Abstract
Context: Sinomenium acutum (Thumb.) Rehd. et Wils. (Menispermaceae, SA) has been used as a traditional Chinese medicine in the treatment of various diseases for hundreds of years; it possesses favorable effects against autoimmune diseases, especially rheumatoid arthritis (RA). A great number of investigations have been done on SA in the last decade, but they are usually scattered across various publications.
Objective: The purpose of this article is to summarize and review the published scientific information about the chemical constituents, pharmacological effects, pharmacokinetics, and clinic applications of this plant since 2000.
Results: The information for 89 cases included in this review was compiled. The SA contains alkaloids, sterols, phospholipids, and some other components. A great deal of pharmacological and clinic research has been done on sinomenine, a main compound from SA, which mainly focuses on the immune system, cardiovascular system, and nervous system.
Conclusion: Previous studies strongly support its potential as an effective adaptogenic herbal remedy. There is no doubt that SA is being widely used now and will have extraordinary potential for the future.
Introduction
Sinomenium acutum (Thumb.) Rehd. et Wils. (Menispermaceae, SA) was first recorded as an herbal medicine in Ben-Cao-Gang-Mu (Compendium of Materia Medica). Its main active chemical component is sinomenine, and the pharmacological profile includes immunosuppression arthritis amelioration, anti-inflammation, and protection against hepatitis induced by lipopolysaccharide (LPS). With the increasingly extensive research, the intense interest has been focused on the applications and functions of SA. This paper introduced the chemical constitutions, the pharmacological effects, and the clinic applications for the further study and exploitation of SA.
Chemical constituents
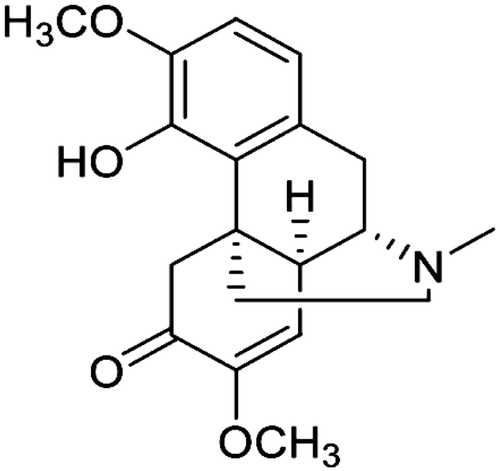
The chemical research has been conducted since last century and lays the foundation for the pharmacological research. Previous phytochemical studies demonstrate that SA contains alkaloids, sterols, lipids, and some other components. Recent research shows that it also contains phenols and terpenes. These compounds are listed in and the chemical structure of sinomenine is showed in .
Table 1. Compounds isolated from SA.
Pharmacological effects
The SA possesses various pharmacological activities due to its complex chemical compositions. The current investigations mainly focus on the immune system, cardiovascular system, and nervous system.
Effects on the immune system
Effects on mononuclear cells
Sinomenine, the main component of the plant, has been widely used in the treatment of autoimmune diseases, especially rheumatoid arthritis (RA). This therapeutic effect may be partially related to its impact on peripheral blood mononuclear cells (PBMC), as sinomenine can downregulate gene expression of IL-1β, IL-8 cytokines of the PBMC (CitationLiu et al., 2002). Sinomenine can reduce PGE2 production in LPS-stimulated human monocytes more than in nonstimulated human monocytes, suggesting that sinomenine selectively inhibits the activity of cyclooxygenas-2 (COX-2), which may be directly related to suppressing cyclooxygenase activity (CitationWang et al., 2003). Sinomenine can downregulate the synthesis of cytokines of mononuclear macrophage system, which may be its anti-inflammatory and antirheumatic mechanisms of action (CitationLi et al., 2004). Another study demonstrates that sinomenine can induce apoptosis of macrophages through activation of extracellular signal-regulated kinase (ERK), which contributes to its immunosuppressive function (CitationHe et al., 2005). Sinomenine is also observed to enhance the phagocytosis ability of macrophage through downregulating the expression of IL-6 and TNF-α in macrophages (CitationZou et al., 2008).
