Abstract
Context: Amphibian skins have wide variety of biologically active compounds associated with the natural defenses of these animals.
Objectives: To study the in vitro anticancer activity of methanol extracts of the skin of Rhinella jimi Stevaux (Anura: Bufonidae).
Material and methods: The extract was obtained by cold methanol extraction for 96 h using dried skins (295 mg). The methanol skin extract was dried under reduced pressure, giving a 5.5% yield. In order to test for growth-inhibitory activity, in vitro tests were performed with the following cancer cell lines using concentrations ranging between 0.25–250 µg/mL of the extract by 48 h: K562 (leukemia), MCF-7 (breast), NCI-ADR (breast with MDR phenotype), UACC-62 (melanoma), NCI460 (lung), PCO3 (prostate), HT-29 (colon), OVCAR (ovary), and 786-0 (kidney).
Results: The methanol extract of R. jimi produced a growth inhibition in a dose-dependent manner against the most of the assayed cell lines. In addition to the growth inhibition, the extract induced the cell death in the ovary and colon lines (EC50 0.125 and 0.2 µg/mL, respectively), demonstrating 100% of inhibition with 2.5 µg/mL. However, prostate and leukemia cell lines demonstrated less sensitivity, with EC50 of 24 and 235 µg/mL, respectively. This is the first report about the anticancer activity by natural products from the skin of R. jimi.
Conclusions: The methanol extracts of R. jimi significantly affected the growth of several cell lines, demonstrating that these compounds are a potential source of substances that could be utilized in cancer treatments.
Introduction
Amphibian skins are morphologically, biochemically, and physiologically complex organs, having important functions associated to the survival of these animals as in the respiration, water balance, excretion, temperature control, reproduction, antipredator camouflage, and antimicrobial and antifungal resistance (CitationMortari et al., 2004). Several biologically active substances such as biogenic amines, steroids, peptides and lipophilic alkaloids have been reported in amphibian skins, and these substances demonstrate a large spectrum of pharmacological properties, providing chemical defenses against predators (CitationGomes et al., 2007a; CitationDaly et al., 2004). Among the many classes of compounds in amphibian skins, alkaloids have received considerable attention. An example is the alkaloid bufalin, component of the remedy Chan Su and used by the traditional medicine due the antineoplastic activity (CitationGomes et al., 2007b).
Alkaloids are utilized by the pharmaceutical industries in the form of derivates or as starting materials for synthesizing drugs (CitationHenriques et al., 2007). According to CitationDaly et al. (2005), alkaloids are generally acquired by amphibians through their diets of arthropods (mainly insects).
Rhinella jimi Stevaux (Anura: Bufonidae) is a toad species recently described for the central-western and northwestern regions of Brazil. R. jimi is geographically restricted to northwestern of Brazil (Stevaux, Citation2002), and information about its ecology and natural history has been limited to a few publications describing its periods of activity (CitationSilva et al., 2007) and its parasitic infections (CitationAnjos et al., 2008). In addition to their pharmacological interest, toad skin secretions (aromatic amines) can be useful to taxonomic studies (biochemical taxonomy) and to evaluate the evolutionary relationships, especially in the genus Rhinella (CitationMaciel et al., 2003).
In light of our limited knowledge of the ecology and biological characteristics of R. jimi, we examined the composition of the principal bioactive substances present in the skin methanol extract of this toad and their in vitro pharmacological potential against cancer cell lines.
Materials and methods
Skins
The project was approved by the Brazilian Institute for the Environment and Natural Resources (IBAMA) to the collection of specimens (066/06-COFAN IBAMA/RAN/02007.001009/04-37). The skins from 37 specimens of R. jimi were removed during the rainy season and deposited in the Zoological Collection of the Universidade Regional do Cariri (LZ-URCA 0469–500, 0503, 0504. 0506–508). These specimens had been collected in the municipalities of Caririaçu (7°2′S × 39°17′W), Crato (7°14′S × 39°24′W), Mauriti (7°23′S × 38°46′W), and Várzea Alegre (6°47′S × 39°17′W) in Ceará State, as well as from the municipality of Exú (7°30′S × 39°43′W) in Pernambuco State, Brazil. All these sites are included inside the biome called “Caatinga” (dryland vegetation, with stunted trees and cacti), typical of northeastern Brazil, as well as some areas of “Cerrado” (savanna vegetation). The climate in this semi-arid region is hot and dry, with a rainy period limited to 3–4 months (CitationIPECE, 2005).
