Abstract
Context: Bellis perennis L. (Asteraceae) has been used traditionally in the treatment of bruises, broken bones, and wounds by European people.
Objective: To investigate the wound healing activity of B. perennis flowers in Wistar albino rats.
Materials and methods: Dried B. perennis flowers were extracted with ethanol, then fractioned with n-butanol and an oinment was prepared. Twelve male adult Wistar rats were used. Six wounds were created for each animal by using circular excision wound model. The first two wounds were treated topically with HOTBp (hydrophilic ointment treatment containing n-butanol fraction). The second two wounds were control group and not treated with anything. The third two wounds were treated only with HOT (hydrophilic ointment treatment without n-butanol fraction). Treatments were applied once a day and lasted for 30 days. Wound samples were excised on days 5th, 10th and 30th. The percentage of wound healing was calculated by Walker’s formula after measurement of the wound area and the tissue samples were examined histopathologically.
Results: The percentages of wound closure (HOTBp: 100%; HOT: 85% and control: 87%) and histopathological observations showed that there were statistically significant differences between HOTBp, HOT and control groups (p < 0.05) at 30th day.
Discussion and conclusion: Topically administered ointment prepared from the n-butanol fraction of B. perennis flowers has a wound healing potential without scar formation in circular excision wound model in rats. Thus, traditional usage of wound healing activity of B. perennis was scientifically verified for the first time.
Keywords::
Introduction
Bellis perennis L. (common daisy) is a herbaceous perennial herb in the family Asteraceae, which grows in meadows, lawns and other grassy areas (CitationPanda, 2004). It is native to western, central, and northern Europe, but is commonly found as an invasive plant in North America (CitationTutin et al., 1976). Common daisy is known as a traditional wound herb (CitationLeporatti & Ivancheva, 2003; CitationAl-Douri & Al-Essa, 2010) and it had been used for the treatment of bruises, broken bones, and wounds by Crusaders in the Middle Ages (CitationMitich, 1997). B. perennis has also been used in the treatment of sore throat (CitationUysal et al., 2010), headache (CitationUzun et al., 2004), common cold, stomachache, eye diseases, eczema, skin boils, gastritis, enteritis, diarrhea, bleeding, rheumatism, inflammation, and infections of the upper respiratory tract in traditional medicine (CitationCakilcioglu et al., 2010). Furthermore, astringent, expectorant, diuretic, booster, purgative and diaphoretic properties have been recorded (CitationGrieve, 1982; CitationBaytop, 1999; CitationDuke et al., 2002).
The main constituents are triterpenoid saponins (CitationHiller et al., 1988; CitationSchopke et al., 1991; CitationLi et al., 2005; CitationMorikawa et al., 2008; CitationYoshikawa et al., 2008), essential oils (CitationAvato et al., 1997; CitationKavalcioglu et al., 2010) and flavonoids (CitationGudej & Nazaruk, 2001). Antibacterial (CitationAvato et al., 1997; CitationKavalcioglu et al., 2010), antifungal (CitationDesevedavy et al., 1989; CitationAvato et al., 1997; CitationKavalcioglu et al., 2010), antihyperlipidemic (CitationMorikawa et al., 2010a), antioxidant (CitationKavalcioglu et al., 2010), postpartum antihemorrhagic (CitationOberbaum et al., 2005), pancreatic lipase inhibitor (CitationMorikawa et al., 2010b) and cytotoxic activities against HL-60 human promyelocytic leukemia cells (CitationLi et al., 2005) of B. perennis have also been investigated.
Wound healing involves a series of events namely chemotaxis, cell division, revascularization, synthesis of new extracellular matrix, and the formation and remodeling of the scar tissue (CitationHanna & Giacopelli, 1997). Regeneration involves the restitution of tissue components identical to those removed or killed. By contrast, repair is a fibro proliferative response that “patches” rather than restores a tissue. If tissue injury is severe or chronic, and results in damage of both parenchymal cells and the stromal framework of the tissue, healing cannot be accomplished by regeneration. Under these conditions, the main healing process is repair by deposition of collagen and other extracellular matrix components, causing the formation of a scar. The term scar is most often used in connection to wound healing in the skin, but is also used to describe the replacement of parenchymal cells in any tissue by collagen, as in the heart after myocardial infarction (CitationKumar et al., 2010).