Effects on T cells
T cells, a kind of effector cell, can regulate the immune responses and plays an important role in the pathogenesis of autoimmune diseases. Sinomenine may play an immunosuppresive role in rat renal allograft models through inhibiting CD4+ T-cell proliferation and downregulating the levels of INF-γ, TNF-α (CitationWang et al., 2004). CitationLi et al. (2005) reported that sinomenine has the inhibitory effects on abnormal T lymphoctytes activation and the mechanism may mainly be related to the inhibitory effects on Ca2 +-dependant signal pathway of T lymphocytes activation but not protein kinase C (PKC) pathway. Sinomenine can keep the balance of CD4+/CD8+ ratio of T lymphocyte subtype and increase apoptosis ratio of spleen lymphocyte, which may be the mechanism of its immunological inhibitory effect (CitationLiu et al., 2005). Meanwhile, sinomenine suppresses the activation and proliferation of T lymphocytes by blocking the cells at G0/G1 phase, the mechanism which may be relevant to the downregulation of transferring receptor of T lymphocytes and the decrease of intake of iron ion by the cells (CitationChen et al., 2008), but it is uncertain whether the inhibition of proliferation of T lymphocytes is related to activation of ERK. Sinomenine has the potential to counter the shift in the Th1/Th2 balance and thereby produces therapeutic effects on mesangial proliferative nephritis (CitationCheng et al., 2009).
Effects on dendritic cells
Dendritic cells (DC) are the most powerful antigen-presenting cells, which have quite important effects upon the immune system. In addition to the effects mentioned above, sinomenine inhibits the antigen presenting function of DC by decreasing their nuclear factor-κB (NF-κB) activity involved in their maturation cascade and T-cell activation (CitationZhao et al., 2006, Citation2007). Sinomenine can regulate the host immunological status by preventing the maturity of DC, inhibiting the activity of its antigen presentation, and suppressing the secretion of its cytokine (CitationZhao et al., 2007). In vitro, sinomenine inhibits the level of IL-12 secreted by DC in dose-dependent manner (CitationXu & Zhou, 2007). Besides the effects on cytokines of DC, sinomenine in low dose can promote the differentiation of DC, but inhibit the maturation of imDC, weaken its antigen presenting activity in autoimmunnity diseases (CitationYang et al., 2007). The mRNA and protein expressions of CCR5 and CCR7 on the surface of the DC are also decreased by sinomenine. Similar results are observed in the expressions of CXCL9 (MIG) and CXCL10 (IP-10), but not in CXCL11 (ITAC) (CitationYu & Luo, 2009).
Effects on cytokines
Given the significant role of cytokines in inflammation, more attention has been aroused. Some investigators observed that sinomenine (50, 500 μmol/L) attenuates nuclear translocation of NF-κB p65 subunit and the DNA-binding activity of NF-κB of collagen-induced arthrit (CIA) rats in a concentration-dependent manner, which may be one of anti-inflammatory mechanisms (CitationHuang et al., 2007). Sinomenine, the main active constituent of SA, can inhibit the production of IL-1β and TNF-α in the synovial cells reduce the expression of IL-1β and TNF-α mRNA and recover the normal histological features of synovial cells in adjuvant arthritis rats (CitationChen et al., 2008). One of the possible mechanisms of curative effect of sinomenine on arthritis is that it relieves the erosion of the articular by inhibiting the expression of CYR61 (CitationSun et al., 2009). Sinomenine can significantly reduce the level of IL-6. Moreover, it has a synergistic effect in combination with Cyclosprin A (CsA) (CitationLiao et al., 2009). Sinomenine also reduces the cerebral ischemia-reperfusion injury by decreasing the expression of P-selectin and ICAM–1-mediated inflammatory reaction in diabetic rats (Zh CitationOu et al., 2009).
