Abstract
Context: Hyssopus angustifolius M. Bieb. (Lamiaceae) is one of the most important medicinal plants in Iranian traditional medicine for the treatment of lung inflammation, laryngitis and cough relief. Much attention has been paid to this medicinal plant because of its traditional uses.
Objective: The present study examined the antioxidant and antihemolytic activities of ethyl acetate extract of stems, leaf and flowers of Hyssopus angustifolius.
Materials and methods: Antioxidant activity of extracts was evaluated by employing six different models, i.e., DPPH, nitric oxide and hydrogen peroxide scavenging, metal chelating and reducing power activities and hemoglobin-induced linoleic acid system. Also, antihemolytic activity was evaluated against hydrogen peroxide-induced hemolysis.
Results: Flowers extract showed the better activity than leaf and stems extracts in DPPH radical scavenging activity (IC50 was 275.4 ± 7.6 μg mL−1). Leaf, stems and flowers extracts showed good nitric oxide scavenging activity (IC50 were 376.6 ± 11.4 µg mL−1 for flowers, 297.6 ± 9.6 μg mL−1 µg mL−1 for leaves and 837.8 ± 19.2 µg mL−1 for stems). The leaf extract exhibited better hydrogen peroxide scavenging and Fe2+ chelating activity than stems and flowers extracts. In hemoglobin-induced linoleic acid system, all of the extracts exhibited very good activity. Also, extracts show weak reducing power activity. The ethyl acetate extract of leaf showed better antihemolytic activity than the flower and stems (IC50 was 94.0 ± 2.4 μg mL−1).
Discussion and conclusion: These findings give a scientific basis to the traditional usage of Hyssopus angustifolius, also showing its potential as rich sources of natural antioxidant compounds.
Keywords::
Introduction
Recently, there has been interest in the use of antioxidative nutritional substances (CitationGigante et al., 2007; CitationSimpore et al., 2006). Numerous studies showed that using of some vitamins, minerals, and other food constituents with antioxidant activity can protect our body against some diseases caused by oxidative imbalance and free radicals such as cardiovascular disease, cancer, and the aging process (CitationWu et al., 2005; CitationHsia et al., 2007; CitationLuchsinger et al., 2007; CitationMarcason, 2007). Antioxidants can decrease the chance of getting the above-mentioned diseases via inhibition of reactive oxygen species that originated from aerobic metabolism (CitationAbdel-Wahhab et al., 2005). Natural products are very rich sources of natural antioxidant agents.
Hyssopus angustifolius M. Bieb. (Lamiaceae) is a medicinal plant that used in Iranian folk medicine and cultivated in central and south area of Europe (CitationOmidbaigi, 2000). In Iranian folk medicine, this species used in tea blends to diminish coughing and induce sedative effects, and to reduce catarrh (CitationKhazaie et al., 2008). To the best of our knowledge there are no reports about biological activities of ethyl acetate extract of different parts of Hyssopus angustifolius.
Materials and methods
Chemicals
Ferrozine, linoleic acid, trichloroacetic acid, 1,1-diphenyl-2-picryl hydrazyl, potassium ferricyanide, sodium nitrite, and hydrogen peroxide were purchased from Sigma Chemical Co. (USA). Gallic acid, quercetin, butylated hydroxyanisole, vitamin C, sulfanilamide, N-(1-naphthyl) ethylenediamine dihydrochloride, EDTA and ferric chloride were purchased from Merck Co. (Germany). All other chemicals were of analytical grade or purer.
Plant materials
Whole plants of Hyssopus angustifolius were collected from Elburz Mountains, Iran, in spring 2010. The plant was identified by Dr. Bahman Eslami (Assistant Professor of Plant Systematic and Ecology, Department of Biology, Islamic Azad University, Branch of Ghaemshahr, Iran) and authenticated at the Herbarium of Department of Biology in the University of Mazandaran (a voucher specimen No 975).
Extraction
Approximately 100 g of the samples powder were placed in a Soxhlet extractor and extracted with ethyl acetate for 8 h. The solvent was recovered by distillation in vacuo, and the residue, stored in the desiccator, was used for subsequent experiments.
