of isorhamnetin, rhamnetin, and quercetin on the concentrations of cholesterol and lipoperoxide in the serum and liver and on the blood and liver antioxidative enzyme activities of rats. Biosci Biotechnol Biochem, 59, 595–601.
- Inukai N, Uchida M, Miyazaki Y, Suzuki T, Yoshikawa H, Tanaka K, Morita H, Takizawa T. (2010). Nitric oxide production and its contribution to hepatocyte proliferation in normal juvenile rats. J Vet Med Sci, 72, 861–867.
- Kajikawa S, Imada K, Takeuchi T, Shimizu Y, Kawashima A, Harada T, Mizuguchi K. (2011). Eicosapentaenoic acid attenuates progression of hepatic fibrosis with inhibition of reactive oxygen species production in rats fed methionine- and choline-deficient diet. Dig Dis Sci, 56, 1065–1074.
- Kamada C, da Silva EL, Ohnishi-Kameyama M, Moon JH, Terao J. (2005). Attenuation of lipid peroxidation and hyperlipidemia by quercetin glucoside in the aorta of high cholesterol-fed rabbit. Free Radic Res, 39, 185–194.
- Kim JH, Park SH, Bae SS, Hong KW, Kim YD, Park KP, Choi BT, Shin HK. (2011). Combinatorial effect of probucol and cilostazol in focal ischemic mice with hypercholesterolemia. J Pharmacol Exp Ther, 338, 451–457.
- Korou LM, Agrogiannis G, Pantopoulou A, Vlachos IS, Iliopoulos D, Karatzas T, Perrea DN. (2010). Comparative antilipidemic effect of N-acetylcysteine and sesame oil administration in diet-induced hypercholesterolemic mice. Lipids Health Dis, 9, 23.
- Kumar SA, Sudhahar V, Varalakshmi P. (2006). Protective role of eicosapentaenoate-lipoate (EPA-LA) derivative in combating oxidative hepatocellular injury in hypercholesterolemic atherogenesis. Atherosclerosis, 189, 115–122.
- Lee KH, Park E, Lee HJ, Kim MO, Cha YJ, Kim JM, Lee H, Shin MJ. (2011). Effects of daily quercetin-rich supplementation on cardiometabolic risks in male smokers. Nutr Res Pract, 5, 28–33.
- López-Revuelta A, Sánchez-Gallego JI, Hernández-Hernández A, Sánchez-Yagüe J, Llanillo M. (2006). Membrane cholesterol contents influence the protective effects of quercetin and rutin in erythrocytes damaged by oxidative stress. Chem Biol Interact, 161, 79–91.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193, 265–275.
- Mahley RW, Holcombe KS. (1977). Alterations of the plasma lipoproteins and apoproteins following cholesterol feeding in the rat. J Lipid Res, 18, 314–324.
- McCord JM, Fridovich I. (1969). Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem, 244, 6049–6055.
- Mihara M, Uchiyama M. (1978). Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem, 86, 271–278.
- Monte MJ, Jimenez R. (1993). Effects of a hypercholesterolaemia-inducing diet on biliary electrolytes and lipid secretion in the rat. Int J Exp Pathol, 74, 203–210.
- Morales AI, Vicente-Sánchez C, Jerkic M, Santiago JM, Sánchez-González PD, Pérez-Barriocanal F, López-Novoa JM. (2006). Effect of quercetin on metallothionein, nitric oxide synthases and cyclooxygenase-2 expression on experimental chronic cadmium nephrotoxicity in rats. Toxicol Appl Pharmacol, 210, 128–135.
- Nepal S, Malik S, Sharma AK, Bharti S, Kumar N, Siddiqui KM, Bhatia J, Kumari S, Arya DS. (2011). Abresham ameliorates dyslipidemia, hepatic steatosis and hypertension in high-fat diet fed rats by repressing oxidative stress, TNF-α and normalizing NO production. Exp Toxicol Pathol.
- Ohta Y, Kongo M, Kishikawa T. (2003). Melatonin exerts a therapeutic effect on cholestatic liver injury in rats with bile duct ligation. J Pineal Res, 34, 119–126.
- Perez-Vizcaino F, Duarte J. (2010). Flavonols and cardiovascular disease. Mol Aspects Med, 31, 478–494.
- Prasad K, Kalra J. (1993). Oxygen free radicals and hypercholesterolemic atherosclerosis: Effect of vitamin E. Am Heart J, 125, 958–973.
- Reitman S, Frankel S. (1957). A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol, 28, 56–63.
- Roeschlau P, Bernt E, Gruber W. (1974). Enzymatic determination of total cholesterol in serum. Z Klin Chem Klin Biochem, 12, 226.
- Romero M, Jiménez R, Hurtado B, Moreno JM, Rodríguez-Gómez I, López-Sepúlveda R, Zarzuelo A, Pérez-Vizcaino F, Tamargo J, Vargas F, Duarte J. (2010). Lack of beneficial metabolic effects of quercetin in adult spontaneously hypertensive rats. Eur J Pharmacol, 627, 242–250.
- Scheuer H, Gwinner W, Hohbach J, Gröne EF, Brandes RP, Malle E, Olbricht CJ, Walli AK, Gröne HJ. (2000). Oxidant stress in hyperlipidemia-induced renal damage. Am J Physiol Renal Physiol, 278, F63–F74.
- Sithisarn P, Supabphol R, Gritsanapan W. (2006). Comparison of free radical scavenging activity of Siamese neem tree (Azadirachta indica A. Juss var. siamensis Valeton) leaf extracts prepared by different methods of extraction. Med Princ Pract, 15, 219–222.
- Teitz NN. (1987). Assay for gamma glutamyl transpeptidase activity in serum. In: Fundamentals of Clinical Chemistry, third edition. Philadelphia: W.B. Saunders /Co. p. 391.
- Vessal M, Hemmati M, Vasei M. (2003). Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol, 135C, 357–364.
- Vicente-Sánchez C, Egido J, Sánchez-González PD, Pérez-Barriocanal F, López-Novoa JM, Morales AI. (2008). Effect of the flavonoid quercetin on cadmium-induced hepatotoxicity. Food Chem Toxicol, 46, 2279–2287.
- Warnick GR, Benderson J, Albers JJ. (1982). Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem, 28, 1379–1388.
- Yamamoto Y, Oue E. (2006). Antihypertensive effect of quercetin in rats fed with a high-fat high-sucrose diet. Biosci Biotechnol Biochem, 70, 933–939.
- Yang RL, Li W, Shi YH, Le GW. (2008). Lipoic acid prevents high-fat diet-induced dyslipidemia and oxidative stress: A microarray analysis. Nutrition, 24, 582–588.
- Zhao L, Wu J, Wang Y, Yang J, Wei J, Gao W, Guo C. (2011). Cholesterol metabolism is modulated by quercetin in rats. J Agric Food Chem, 59, 1104–1108.
- Zou JG, Wang ZR, Huang YZ, Cao KJ, Wu JM. (2003). Effect of red wine and wine polyphenol resveratrol on endothelial function in hypercholesterolemic rabbits. Int J Mol Med, 11, 317–320.