In addition, sinomenine suppresses the production of proinflammatory cytokines IL-1β and IL-6 in serum, inhibits protein expression and activities of MMP-2 and MMP-9, and elevates protein expression and activities of TIMP-1 and TIMP-3 in rat paw tissues (CitationZhou et al., 2008). The levels of CD147, MMP-2, and MMP-9 of A-THP-1 cells are markedly downregulated at the most notable concentrations of 0.25 mmol/L and 1.00 mmol/L (p < 0.01) of sinomenine (CitationOu et al., 2009). These results suggest a possible mechanism of the inhibitory effect of sinomenine on cell invasion and migration ability. Recent study shows that sinomenine can suppress IL-1β-induced mRNA and protein expressions of MMP-1, MMP-3, MMP-9, and MMP-13 in SW1353 cells and human osteoarthritic (OA) chondrocytes (CitationHuang et al., 2010), suggesting that sinomenine may act as an agent for pharmacological intervention in the process of OA. Heme oxygenase-1 (HO-1), a rate-limiting enzyme that oxidizes heme to biliverdin and carbon monoxide, has been proved to have anti-inflammatory properties in multiple inflammatory responses (Tranter & Jones, 2010). CitationSong et al. (2009) investigated the effect of sinomenine on HO-1 induction and its hepatocellular protective effect. The result showed that sinomenine pretreatment is able to induce HO-1 expression in donor livers in a dose-dependent manner. The research on the effects of sinomenine on the bone marrow-derived mast cell (BMMC) by Oh et al. reveals that sinomenine inhibits the PMA plus A23187-induced production of IL-6, PGD(2), LTC4, β-Hex, and COX-2 protein (CitationOh et al., 2011), indicating its potential for the treatment of allergy.
Effects on the cardiovascular system
Effects on decompression
Previous investigations demonstrate not only the total alkali of SA but also that sinomenine has antianginal effects on decompression. Intravenous injection or oral application of the total alkaloid from SA shows hypotensive effects in normal rats, dogs, anesthesia cats, and chronic renal hypertension dogs. Sinomenine can produce the same effect. It is speculated that the anti-adrenaline, the blocking ganglion effect, and the inhibition of central pressor reflex may contribute to its anti-hypertensive behavior. Sinomenine markedly inhibits vascular smooth muscle cell (VSMC) proliferation and DNA synthesis in the dose-dependent manner (CitationLi et al., 2000). Sinomenine in combination with low-dose T cell-targeted immunosuppression is associated with inhibition of intragraft expression of mediators involved in angiogenesis, vascular tone, and tissue remodeling (CitationMark et al., 2003). The possible mechanism of the vasorelaxation caused by sinomenine is the inhibitions of Ca2+ channel and PK-C activity and the activations of NO and prostaglandin (PG) I2 syntheses in endothelium (CitationNishida & Satoh, 2006). The vasorelaxation is also related to the decreasing of Ca2+ caused by the opening of ATP-sensitive K+ channels (CitationLee et al., 2007).
Effects on arrhythmia
The main constituent of SA, sinomenine, shows significant antagonism against arrhythmia induced by various experimental factors. Sinomenine can shorten the arrhythmia period induced by picrotoxin in rabbit and protect rat against arrhythmia induced by BaCl2. Sinomenine is found to recover the arrhythmia induced by BaCl2 -Ach into sinus rhythm in mice. In addition, sinomenine also revealed significant antagonism against ischemic arrhythmia (Sun et al., 1990). Furthermore, sinomenine blocks INa and ICa-L in a concentration-dependant manner and probably inhibits INa in its inactive state, which may contribute to sinomenine’s anti-arrhythmic effect (CitationDing et al., 2000).
Effects on the nervous system
Effects on the central nervous system
Sinomenine has been proved to have analgesic, sedative, and anxiolytic effects since the 1960s. Whatever method is used, such as body-torsion, hot-plate method, or electrical stimulation procedure, sinomenine showed analgesic effects on laboratory mice. The mechanism may be that sinomenine can inhibit the synthesis and release of PGE. Other effective alkaloid components in SA have similar pharmacological activity. Sinoacutine can increase the mouse pain threshold induced by hot plate or electrical stimulation of the toes, and reduce the writhing times caused by glacial acetic acid. It can also enhance cooperatively pentobarbital-induced hypnosis and sedation in mice.