Phytochemical screening
Total phenolic and flavonoid contents
Total phenolic content was determined by employing the Folin-Ciocalteau method (CitationEbrahimzadeh et al., 2010). The sample (0.5 mL) at 1.6 mg mL−1 was mixed with Folin-Ciocalteau reagent (0.2 N, 2.5 mL) for 5 min and sodium carbonate (75 g L−1, 2.0 mL) was then added. The reactions mixture was incubate for 2 h at room temperature and then measured at 760 nm. Results were expressed as gallic acid equivalents. Total flavonoid content was measured by employing the method of CitationEbrahimzadeh et al., (2010). 0.5 mL of sample at 1.6 mg mL−1 was separately mixed with 1.5 mL of methanol, aluminum chloride (10%, 0.1 mL), potassium acetate (1 M, 0.1 mL) and distilled water (2.8 mL) and incubated for 30 min at room temperature. The reaction mixture was measured at 415 nm. Total flavonoid content was calculated as quercetin from a calibration curve.
Antioxidant activity
1, 1-Diphenyl-2-picryl hydrazyl (DPPH) radical scavenging
Samples (25–400 μg mL−1) were added, at an equal volume, to a solution of DPPH (100 μM in methanol). Reactions were incubating for 15 min at room temperature and then were recorded at 517 nm. The experiment was repeated three times. Vitamin C, BHA and quercetin were used as standard controls. IC50 values denote the concentration, which is scavenging 50% of free radical (CitationNabavi et al., 2010).
Reducing power
The reducing power of extracts was determined using the method of Citation(Pulido et al., 2000). Aqueous solutions of samples (25–400 μg mL−1, 2.5 mL) were mixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide (2.5 mL, 1%). Reaction mixtures were incubated at 50°C for 20 min. To stop the reaction, trichloroacetic acid (10%, 2.5 mL) was added to the mixtures, which was then centrifuged at 1000g for 10 min. The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.1%, 0.5 mL), and measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. Vitamin C was used as positive control.
Metal chelating
The activity of extracts to chelate iron ions was estimated by the method of CitationDinis et al. (1994). Each sample (25–400 µg mL−1, 1 mL) was added to a solution of FeCl2 (2 mM, 0.05 mL). For initiated the reactions, ferrozine (5 mM, 0.2 mL) was added and the mixture was shaken vigorously and left standing at room temperature for 10 min. Absorbance of the solutions was then measured at 562 nm. Na2EDTA was used as positive control.
Nitric oxide scavenging
Sodium nitroprusside (10 mM), in phosphate-buffered saline, was mixed with different concentrations of each extract (25–400 µg mL−1), dissolved in water and incubated at room temperature for 150 min. After the incubation period, 0.5 mL of Griess reagent (1% sulfanilamide, 2% phosphoric acid and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride) was added. The absorbance was measured at 546 nm. Quercetin was used as positive control (CitationSreejayan & Rao, 1997).
Hydrogen peroxide scavenging
Each sample (2 mL) (0.1-1 mg mL−1) in distilled water was added to a hydrogen peroxide solution (0.6 mL, 40 mM) in phosphate buffer (pH 7.4). The absorbances of samples at 230 nm were measured after 10 min against a blank solution containing phosphate buffer without hydrogen peroxide and extracts. Vitamin C and quercetin were used as standards (CitationElmastas et al., 2006).
Hemoglobin-induced linoleic acid system
A reaction mixture (2 mL) containing 100 µL of each extract (25–400 µg mL−1), linoleic acid emulsion (1 mmol L−1), phosphate buffer (40 mmol L−1, pH 6.5), and hemoglobin suspension (0.0016%), was incubated at 37°C for 45 min. After the incubation, hydrochloric acid in ethanol (0.6%, 2.5 mL) was added to stop the lipid peroxidation. The amount of peroxide value was measured in triplicate using the thiocyanate method by reading the absorbance at 480 nm after coloring with of FeCl2 (0.02 mol L−1, 100 µl) and of ammonium thiocyanate (0.3 g mL−1, 50 µl). Vitamin C was used as positive control (CitationNabavi et al., 2010).
Antihemolytic activity
Preparation of rat erythrocytes
Male rats in the body weight range of 180–220 g were housed in individual polypropylene cages and had free access to food and water. The animals were fed with standard diet. The animals were anesthetized with ketamine (60 mg/kg) and xylazine (5 mg/kg) given intraperitoneally. Blood samples were collected via retro-orbital puncture in plain plastic tubes. Erythrocytes were isolated and stored according to the method described by CitationNabavi et al. (2010).
Protection against H2O2 induced hemolysis
Antihemolytic activity of the extracts was determined by the method of CitationEbrahimzadeh et al. (2010).
Different concentrations of the each extracts (0.5 mL) were added to erythrocyte suspension (4%, 2 mL) and the volume was made up to 5 mL with saline buffer. Reaction mixtures were incubated for 5 min at room temperature and then 0.5 mL of H2O2 solution in saline buffer was added to induce hemolysis. After incubation (240 min) at room temperature, the reaction mixture was centrifuged at 250g for 10 min and the extent of hemolysis was determined by measuring the absorbance at 540 nm corresponding to hemoglobin liberation.