When a wound occurs, it may be thoroughly exposed to the infections and complications. Consequently, the aim of the wound management is to heal the wound as quick as possible with minimal scar formation (CitationClark, 1991). The agents, which shorten the healing process, are always required in order to contribute for a rapid and better healing of wounds without hypertrophic scar. The present study describes, to our knowledge for the first time, a wound healing properties of B. perennis flowers.
Materials and methods
Plant material
Common daisy (Bellis perennis L.) flowers and pedicels were collected from Abant Izzet Baysal University Campus, Bolu, Turkey in May of 2009. Identification of the species was made by Arzu Ucar Turker using “Flora of Turkey and the East Aegean Islands” (CitationDavis, 1975) and voucher specimens (collection number AUT-1909) were deposited at the Abant Izzet Baysal University (AIBU) Herbarium, Bolu, Turkey.
Preparation of the common daisy n-butanol fraction
Collected common daisy flowers and pedicels were dried in an oven at 40°C and then ground into a powder. Each 25 g plant sample of B. perennis flower was extracted with 250 ml ethanol (96%) at 60°C for 18 h and then filtered. Ethanol was evaporated under vacuum and residue was dissolved in water. n-Butanol was added into the extract in separation funnel and aqueous portion was discarded. The remaining fraction was flash evaporated to remove the n-butanol and the residue was suspended in water. Frozen n-butanol fraction was lyophilized by using a freeze-dryer at −65°C and residue used for ointment preparation. The yield of fraction (w/w) was 10% (Yield (%) = weight of extract (g)/25 g of powdered plant sample × 100).
Preparation of hydrophilic ointment loaded with B. perennis extract
Ethyl esters wax (12.5 g), white wax (12.5 g), mineral oil (56 g), sodium borate (0.5 g) and purified water (19 g) were accurately weighed to obtain approximately 100 g of hydrophilic cold cream (USP XXI) formulation. Ethyl esters and white waxes were reduced to small pieces and melted on a steam bath. Mineral oil was added, and the mixture was heated until the temperature reached 70°C to form the oil phase. On the other hand, sodium borate was dissolved in purified water, warmed to 70°C. The n-butanol fraction (2.5 g) of B. perennis in powder form was added to the aqueous phase to dissolve the extract, mixed and gradually added onto the melted oil phase. The two phases were stirred rapidly to obtain a homogeneous mixture of the phases yielding cold cream containing 2.5% (w/w) plant fraction.
Animal care
Adult male Wistar albino rats (200–250 g) were obtained from our laboratory colony maintained at the Abant Izzet Baysal University (AIBU). They were exposed from birth to 12 h of light, 12 h of darkness, lights off at 18:00 h. Animals were maintained in plastic cages (16 × 31 × 42 cm) with pine shavings used as bedding. Food pellets and tap water were accessible ad libitum. The procedures in this study were carried out in accordance with the Animal Scientific procedure and approved by the Institutional Animal care and Use Committee. All lighting was provided by cool-white fluorescent tubes controlled by automatic programmable timers. Ambient temperatures in the animal facilities were held constant at 22 ± 2°C in air-ventilated rooms.
Circular excision wound model
Twelve male Wistar albino rats having same age and weight were selected. Before surgery, the rats were anesthetized subcutaneously with ketamine (20 mg/kg, Sigma Chemical Company, St. Louis, Missouri, USA) and intraperitoneally with pentobarbital (32.5 mg/kg, Sigma Chemical Company, St. Louis, Missouri, USA). The depth of anesthesia was monitored by the frequent testing of leg reflexes and muscle tonus. The back hairs of the rats were depilated by careful shaving. The circular wound was created with a 4 mm punch biopsy by excising only the skin on the dorsal interscapular region of each animal and wounds were left open (CitationTramontina et al., 2002). Six wounds were created for each animal. The first two wounds were treated topically with the HOTBp (hydrophilic ointment treatment containing the n-butanol fraction of B. perennis). The second two wounds were control group and not treated with anything. The third two wounds were treated only with the HOT (hydrophilic ointment treatment without n-butanol fraction). Treatments were applied once a day and lasted for 30 days.