Effects on neurotransmitters
Sinomenine has positive effects on morphine-dependent rats, which may be relevant to monoamines neurotransmitter regulation disorders. The rats given increasing doses of morphine will produce a physical dependence. Sinomenine can significantly inhibit withdrawal syndrome in morphine-dependent mice and relieve body weight loss of mice, which may be related to modulating neurotransmitters (CitationWang et al., 2002). Sinomenine can inhibit the withdrawal contracture of in vitro ileum from morphine-dependent guinea pigs (CitationHu et al., 2003) and has an effect on the NO/nNOS system in the cerebellum and spinal cord, which may contribute to its alleviation of morphine-withdrawal symptoms (CitationLiu et al., 2007). Sinomenine treatment can regulate the mRNA expressions of HO2 and soluble guanylyl cyclase-α-1 subunit (sGC-α-1) genes, which may be one of the molecular mechanisms of its action on the morphine-dependent mice (CitationZhen et al., 2008). Alcohol extracts from SA and sinomenine can decrease the concentration of the neurotransmitters and elevate the intracellular calcium level and inhibit the decrease of Ca2+ induced by naloxone1 (CitationZhang et al., 2009).
Sinomenine exhibits an anxiolytic-like effect (CitationChen et al., 2005), and Qingfeng capsule, a SA extract, also has a significant effect against anxiety of morphine-dependent rats (CitationGao et al., 2007). Sinomenine can inhibit the microglial NADPH oxidase (PHOX) activity, suggesting a novel therapy to treat inflammation-mediated neurodegenerative diseases (CitationQian et al., 2007).
CitationMo et al. (2004) reported that the SA and sinomenine can suppress the acquisition and expression of place preference induced by morphine via the decrease of cAMP level in the brain. The formation and relapse of morphine dependence may cause the changes of neurosteroids in rat frontal cortex (Fc) and hippocampus (Hc) (CitationWang et al., 2006). Besides, the SA and sinomenine markedly decrease the content of histamine (HA) in mouse brain (CitationMo & Zhou, 2006). The study of the conditioned place preference (CPP) in mice shows that the SA and sinomenine result in significantly reduced time of stay in morphine-paired compartment and brain HA level (p < 0.01) (CitationMo et al., 2006). Furthermore, the SA extract can inhibit dopamine transporter (DAT)/norepinephrine transporter (NET) activators and/or serotonin transporter (SERT), and improve neuropsychological disorders possibly through regulation of monoamine transporters (CitationZhao et al., 2009). The SA extract reveals a therapeutic effect on CA1 hippocampal neurons in morphine withdrawal mice (CitationZhu et al., 2009).
Other effects
Besides the effects mentioned above, sinomenine plays a significant role in the treatment of hyperosteogeny, inhibition of smooth muscle, and promotion of gastrointestinal motility. Sinomenine can significantly inhibit the proliferation response of splenocytes induced by MBP(68-82), and TNF-α and IFN-γ, secreted by splenocytes induced by MBP(68-82), are also inhibited by sinomenine in a dose-dependent manner, which support the presumption that sinomenine may be a promising new therapeutic intervention in multiple sclerosis (MS) (CitationZeng et al., 2007).
Besides, opioid μ-receptor (OMR) is a primary target site for analgesics, and drugs of abuse and receptor phosphorylation is thought to be a pivotal initial event in agonist regulation of the OMR (CitationJohnson et al., 2005). CitationWang et al. (2008) found that sinomenine has an ability to activate OMR, implicating the potential of sinomenine to be applied in clinic. There is data analysis suggesting that sinomenine significantly promotes apoptosis of human lung cancer NCI-H460 cells through the mitochondrial pathway (CitationJiang et al., 2010).
Pharmacokinetics
Many studies have investigated the pharmacokinetics of sinomenine. It has been confirmed that sinomenine hydrochloride (SM·HCl) is an effective treatment for RA. A 24 h SM·HCl sustained release preparation (once daily) is developed, which can prolong the absorption phase and reduce the degree of concentration fluctuation in vivo by managing the drug release characteristics of dosage forms in vitro (CitationSun et al., 2005). The SM·HCl (90 mg/kg) significantly improves the bioavailability of paeoniflorin in rats (CitationLiu et al., 2005a), while coadministration of paeoniflorin does not affect the pharmacokinetic parameters and tissue distribution of sinomenine (CitationLiu et al., 2005b). The mechanism is suggested that sinomenine decreases the efflux transport of paeoniflorin by P-glycoprotein (CitationChan et al., 2006).