Methanol extracts of the skin and chemical prospection
The extract was obtained by cold methanol extraction for 96 h of dried skins (295 mg) that had been kept at room temperature. The methanol skin extract was dried under reduced pressure, with a yield of 5.5%. The methanol extract was chemically prospected according to the method of CitationMatos (1997). Tests were performed for the identification of anthrocyanic heterosides, saponins, tannins, flavonoids, steroids, triterpenoids, organic acids and alkaloids. The tests were based on the visual observation of a change in color or formation of precipitate after the addition of specific reagents.
Growth-inhibition assays
To evaluate the growth-inhibitory activity, in vitro tests were performed with the following cancer cell lines: K562 (leukemia), MCF-7 (breast), NCI-ADR (breast with multiple drug resistance phenotype), UACC-62 (melanoma), NCI460 (lung), PCO3 (prostate), HT-29 (colon), OVCAR (ovary), and 786-0 (kidney). These cell lines were cultivated in RPMI with 5% heat-inactivated fetal bovine serum (FBS) in an atmosphere of 5% of CO2 at 37°C in a humidified environment (CitationMonks et al., 1991; CitationSkehan et al., 1990). Stock solutions were prepared from the methanol extracts of R. jimi skin by diluting them with dimethylsulfoxide (DMSO) to a concentration of 1 mg/mL. These solutions were subsequently diluted with 400 volumes of RPMI/FBS/gentamicin to avoid any influence of DMSO (less than 10%) on the cell cultures. The cell suspensions in RPMI/FBS/gentamicin were plated at their respective inoculation densities in 96-well culture plates. These plates were then incubated for 24 h for re-acclimation at 37°C in an atmosphere of 5% CO2 in a humidified environment. After this period, a control plate was fixed by adding trichloroacetic acid (TCA) to determine the quantity of cells present when the drugs were first added (CitationMonks et al., 1991; CitationSkehan et al., 1990). The toad skin extract was then added to the other plates at concentrations of 0.25, 2.5, 25, and 250 µg/mL and incubated for 48 h. After 48 h, the plates were centrifuged for 3 min at 2000 rpm and adhered cells were fixed with 50 µL of 50% TCA; the cells in suspension (leukemia) were fixed with 80% TCA. To complete cell fixation, the plates were incubated for 1 h at 4°C, submitted to four consecutive washes with distilled water to remove any residues, and then allowed to air dry at room temperature. Once dried, the plates were stained by adding 50 µL of sulphorodamine B stain (0.4% weight/volume dissolved in 1% acetic acid) and incubated at 4°C for 30 min. After staining, the plates were washed four times with a 1% acetic acid solution; all of the residues of the washing solution were then removed and the plates were again dried at room temperature. Stain bound to the cell proteins was dissolved with a 10 µM solution of pH 10.5 Trizma base for 5 min using ultrasound. Absorbance was measured at 560 nm using a spectrophotometric microplate reader. Each assay was realized in triplicate and the percentage of growth inhibition was determined by calculating the averages of the spectrophotometric readings (after correcting for the blanks).
Statistical analysis
The EC50 values (concentration of extract needed to necessary for produce of 50% maximal effect) were determined by linear regression analysis of the using Prisma Software 5.0.
Results
Bioprospection of the methanol extract of R. jimi revealed a presence of alkaloids, as well as triterpenes, steroids, and saponins. The results of the cytotoxicity testing were expressed in terms of the percentages of growth inhibition at different methanol extract concentrations (). The chemotherapeutic drug doxorubicin was used as a positive control (). Samples were considered active if they produced more than 50% growth inhibition (cytostatic activity) (below the dotted line indicated in ). Cytocidal activity (cell death) corresponded to inhibition values below the zero line.