Estimation of wound healing (wound closure)
Curative effect on the wound (wound closure) was evaluated by tracing the outer margins of the wound on each rat. Wound areas were traced manually and calculated in square millimeters. Wound area was measured at 1st, 5th, 10th and 30th days after wounding and the wound closure rate was expressed as the percentage of wound area compared with that on post operative day (POD) (100%). The percentage of wound healing was calculated by Walker’s formula after measuring the wound area (CitationWalker & Mason, 1968). The percentage of wound healing was computed at the beginning of experiments and the next 5th, 10th, and 30th days.
Histopathology
The full thickness of skin specimens from each group were collected for histopathological examination. Samples were fixed in 10% buffered formalin, processed and embedded in paraffin, and then serially cut. The sections were stained with hematoxylin & eosin (H&E) and Gomori trichrome stains. Histological evaluation was done by a pathologist in a blind randomly numbered fashion. Re-epithelization, the formation of granulation tissue collogen deposition and connective tissue remodeling were analyzed. The amount of collagen was rated on a subjective scale of 0 to 3, with 0, representing no collagen; 1, little collagen; 2 moderate collagen; 3, abundant collagen. It was searched whether hypertrophic scar formation for the thirtieth day of the study.
Statistical analysis of the data
All data [3 treatments (control, HOT and HOTBp) × 3 days (5, 10, 30)] were analyzed by analysis of variance (ANOVA) with the last factor as a within subject or repeated design using SPSS version 15 (SPSS Inc., Chicago, IL, USA). Values were considered statistically significant at p ≤ 0.05. Data are presented as mean ± standard error (SE) after back transforming from ANOVA results. Kruskal-Wallis test for multiple comparisons and Mann-Whitney U test for binary comparisons were used for histopathological data.
Results and discussion
Although there are some studies representing the biological activities of B. perennis, there is no study about wound healing effect of this species. We, therefore, aimed to evaluate the vulnerary activity of B. perennis by an open wound procedure in rats.
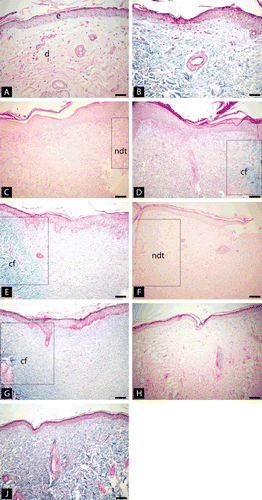
and illustrate the normal histological skin tissue samples at the beginning of the experiment. Same tissue properties were observed in all groups of histological samples at the 5th and the 10th days. Late phase granulation tissues which were characterized with the fibroblastic proliferation and scattered lymphocytes were existed in the wound at the fifth day (). The collagen fibers started to emerge slightly in the granulation tissue (). Collagen fibrils became more abundant at the tenth day (). In the thirtieth day, granulation tissues were converted into hypertrophic scar formations in all animals of the control and HOT groups ( and ). On the other hand, in HOTBp group, the hypertrophic fibrous scar tissues disappeared completely and an absolute amelioration was determined in all animals ( and ). The differences among groups at the 30th day were statistically significant (p = 0.004). Binary comparisons between HOTBp group and control group, and HOTBp and HOT group were also statistically significant (p = 0.008).