Statistical analysis
Experimental results are expressed as means ± S.D. All measurements were in triplicate. The data were analyzed by an analysis of variance (p < 0.05) and the means separated by Duncan’s multiple range tests. The IC50 values were calculated from linear regression analysis.
Results and discussion
Total phenolic content of extracts are reported as gallic acid equivalents by reference to a standard curve (y = 0.0054x + 0.0628, r2 = 0.987). The total phenolic content of the ethyl acetate extract of flowers, leaves and stems were 134.4 ± 8.0, 122.6 ± 7.9 and 97.0 ± 6.4 mg gallic acid equivalent/g of extract, respectively. Also, total flavonoid contents of ethyl acetate extract of flowers, leaves and stems were in the order of 67.2 ± 2.0, 76.1 ± 3.1 and 44.1 ± 1.3 mg quercetin equivalent/g of extract powder, respectively, by reference to a standard curve (y = 0.0063x, r2 = 0.999). Phenol and polyphenolic contents in natural products have significant biological activities (Citationvan Acker et al., 1996).
In DPPH radical scavenging activity model, ethyl acetate extracts showed lower activity than standard compound. IC50 values of the samples was in the order of flowers (275.4 ± 7.6 μg mL−1) > leaves (297.0 ± 9.6 μg mL−1) > stems (396.4 ± 10.4 μg mL−1), respectively. Also IC50 values for ascorbic acid, quercetin and BHA were 5.05 ± 0.1, 5.28 ± 0.2 and 53.96 ± 3.1 μg mL−1, respectively. The polyphenolic content in the extracts may correlate to their good DPPH radical scavenging activity. It has been assumed that phenol and flavonoid can quench DPPH radical via hydrogen- or electron-donation mechanism and changes its color from violet to yellow. Antioxidant is defined as any substances which are able to perform this reaction.
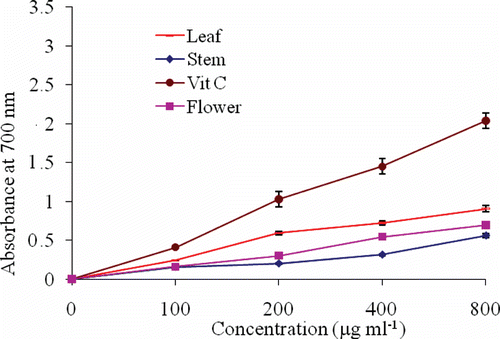
In the reducing power model, electron donor compound in the extracts can reduce Fe3+ to Fe2+. Perl’s Prussian blue color form by Fe2+ complex can be monitored by measuring of absorbance of sample at 700 nm (CitationEbrahimzadeh et al., 2010). Increasing in absorbance at 700 nm indicates an increase in reducing ability. Dose response curves for the reducing power of extracts are shown in . Vitamin C has better electron donating activity than tested samples (p < 0.001).
Nitric oxide generated from sodium nitroprusside in aqueous solution at physiological pH interacts with oxygen leading to the production of nitrite ions that can be monitored using griess reagent. Nitric oxide scavengers challenge with oxygen, leading to reduced production of nitrite ions. The IC50 values for nitric oxide radical scavenging activity of extracts were in order to: leaves (297.6 ± 9.6 μg mL−1) > flowers (376.6 ± 11.4 μg mL−1) > stems (837.8 ± 19.2 μg mL−1). Although quercetin showed better nitric oxide scavenging activity than extracts (IC50 = 17.01 ± 0.03 µg mL−1, p < 0.001), there are numerous reports on its carcinogenic activity (CitationDunnick & Hailey, 1992). Nitric oxide has an important role in the initiation or progression of many diseases such as inflammation, cancer and other pathological conditions (CitationNabavi et al., 2010). Natural products with nitric oxide scavenging ability may be interest in preventing the above-mentioned diseases.