Figure 1. Percentages of growth inhibition of cancerous cells exposed to different concentrations of methanol extracts of Rhinellajimi skin.Effects of methanol extracts of Rhinella jimi skin on the proliferation of cancer cells after exposure for 48 h. Cell growth was analysed according to the techniques described in the materialand methods section. UACC-62, melanoma cells; MCF-7, breast cancer cells; NCIADR/RES, adryamycin-resistant ovarian cancer cells; 786-0, kidney cancer cells; NCI-H460, lung, non-small cancer cells; PC-3, prostate cancer cells; OVCAR-03, ovarian cancer cells; HT-29, colon cancer cells; K562, erythromyeloblastoid leukemia cells.
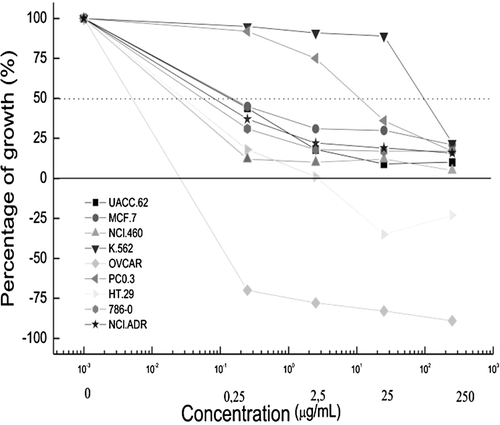
Figure 2. Evaluation of the effects of the chemotherapeutic drug doxorrubicin (positive control) against cancerous cells. Effects of chemotherapeutic drug doxorubicin (positive control) on the proliferation of cancer cells after exposure for 48 h. UACC-62, melanoma cells; MCF-7, breast cancer cells; NCIADR/RES, adryamycin-resistant ovarian cancer cells; 786-0, kidney cancer cells; NCI-H460, lung, non-small cancer cells; PC-3, prostate cancer cells; OVCAR-03, ovarian cancer cells; HT-29, colon cancer cells; K562, erythromyeloblastoid leukemia cells.
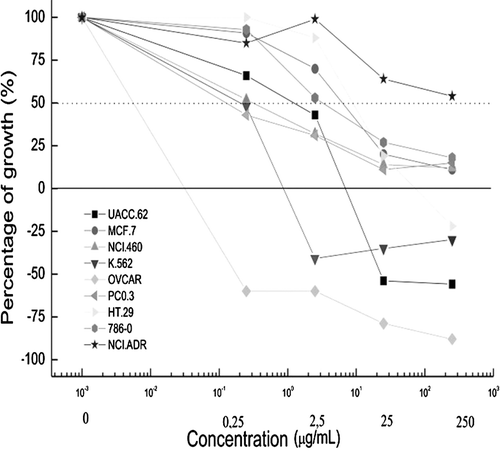
The extract induced cell death in the ovary and colon lines, however, prostate and leukemia cell lines demonstrated less sensitivity (). The methanol extract of R. jimi produced an optimal activity profile, producing a growth inhibition in a dose-dependent manner against most of the assayed cell lines, showing better results than doxorubicin ( and ; ). When comparing the concentration of the extract that inhibited 50% of the cell line population (EC50), it was demonstrated that the extract was more effective than the doxorrubicin against 6 of the cell lines assayed.
Table 1. Comparisons of anti-proliferative activity of methanol extracts of Rhinella jimi and the chemotherapeutic drug doxorrubicin (positive control) against cancerous cells (μg/mL).
Table 2. Comparisons of EC50 of methanol extracts of Rhinella jimi and the chemotherapeutic drug doxorrubicin (positive control) against cancerous cells (μg/mL).
Discussion
Many compounds obtained from the skins of amphibians belonging to the family Bufonidae demonstrated inhibitory activity against cancerous cells (CitationGiri et al., 2006; CitationGomes et al., 2007a; CitationHalliday et al., 2009). The main explanation for the cytotoxicity of methanol extracts of R. jimi skin can be related to the presence of compounds such alkaloids and cardiotonic steroids. These compounds have been reported in the gland secretions of these toads and include telocinobufagin and helebrigenin that have been shown to have anti-leshmaniasis and anti-trypanosomal activities (CitationTempone et al., 2008; CitationJared et al., 2009). Another species of the genus Rhinella has been shown to inhibit the growth of abnormal kidney cells in dogs (CitationGomes et al., 2007b), demonstrating that these compounds can be well distributed among the genus and indicating that the presence of these substances in toad skins is related to defense mechanisms against predators (CitationCoutinho et al., 2008).