Figure 1. Histological sections of cutaneous wound site obtained from the controls, HOT and HOTBp lesions of the rats (A) normal histological skin tissue in the first day. (H&E, ×200) (B) normal histological skin tissue in the first day samples stained with Gomori trichrome stain (×200); (C) Late phase granulation tissues which were characterized with fibroblastic proliferation and scattered lymphocytes in a fifth day subject. Normal dermal tissue is seen at right corner [(rectangular) (Hematoxylin and eosin, ×100); (D) The collagen fibers started to emerge slightly in the granulation tissue in the 5th day samples (Gomori trichrome stain, ×100); (E) a lesion at a 10th day subject. Collagen fibrils is seen as more abundant than the 10th day sample (Gomori trichrome stain, ×100), (F) a sample of control lesion in the 30th day. Healing with fibrous scar formations is seen (Hematoxylin and eosin, ×100) (G) same tissue of F with Gomori trichrome stain (×100) (H, J) Near normal skin tissue without fibros scar tissue seen in a 30th day sample treated with Bellis perennis (Hematoxylin and eosin, Gomori trichrome stain, respectively, ×100). (e) epidermis, (d) dermis, (ndt) normal dermal tissue, (cf) collagen fibers.
represents the percentages of wound healing after ointment treatments in control, HOT and HOTBp groups. There was no statistically significant difference (p > 0.05) in the means of wound healing percentages between control and HOT animals. Wound healing percentage measurement showed a significant increment (p < 0.05) in the HOTBp group compared to the controls and HOT at the 30th day. Hydrophilic ointment loaded with B. perennis extract accelerated the wound healing processes at the 30th day of the experiment ().
Figure 2. Wound closure percentages of the control, HOT and HOTBp groups at the 5th, 10th and the 30th days of the experiment.
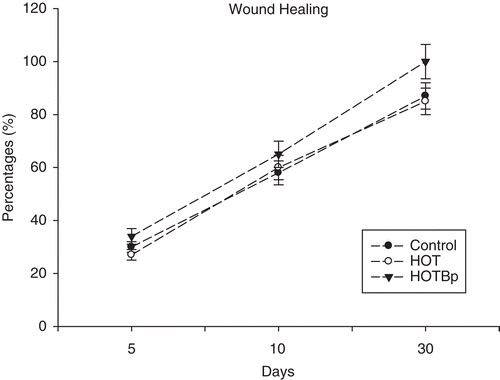
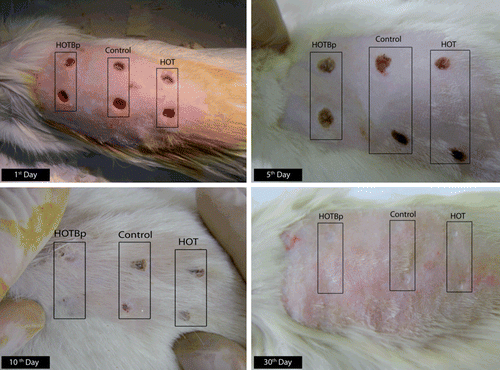
Figure 3. Photographs of the different rats in different days of the experiment. HOT: hydrophilic ointment treatment, HOTBp: hydrophilic ointment loaded with B. perennis fraction.
The inflammatory phase of the healing begins immediately after wounding and lasts approximately four days (CitationAlbritton, 1991). The proliferating phase begins toward the end of the inflammatory phase and lasts as long as three weeks. Collagen synthesis starts by the 3th to 5th day post injury (CitationCooper, 1990). In the present study, the 5th and the 10th day samples were similar in all groups when the inflammatory and proliferating phases took place. In the 5th day histopathology, a granulation tissue was existed and the collagen started to emerge. At the 10th day, collagen amount increased. On the other hand, the difference among the groups arises at the 30th day of the experiment when the remodeling phase was going on.
CitationKanzaki et al. (1998) investigated the accelerator effects of saponins on the extracellular matrix metabolism, the activation and synthesis of TGF-B1, and the modification of TGF-B receptor expressions in fibroblasts. They suggested that saponins stimulate the wound healing process through the changes of the extracellular matrix metabolism and is accompanied by modification of TGF receptor expressions in fibroblasts (CitationKanzaki et al., 1998). One of the main constituents of the B. perennis is triterpenoid saponins (CitationHiller et al., 1988; CitationSchopke et al., 1991; CitationLi et al., 2005; CitationMorikawa et al., 2008; CitationYoshikawa et al., 2008). Wound healing potential of B. perennis may be attributed to the saponins which might be an additive effect that accelerates the progress probably during the phases of wound healing.