Leaf extract showed better iron chelating activity than others. Iron ion chelating activity of extracts was in the order of leaves (603.5 ± 11.2 µg mL−1) > flowers (776.0 ± 12.4 µg mL−1) > stems (942.2 ± 16.0 µg mL−1), respectively. EDTA showed potent activity that was better than extracts (IC50= 18 ± 0.5 µg mL−1, p < 0.001). Iron chelators can remove tissue iron via forming soluble, stable complexes that can excreted in the feces and/or urine. Chelation therapy is one of the most common ways to reduce iron related complications in human and so improve life quality and overall survival in many diseases such as thalassemia major (CitationHebbel et al., 1990). Deferoxamine is an iron chelator drug used for chelation therapy in treatment of iron overload in thalassemia patients. Overdose of deferoxamine may cause some adverse effects (CitationPorter, 1997). Another drug, deferiprone, is orally absorbed bi-dentate iron chelator that can reduce iron via increase in urinary iron excretion, improve negative iron balance and reduce hepatic iron levels. But arthritis, nausea and agranulocytosis are common side effects of this drug. For the above-mentioned reason urgent needs to find an active chelator regimen that is as effective as deferoxamine and deferiprone and has a lower side effect remain (CitationPorter, 1997).
In recent years, searches in natural products to there has been extensive investigate iron chelators with lower adverse effect (CitationGrazul & Budzisz, 2009). Also, many studies showed that iron chelators and hydroxyl-radical scavengers can protect against acute renal failure especially aminoglycoside antibiotic-mediated nephrotoxicity (CitationNabavi et al., 2012). Iron chelators can suppress these procedures. CitationDinis et al., (1994) method has been used for determination of ferrous ion chelating activity. Ferrozine can quantitatively form complexes with Fe2+. In the presence of other chelating agents, the complex formation is disrupted with the result that the red color of the complexes decreases. In iron chelation assay, all of the extracts and EDTA interfered with the formation of ferrous and ferrozine complex, suggesting that it has chelating activity and captures ferrous ion before ferrozine.
Samples show hydrogen peroxide scavenging in a concentration dependent manner. The IC50 values of hydrogen peroxide scavenging activity of extracts were in the order of leaves (297.5 ± 5.9 μg mL−1) > stems (377.5 ± 6.4 μg mL−1) > flower (425.1 ± 7.7 μg mL−1), respectively. The IC50 values for vitamin C and quercetin were 21.4 ± 1.1 and 52 ± 2.6 μg mL−1, respectively. Hydrogen peroxide scavenging ability of sample may be originated from phenolic compounds and/or other active components which can donate electrons to hydrogen peroxide and neutralizing it to water (CitationElmastas et al., 2006). Hydrogen peroxide itself is not very reactive, but it can increase hydroxyl radical formation in the cell and cause cytotoxicity (CitationEbrahimzadeh, 2010).
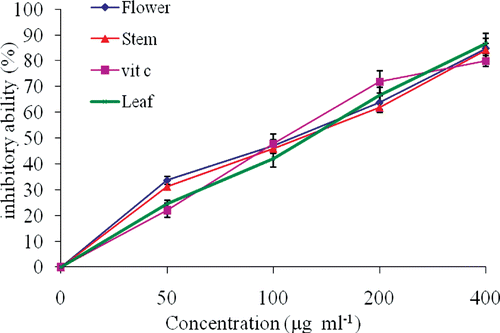
Tested extracts show good activities in hemoglobin-induced linoleic acid emulsion (). Erythrocytes are major targets for free radical attack owing to the presence of both high membrane concentration of polyunsaturated fatty acids and the oxygen transport associated with redox active hemoglobin, which are potent promoters of free radicals. Specially, linoleic acid and arachidonic acid are targets of lipid peroxidation (CitationYu, 2001). The inhibition of lipid peroxidation by samples may be due to their free radical scavenging activities. Lipid peroxidation indirectly initiated by superoxide because superoxide anion acts as a precursor of singlet oxygen and hydroxyl radical (CitationEbrahimzadeh et al., 2010). Hydroxyl radicals attack to membrane lipids via removing of hydrogen atoms that causes lipid peroxidation. There were no significant differences between extracts and vitamin C (p > 0.05).
Figure 2. Antioxidant activity of extracts against hemoglobin-induced lipid peroxidation. Vitamin C was used as positive control.
The antihemolytic activity of extracts were tested and it was found they did not show any harmful effects on erythrocytes. IC50 of antihemolytic activity of extracts was in the order of leaves (94.0 ± 2.4 μg mL−1) > stems (128.6 ± 3.1 μg mL−1) > flowers (137.2 ± 3.9 μg mL−1) vs. vitamin C (235 ± 9 µg mL−1), respectively. All of the extract showed better activity than vitamin C (p < 0.001). Previous studies showed a correlation between antihemolytic activity and flavonoid content and the good activity of extracts may be the result of high flavonoid, especially the flavonol group (CitationChaudhuri et al., 2007).
Conclusion
The present study shows biological activities of the ethyl acetate extract of flowers, leaves and stems of Hyssopus angustifolius. All of the extracts show different levels of antioxidant and antihemolytic activities in the studied models. Other studies on in vivo biological and toxicological activities and/or using this species in clinical study are needed. The results of this study can be benefit as a starting point for further applications of this species or its bioactive natural compounds in natural pharmaceutical formulation.