The investigations on the metabolism and excretion of sinomenine have attracted a lot of interest. The pharmacokinetics and tissue distribution investigations of sinomenine in rats indicate that the liver and kidneys may be the main organs of metabolism and elimination of sinomenine, and sinomenine has a potent binding ability with albumin (CitationLiu et al., 2005c). Demethyl-sinomenine and hydroxylated-sinomenine, two metabolites of sinomenine by phase I metabolic pathway, are found in human plasma (Yao et al., 2005). Three major urinary metabolites of sinomenine are obtained in rats after intragastric administration (CitationChen et al., 2007). Two unknown substances, supposed to be the demethyl and hydroxylated derivatives of sinomenine, are found in skin microdialysates of the rats administered sinomenine (CitationZhen et al., 2007). These investigations indicate that sinomenine can be metabolized by phase I metabolic enzymes not only in liver or intestine but also in skin. The microdialysis study about dynamic determination of sinomenine in skin has proved it (CitationLing et al., 2008).
Cytochrome P450s plays an important role in the biotransformation of many endogenous and exogenous substances. Sinomenine (50 μmol/L) is able to inhibit the activity of CYP2C19 in human microsomes but enhance the elimination of mephenytoin at a normal clinical dose in vivo (CitationYao et al., 2007). Topical bioequivalence of liposome gelpatch of sinomenine is higher than that of normal gelpatch of sinomenine (CitationLing et al., 2008). The experimental studies using beagle dog models demonstrate that the absolute bioavailability of sinomenine is low, and the elimination of sinomenine tablet is fast (CitationChen et al., 2009). After the quantitative measurement of sinomenine, CitationLong et al. (2010) found that the numerical values are linear over the concentration range of 0.1–100 μg/mL for sinomenine in plasma and over the range of 0.01–5.00 μg/g for sinomenine in brain tissue. The extraction and recovery of sinomenine from plasma and brain tissue are 72.48–80.26% and 73.75–80.26%, respectively. Sinomenine has a neuroprotective effect on H2O2-induced injury in PC12 cells in vitro. Sinomenine HCl can also increase absorption of some drugs (such as cimetidine, vitamin C, rutin, luteolin, and insulin) across the intestinal epithelium by means of one or more mechanisms, including a transient opening of the tight junctions to allow for paracellular transport and/or inhibition of active drug efflux transport (CitationLu et al., 2010).
Clinical applications
Treatment of RA
The treatment of RA is one of the major clinical applications of sinomenine. The combination of SA and tripterygium glycosides (TG) can reduce adverse reactions of TG in the clinical treatment of RA. The main alkaloid constituents of the plant combined with methotrexate can decrease the dose of the latter and increase the appropriateness when treating RA (CitationLao, 2000). The hydrochloride preparation of sinomenine has been applied in clinic in China and Japan. It is observed that sinomenine has a good therapeutical effect on RA. The significant effects of sinomenine on the indexes of erythrocyte sedimentation rate (ESR), rheumatoid factors (RF), IgA, and IgM are also observed. These data suggest that this preparation shows higher efficacy and low side effects with total effective rate of 91.52% (CitationYang & Yang, 2003). Zhengqing Fengtongning retard tablets, whose active principle is SM·HCl, can decrease the indexes of ESR and RF dramatically, whose therapeutic effect may be equal to methotrexate (CitationLiu et al., 2006). When combined with small dose methotrexate, Zhengqing Fengtongning retard tablets shows a similar treatment effect but significantly less side effect in comparison with the use of normal dose methotrexate alone (CitationHuang & Wang, 2010). It shows the better effects on the ERS, RF, RP, IgG, and IsA of patients in the treatment group (received sinomenine) than in the control group (received TG tablets), suggesting that sinomenine has better therapeutic effect for RA than TGs (CitationHuang et al., 2007). Compared with nonsteroidal anti-inflammatory drugs (NSAIDs), sinomenine is more effective in amelioration of morning stiffness (p < 0.00001), painful joints (p = 0.03), and erythrocyte sedimentation rate (p < 0.00001) with less adverse events of the digestive system and similar adverse events of the nervous system. These results indicate that sinomenine is a valuable remedy to treat RA clinically (CitationXu et al., 2008). Sinomenine can work as both NSAID and disease-modifying antirheumatic drug (CitationLin et al., 2009).