Flavonoids are known to promote the rapid wound healing due to their antimicrobial, antioxidant, anti-inflammatory and astringent properties (CitationTsuchiya et al., 1996; CitationOkuda, 2005; CitationNayak et al., 2009). The flavonoid ingredient of B. perennis (CitationGudej & Nazaruk, 2001) may contribute to the vulnerary activity. Inhibition of lipid per oxidation effect by flavonoids, is supposed to increase the viability of collagen fibrils, by activating the DNA synthesis and preventing the cell damage (CitationShetty et al., 2008).
Complications in wound healing can arise from abnormalities in any of the basic components of the repair process. Immoderate formation of the components of the repair process is one of the most important complications in wound healing. The accumulation of excessive amounts of collagens may give rise to a raised scar known as a hypertrophic scar; if the scar tissue grows beyond the boundaries of the original wound and does not regress, it is called a keloid. Keloid formation appears to be an individual predisposition (CitationKumar et al., 2010). Hypertrophic scar or keloid is known to be difficult to treat, and prevention is the best approach (CitationSanders & Dickson, 1982).
Scar formation and remodeling to begin in the proliferating phase of repair with the collagen synthesis by fibroblasts. Fetal skin has the ability to heal without a scar. This scar less healing is highly attributed to the absence of acute inflammation and a lack of immune cell infiltration in the first and the second trimesters of the fetus (CitationRowlatt, 1979). Neutrophils, macrophages, T cells and B cells are all absent in scar less fetal wounds, and fetal platelets are less reactive (CitationAdolph et al., 1993; CitationHopkinson-Woolley et al., 1994). On the other hand, the fibrotic healing in the third trimester is attributed to the presence of inflammatory and immune cells (CitationCass et al., 1997). In the present study, B. perennis ointment administered group showed complete wound healing without the hypertrophic scar. However, HOT and control groups showed healing with hypertrophic fibrous scar. Complete wound healing with B. perennis may be attributed to its photochemical character which prevents excessive collagen synthesis and/or improved tissue remodeling formation. The infections are the most important cause of disordered healing. It results in persistent inflammation which may result in excessive collagen deposition. The antimicrobial (CitationDesevedavy et al., 1989; CitationAvato et al., 1997; CitationKavalcioglu et al., 2010) effects of the B. perennis may prevent the wound infection, the inflammation and the excessive collagen synthesis.
The remodeling begins at approximately 21 days post-injury and continues until 1 to 2 years after the injury (CitationCooper, 1990; CitationAlbritton, 1991; CitationCanter, 1991). Early in this phase, fibroblasts continue to produce collagen. The collagen bundles are synthesized during the proliferating phase and they are arranged into parallel position. Wound contraction and the ultimate strength of the healed wound is determined by the amount of collagen synthesis and the extent to which cross-linking has occurred between collagen bundles. In the present study, in HOTBp group, the hypertrophic fibrous scar tissue disappeared completely, and an absolute amelioration was recorded in all animals whereas, the collagenous amount was less and amelioration was not completed in the scar tissue appearance in control and HOT groups in the sections of the 13th day. The results of the present experiment have indicated that the data coming from the wound closure and the histopathology are consistent with to each other.
Conclusion
Although B. perennis (common daisy) is a vulnerary herb with a long history of use in folk medicine, there is no study in terms of the wound healing potential of this plant in a controlled laboratory experiment. Our findings demonstrate that the ointment including the n-butanol fraction of B. perennis flowers have been accelerating effect on the remodeling of the wound and this ointment may be applied on the wound in order to obtain a scar less wound healing. With this study, B. perennis gained a scientific justification as a vulnerary medicinal herb.