The methanol extracts of R. jimi significantly affected the growth of the cell lines HT-29 (colon), 786-0 (kidney) and NCL.ADR (cancerous ovarian cells resistant to adriamicina). Natural products of other amphibian as Bombina variegate have demonstrated interesting results against several types of neoplastic cell lines (CitationDoyle et al., 2003; CitationKamano et al., 2002). The extract of the skin and the secretions of other amphibian species Bufo bufo gargarizans Cantor (Bufonidae) are used in the preparation of the Chinese traditional remedy Chan Su, used against several illnesses and with an antineoplastic activity reported by several articles, mainly due the alkaloid bufagin, a natural product that can affect several mechanisms involved on the cell proliferation (CitationHashimoto et al., 1997; CitationAkiyama et al., 1999; CitationYeh et al., 2003; CitationNasu et al., 2005).
Our results shown that compounds obtained from the skin of R. jimi are a potential source of substances with antineoplastic activities, demonstrating the necessity of more detailed studies to identify the compounds and the specific mechanisms affected by these natural products.
Acknowledgements
The authors are grateful to FUNCAP (Fundação Cearense de Apoio ao Desenvolvimento Científico and Tecnológico) for financial support in the form of research grants (process No. 9913/06 – Contract 0006-00/2006); CAPES for the Master’s scholarship awarded to Samuel Vieira Brito and Felipe Silva Ferreira; the Brazilian Institute for the Environment and Natural Resources (IBAMA) for permission to collect specimens (066/06-COFAN IBAMA/RAN/02007.001009/04-37); the Centro Pluridisciplinar de Pesquisas Químicas, Biológicas e Agrícolas (CPQBA) of the Universidade Estadual de Campinas – UNICAMP for performing the tests with human cancer cell lines; and FIOCRUZ for furnishing the bacterial strains.
Declaration of interest
The authors report no conflicts of interest.
References
- Akiyama M, Ogura M, Iwai M, Iijima M, Numazawa S, Yoshida T. (1999). Effect of bufalin on growth and differentiation of human skin carcinoma cells in vitro. Hum Cell, 12, 205–209.
- Anjos LA, Silva LEM, Almeida WO, Costa JGM, Vasconcellos A. (2008).Chaunus jimi (NCN) endoparasites Herpetolo Rev, 39, 337–338.
- Coutinho HD, Lôbo KM, Bezerra DA, Lôbo I. (2008). Peptides and proteins with antimicrobial activity. Indian J Pharmacol, 40, 3–9.
- Daly JW, Noimai N, Kongkathip B, Kongkathip N, Wilham JM, Garraffo HM, Kaneko T, Spande TF, Nimit Y, Nabhitabhata J, Chan-Ard T. (2004). Biologically active substances from amphibians: Preliminary studies on anurans from twenty-one genera of Thailand. Toxicon, 44, 805–815.
- Daly JW, Spande TF, Garraffo HM. (2005). Alkaloids from amphibian skin: A tabulation of over eight-hundred compounds. J Nat Prod, 68, 1556–1575.
- Doyle J, Brinkworth CS, Wegener KL, Carver JA, Llewellyn LE, Olver IN, Bowie JH, Wabnitz PA, Tyler MJ. (2003). nNOS inhibition, antimicrobial and anticancer activity of the amphibian skin peptide, citropin 1.1 and synthetic modifications. The solution structure of a modified citropin 1.1. Eur J Biochem, 270, 1141–1153.
- Giri B, Gomes A, Debnath A, Saha A, Biswas AK, Dasgupta SC, Gomes A. (2006). Antiproliferative, cytotoxic and apoptogenic activity of Indian toad (Bufo melanostictus, Schneider) skin extract on U937 and K562 cells. Toxicon, 48, 388–400.
- Gomes A, Giri B, Kole L, Saha A, Debnath A, Gomes A. (2007a). A crystalline compound (BM-ANF1) from the Indian toad (Bufo melanostictus, Schneider) skin extract, induced antiproliferation and apoptosis in leukemic and hepatoma cell line involving cell cycle proteins. Toxicon, 50, 835–849.