Treatment of glomerular diseases
Glomerular disease is a common disease in the clinic, which is the main factor accounting for chronic renal failure (CRF). It has been described that sinomenine preparation can decrease not only proteinuria excretion but also hematuria, and the negative effects are significantly less than TG tablet. The therapeutic effects may be associated with the immunodepression, anti-inflammatory action, and anticoagulation of sinomenine. A clinical trial was carried out involving 28 patients with glomerulonephritis who were treated with sinomenine preparation for 3 months. Results display proteinuria (TUPr) is decreased significantly (p < 0.01) and plasma albumin (ALB) is increased (p < 0.05). The occurrence rate of adverse reactions is 14.28% for the sinomenine group and 50% for the Tripterygium wilfordii Hook. F. (Celastraceae) group, indicating a statistical difference (p < 0.05) (CitationSun et al., 2007).
Other diseases treatment
Sinomenine has a certain therapeutic effect on some organic atrial or ventricular arrhythmia, especially on otherwise untreatable arrhythmia. In addition, sinomenine is widely used in the treatment of various other diseases (CitationLi et al., 2009), such as hyperosteogeny, ankylosing spondylitis, Bi syndrome, systemic lupus erythematosus, and the detoxification of heroin addicts.
Conclusion
The SA has been utilized to prevent and treat various diseases especially RA in Chinese medicine for over hundreds of years. Sinomenine, a natural compound from SA has shown the immunosuppressive, analgesic, sedative, and anxiolytic-like effects. With relatively few side effects and favorable therapeutical effects, SA has been used in the treatment of RA, glomerular diseases, and ventricular arrhythmia in clinical trials.
However, further studies are required for SA development. The chemical constituents are still not complete. Although there are many investigations on sinomenine, other known numerous compounds hardly investigate on their pharmacological effects and clinical applications. In addition, few molecular mechanisms are known and the definitive target protein bound by sinomenine still remains undetermined. Moreover, toxicological data are imperfect, which has an adverse effect on the clinical applications of SA.
Many of studies have suggested the potential to be an effective herbal remedy of SA. The investigations about the effects of SA on the immune system, cardiovascular system, and nervous system bring great benefits to human health. Nonetheless, the authors are looking forward to seeing further research of this extremely potential therapeutic agent.
Declaration of interest
This work was supported by the National Natural Science Foundation of China (No. 81173462) and the Open Research Fund of State Key Laboratory Breeding Base of Systematic Research, Development and Utilization of Chinese Medicine Resources.
References
- Bao GH, Qin GW, Wang R, Tang XC. (2005). Morphinane alkaloids with cell protective effects from Sinomenium acutum. J Nat Prod, 68, 1128–1130.
- Bao GH, Wang XL, Tang XC, Chiu P, Qin GW. (2009). Sinoracutine, a novel skeletal alkaloid with cell-protective effects from Sinomenium acutum. Tet Lett, 50, 4375–4377.
- Ban XH, Huang ZY, Li Y, Zhou L, Zahng JM, Yang XS, Zhang YH. (2008). Studies on the chemical constituents of Sinomenium acutum. Lishizhen Med Mat Med Res, 19, 1831–1832.
- Chan K, Liu ZQ, Jiang ZH, Zhou H, Wong YF, Xu HX, Liu L. (2006). The effects of sinomenine on intestinal absorption of paeoniflorin by the everted rat gut sac model. J Ethnopharmacol, 103, 425–432.
- Chen WM, Qiu F, Wu LJ, Yao XS. (2005). A new alkaloid from Sinomenium acutum. Chinese Chem Lett, 16, 1481–1483.
- Chen GX, Li XJ, Liu QP Liu XL, Huang KE Chen JF. (2008). Effect of sinomenine on activation and proliferation of T lymphocytes. J Guangzhou Univ TCM, 25, 425–428.
- Chen FJ, Ye L, Hu W, Zhang RY, Chen YH. (2008). Effect of sinomenine on immune function of adjuvant arthritis rats. J Guangzhou Univ TCM, 25, 421–424.
- Chen SW, Mi XJ, Wang R, Wang WJ, Kong WX, Zhang YJ, Li YL. (2005). Behavioral effects of sinomenine in murine models of anxiety. Life Sci, 78, 232–238.