Declaration of interest
The authors report no conflicts of interest.
References
- Adolph VR, DiSanto SK, Bleacher JC, Dillon PW, Krummel TM. (1993). The potential role of the lymphocyte in fetal wound healing. J Pediatr Surg, 28, 1316–1320.
- Albritton JS. (1991). Complications of wound repair. Clin Podiatr Med Surg, 8, 773–785.
- Al-Douri NA, Al-Essa LY. (2010). A survey of plants used in Iraqi traditional medicine. J J Pharm Sci, 3, 100–8.
- Avato P, Vitali C, Mongelli P, Tava A. (1997). Antimicrobial activity of polyacetylenes from Bellis perennis and their synthetic derivatives. Planta Med, 63, 503–507.
- Baytop T. (1999). Therapy with Medicinal Plants in Turkey (Past and Present). Istanbul, Turkey: Nobel Medical Bookhouse.
- Cakılcıoglu U, Sengun MT, Turkoglu I. (2010). An ethnobotanical survey of medicinal plants of Yazıkonak and Yurtbası districts of Elazığ province, Turkey. J Med Plant Res, 4, 567–572.
- Canter KG. (1991). Conservative management of wounds. Clin Podiatr Med Surg, 8, 787–798.
- Cass DL, Bullard KM, Sylvester KG, Yang EY, Longaker MT, Adzick NS. (1997). Wound size and gestational age modulate scar formation in fetal wound repair. J Pediatr Surg, 32, 411–415.
- Clark RA. (1991). Growth factors and wound repair. J Cell Biochem, 46, 1–2.
- Cooper DM. (1990). Optimizing wound healing. A practice within nursing’s domain. Nurs Clin North Am, 25, 165–180.
- Davis PH. (1975). Flora of Turkey and the East Aegean Islands, Vol 5. Edinburgh: Edinburgh University Press.
- Desevedavy C, Amoros M, Girre L, Lavaud C, Massiot G. (1989). Antifungal agents: in vitro and in vivo antifungal extract from the common daisy, Bellis perennis. J Nat Prod, 52, 184–185.
- Duke JA, Bogenschutz-Godwin MJ, DuCellier J, Duke PA. (2002). Handbook of Medicinal Plants, 2nd ed. Boca Raton: CRC Press.
- Grieve M. (1982). A Modern Herbal, Vol 1. New York: Dover Publications.
- Gudej J, Nazaruk J. (2001). Flavonol glycosides from the flowers of Bellis perennis. Fitoterapia, 72, 839–840.
- Hanna JR, Giacopelli JA. (1997). A review of wound healing and wound dressing products. J Foot Ankle Surg, 36, 2–14; discussion 79.
- Hiller K, Schopke T, Wray V, Schulten HR. (1988). The structure of the major saponins from Bellis perennis L. Pharmazie, 43, 850–852.
- Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. (1994). Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci, 107 (Pt 5), 1159–1167.
- Kanzaki T, Morisaki N, Shiina R, Saito Y. (1998). Role of transforming growth factor-beta pathway in the mechanism of wound healing by saponin from Ginseng Radix rubra. Br J Pharmacol, 125, 255–262.
- Kavalcioglu N, Açik L, Demirci F, Demirci B, Demir H, Baser KH. (2010). Biological activities of Bellis perennis volatiles and extracts. Nat Prod Commun, 5, 147–150.
- Kumar V, Abbas AK, Fausto N, Aster JC. (2010). Robins and Cotran Pathologic Basis of Disease. Philadelphia: Saunders-Elsevier Inc.
- Leporatti ML, Ivancheva S. (2003). Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J Ethnopharmacol, 87, 123–142.
- Li W, Asada Y, Koike K, Nikaido T, Furuya T, Yoshikawa T. (2005). Bellisosides A–F, six novel acylated triterpenoid saponins from Bellis perennis (Compositae). Tetrahedron, 61, 2921–2929.