- Gomes A, Giri B, Saha A, Mishra R, Dasgupta SC, Debnath A, Gomes A. (2007b). Bioactive molecules from amphibian skin: Their biological activities with reference to therapeutic potentials for possible drug development. Indian J Exp Biol, 45, 579–593.
- Halliday DC, Venables D, Moore D, Shanmuganathan T, Pallister J, Robinson AJ, Hyatt A. (2009). Cane toad toxicity: An assessment of extracts from early developmental stages and adult tissues using MDCK cell culture. Toxicon, 53, 385–391.
- Hashimoto S, Jing Y, Kawazoe N, Masuda Y, Nakajo S, Yoshida T, Kuroiwa Y, Nakaya K. (1997). Bufalin reduces the level of topoisomerase II in human leukemia cells and affects the cytotoxicity of anticancer drugs. Leuk Res, 21, 875–883.
- Henriques AT, Limberger RP, Kerberv A, Moreno PRH. (2007). Alcalóides: Generalidades e aspectos básicos. In: Simões CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR, eds. Farmacognosia da planta ao medicamento. Porto Alegre: Ed. UFRGS, 651–666.
- IPECE - Instituto De Pesquisa Estratégica Econômica Do Ceará. (2005). Perfil Básico Municipal Fortaleza: Governo Do Estado Do Ceará - Secretária Do Planejamento e Coordenação.
- Jared C, Antoniazzi MM, Jordão AE, Silva JR, Greven H, Rodrigues MT. (2009). Parotoid macroglands in toad (Rhinella jimi): Their structure and functioning in passive defence. Toxicon, 54, 197–207.
- Kamano Y, Nogawa T, Yamashita A, Hayashi M, Inoue M, Drasar P, Pettit GR. (2002). Isolation and structure of a 20,21-epoxybufenolide series from “Ch’an Su”. J Nat Prod, 65, 1001–1005.
- Maciel NM, Schwartz CA, Rodrigues Pires Júnior O, Sebben A, Castro MS, Sousa MV, Fontes W, Ferroni Schwartz EN. (2003). Composition of indolealkylamines of Bufo rubescens cutaneous secretions compared to six other Brazilian bufonids with phylogenetic implications. Comp Biochem Physiol B, Biochem Mol Biol, 134, 641–649.
- Matos FJA (1997) Introdução à fitoquímica experimental Fortaleza: ED-UFC.
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A. (1991). Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst, 83, 757–766.
- Mortari MR, Schwartz EN, Schwartz CA, Pires OR Jr, Santos MM, Bloch C Jr, Sebben A. (2004). Main alkaloids from the Brazilian dendrobatidae frog Epipedobates flavopictus: Pumiliotoxin 251D, histrionicotoxin and decahydroquinolines. Toxicon, 43, 303–310.
- Nasu K, Nishida M, Ueda T, Takai N, Bing S, Narahara H, Miyakawa I. (2005). Bufalin induces apoptosis and the G0/G1 cell cycle arrest of endometriotic stromal cells: A promising agent for the treatment of endometriosis. Mol Hum Reprod, 11, 817–823.
- Silva GR, Santos CL, Alves MR, Sousa SDV, Annuziata BB. (2007). Anfíbios das dunas litorâneas do extremo norte do Piauí, Brasil. Sitientibus, 7, 334–340.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. (1990). New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst, 82, 1107–1112.
- Stevaux MN. 2002 The new species of Bufo (Anura, Bufonidae) in the Northeastern Brazil Rev Bras Zool, 19, 235–242.
- Tempone AG, Pimenta DC, Lebrun I, Sartorelli P, Taniwaki NN, de Andrade HF Jr, Antoniazzi MM, Jared C. (2008). Antileishmanial and antitrypanosomal activity of bufadienolides isolated from the toad Rhinella jimi parotoid macrogland secretion. Toxicon, 52, 13–21.
- Yeh JY, Huang WJ, Kan SF, Wang PS. (2003). Effects of bufalin and cinobufagin on the proliferation of androgen dependent and independent prostate cancer cells. Prostate, 54, 112–124.