Declaration of interest
The authors report no declarations of interest.
References
- Abdel-Wahhab MA, Abdel-Galil MM, El-Lithey M. (2005). Melatonin counteracts oxidative stress in rats fed an ochratoxin A contaminated diet. J Pineal Res, 38, 130–135.
- Chaudhuri S, Banerjee A, Basu K, Sengupta B, Sengupta PK. (2007). Interaction of flavonoids with red blood cell membrane lipids and proteins: Antioxidant and antihemolytic effects. Int J Biol Macromol, 41, 42–48.
- Dinis TC, Maderia VM, Almeida LM. (1994). Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys, 315, 161–169.
- Dunnick JK, Hailey JR. (1992). Toxicity and carcinogenicity studies of quercetin, a natural component of foods. Fundam Appl Toxicol, 19, 423–431.
- Ebrahimzadeh MA, Nabavi SF, Nabavi SM, Eslami B. (2010). Antihemolytic and antioxidant activities of Allium paradoxum. Cent Eur J Biol, 5, 338–45.
- Elmastas M, Gulcinb I, Isildaka O, Kufrevioglub OI, Ibaoglua K, Aboul-Enein HY. (2006). Radical scavenging activity and antioxidant capacity of Bay leaf extracts. J Iran Chem Soc, 3, 258–66.
- Gigante DP, Buchweitz M, Helbig E, Almeida AS, Araújo CL, Neumann NA, Victora C. (2007). Randomized clinical trial of the impact of a nutritional supplement “multimixture” on the nutritional status of children enrolled at preschools. J Pediatr (Rio J), 83, 363–369.
- Grazul M, Budzisz E. (2009). Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord Chem Rev, 253, 2588–98.
- Hebbel RP, Leung A, Mohandas N. (1990). Oxidation-induced changes in microrheologic properties of the red blood cell membrane. Blood, 76, 1015–1020.
- Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, Trevisan M; Women’s Health Initiative Investigators. (2007). Calcium/vitamin D supplementation and cardiovascular events. Circulation, 115, 846–854.
- Luchsinger JA, Tang MX, Miller J, Green R, Mayeux R. (2007). Relation of higher folate intake to lower risk of Alzheimer disease in the elderly. Arch Neurol, 64, 86–92.
- Khazaie HR, Nadjafi F, Bannayan M. (2008). Effect of irrigation frequency and planting density on herbage biomass and oil production of thyme (Thymus vulgaris) and hyssop (Hyssopus officinalis). Ind Crop Prod, 27, 315–21.
- Nabavi SF, Ebrahimzadeh MA, Nabavi SM, Eslami B. (2010). Antioxidant activity of flower, stem and leaf extracts of Ferula gummosa Boiss. Grasas Aceites, 61, 244–50.
- Nabavi SF, Nabavi SM, Moghaddam AH, Naqinezhad A, Bigdellou R, Mohammadzadeh S. (2012). Protective effects of Allium paradoxum against gentamicin-induced nephrotoxicity in mice. Food Funct, 3, 28–29.
- Marcason W. (2007). Is supplementation of B vitamins still recommended to reduce the risk of heart disease? J Am Diet Assoc, 107, 525.
- Omidbaigi R. (2000). Production and processing of medicinal plants, Astan Quds Razavi Publications. Behnashr Co. Mashad, Iran. 3, 27–31.
- Porter JB. (1997). A risk-benefit assessment of iron-chelation therapy. Drug Saf, 17, 407–421.
- Pulido R, Bravo L, Saura-Calixto F. (2000). Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem, 48, 3396–3402.
- Simpore J, Kabore F, Zongo F, Dansou D, Bere A, Pignatelli S, Biondi DM, Ruberto G, Musumeci S. (2006). Nutrition rehabilitation of undernourished children utilizing Spiruline and Misola. Nutr J, 5, 3.
- Sreejayan Rao, MN. (1997). Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol, 49, 105–107.
- van Acker SA, van den Berg DJ, Tromp MN, Griffioen DH, van Bennekom WP, van der Vijgh WJ, Bast A. (1996). Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med, 20, 331–342.
- Wu LC, Ho JA, Shieh MC, Lu IW. (2005). Antioxidant and antiproliferative activities of Spirulina and Chlorella water extracts. J Agric Food Chem, 53, 4207–4212.
- Yu L. (2001). Free radical scavenging properties of conjugated linoleic acids. J Agric Food Chem, 49, 3452–3456.