- Mitich LW. (1997). Engilish daisy (Bellis perennis L.). Weed Technol, 11, 626–628.
- Morikawa T, Li X, Nishida E, Ito Y, Matsuda H, Nakamura S, Muraoka O, Yoshikawa M. (2008). Perennisosides I-VII, acylated triterpene saponins with antihyperlipidemic activities from the flowers of Bellis perennis. J Nat Prod, 71, 828–835.
- Morikawa T, Muraoka O, Yoshikawa M. (2010). [Pharmaceutical food science: Search for anti-obese constituents from medicinal foods-anti-hyperlipidemic saponin constituents from the flowers of Bellis perennis]. Yakugaku Zasshi, 130, 673–678.
- Morikawa T, Li X, Nishida E, Nakamura S, Ninomiya K, Matsuda H, OdaY Muraoka, O, Yoshikawa M. (2010b). Medicinal flowers part 29 acylated oleanane-type triterpene bisdesmosides: perennisaponins G, H I, J, K, L, and M with pancreatic lipase inhibitory activity from the flowers of Bellis perennis. Helv Chim Acta, 93, 573–586.
- Nayak BS, Raju SS, Eversley M, Ramsubhag A. (2009). Evaluation of wound healing activity of Lantana camara L. – a preclinical study. Phytother Res, 23, 241–245.
- Oberbaum M, Galoyan N, Lerner-Geva L, Singer SR, Grisaru S, Shashar D, Samueloff A. (2005). The effect of the homeopathic remedies Arnica montana and Bellis perennis on mild postpartum bleeding – a randomized, double-blind, placebo-controlled study – preliminary results. Complement Ther Med, 13, 87–90.
- Okuda T. (2005). Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry, 66, 2012–2031.
- Panda H. (2004). Handbook on Medicinal Herbs with Uses. India: Asia Pacific Business Press.
- Rowlatt U. (1979). Intrauterine wound healing in a 20 week human fetus. Virchows Arch A Pathol Anat Histol, 381, 353–361.
- Sanders R, Dickson MG. (1982). BCG vaccination scars: an avoidable problem? Br Med J (Clin Res Ed), 285, 1679–1680.
- Schopke T, Wray V, Rzazewska B, Hiller K. (1991). Bellissaponin-BA1 and Bellissaponin-BA2 – acylated saponins from Bellis perennis. Phytochemitry, 30, 627–631.
- Shetty S, Udupa S, Udupa L. (2008). Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of Ocimum sanctum linn in rats. Evid Based Complement Alternat Med, 5, 95–101.
- Tramontina VA, Machado MAN, Kim SH, et al. (2002). Effect of fibrin glue (Tissucol) and fibrin glue prepaired from snake venom on palatal woundhealing in rabbits. J Dent Res, 81, 127.
- Tsuchiya T, Suzuki O, Igarashi K. (1996). Protective effects of chlorogenic acid on paraquat-induced oxidative stress in rats. Biosci Biotechnol Biochem, 60, 765–768.
- Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA. (1976). Flora Europaea, Vol. 4. Cambridge, Great Britain: Cambridge University Press.
- Uysal I, Onar S, Karabacak E, Celik S. (2010). Ethnobotanical aspects of Kapıdag Peninsula (Turkey). Biol Diver Conserv, 3, 15–22.
- Uzun E, Sariyar G, Adsersen A, Karakoc B, Otük G, Oktayoglu E, Pirildar S. (2004). Traditional medicine in Sakarya province (Turkey) and antimicrobial activities of selected species. J Ethnopharmacol, 95, 287–296.
- Yoshikawa M, Li X, Nishida E, Nakamura S, Matsuda H, Muraoka O, Morikawa T. (2008). Medicinal flowers. XXI. Structures of perennisaponins A, B, C, D, E, and F, acylated oleanane-type triterpene oligoglycosides, from the flowers of Bellis perennis. Chem Pharm Bull, 56, 559–568.
- Walker HL, Mason AD Jr. (1968). A standard animal burn. J Trauma, 8, 1049–1051.