- Cheng Y, Zhang J, Hou W, Wang D, Li F, Zhang Y, Yuan F. (2009). Immunoregulatory effects of sinomenine on the T-bet/GATA-3 ratio and Th1/Th2 cytokine balance in the treatment of mesangial proliferative nephritis. Int Immunopharmacol, 9, 894–899.
- Cheng WM, Qiu F, Yao XS. (2007). Three major urinary metabolites of sinomenine in rats. J Asian Nat Prod Res, 9, 13–18.
- Chen W, Zhou Y, Kang J, Li Q, Feng X. (2009). Study on pharmacokinetics and absolute bioavailability of sinomenine in beagle dogs. Zhongguo Zhong Yao Za Zhi, 34, 468–471.
- Ding ZR, Li GS, Jiang XJ, Xu JL, Wang T. (2000). The effect of sinomenine on sodium, L-type calcium current in guinea pig ventricular myocytes. Chinese J Cardiac Pacing Electrophysiol, 14, 39–41.
- Gao XL, Mo ZX, Qiu YH. (2007). Effects of QingFengTeng capsule on anxious behavior in morphine dependent rats. Chin J Drug Depend, 16, 430–434.
- He X, Wang J, Guo Z, Liu Q, Chen T, Wang X, Cao X. (2005). Requirement for ERK activation in sinomenine-induced apoptosis of macrophages. Immunol Lett, 98, 91–96.
- Huang ZY, Zhang YH, Zhou L, Li Y, Yang XS. (2009). Studies on the chemical constituents of Sinomenium acutum (‖). Chin Tradit Herb Drugs, 40, 193–196.
- Huang XL, Hao F, Wang Y, Fang YF. (2007). Inhibition of sinomenine on nuclear factor-κB of synoviocytes in collagen-induced arthritis rats. Acta Acad Med Mil Tert, 29, 1269–1272.
- Hu XD, LI FY. (2003). Effects of sinomenine on morphine withdrawal syndromes in mice rats and guinea pig ileum. Chin J Drug Depend, 12, 28–31.
- Huang BD, He AS, Fu M, Sheng PY, Liao WM. (2010). Sinomenine suppresses expression of interleukin-1beta-induced matrix metalloproteinases in human osteoarthritic chondrocytes. J Med Plants Res, 4, 1830–1836.
- Huang ZS, Wang N. (2010). Clinical observation on treatment of rheumatoid arthritis with Zhengqing Fengtongning Retard Tablets. Hebei Med J, 32, 59–60.
- Huang GD, Li JB, Huang YH, Tang LJ, Zhang S, Li Q. (2007). The Clinical study on sinomenine for 100 patients with rheumatoid arthritis. JETCM, 16, 416–417.
- Jiang T, Zhou L, Zhang W, Qu D, Xu X, Yang Y, Li S. (2010). Effects of sinomenine on proliferation and apoptosis in human lung cancer cell line NCI-H460 in vitro. Mol Med Report, 3, 51–56.
- Jin HZ, Mao YS, Zhou TX, Wang R, Tang XC, Qin GW. (2007). Alkaloids from stems of Sinomenium acutum. Chin J Nat Med, 5, 35–37.
- Jin HZ, Wang XL, Wang HB, Wang YB, Lin LP, Ding J, Qin GW. (2008). Morphinane alkaloid dimers from Sinomenium acutum. J Nat Prod, 71, 127–129.
- Johnson EE, Christie MJ, Connor M. (2005). The role of opioid receptor phosphorylation and trafficking in adaptations to persistent opioid treatment. Neurosignals, 14, 290–302.
- Kato A, Yasui M, Yano N, Kawata Y, Moriki K, Adachi I, Hollinshead J, Nash RJ. (2009). Alkaloids inhibiting l-histidine decarboxylase from Sinomenium acutum. Phytochemistry Letters, 2, 77–80.
- Lao ZY. (2000). Sinomenine combined with methotrexate in treating rheumatoid arthritis. Chin J New Drugs Clin Rem, 19, 254–256.
- Lee PY, Chen W, Liu IM, Cheng JT. (2007). Vasodilatation induced by sinomenine lowers blood pressure in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol, 34, 979–984.
- Li HB. (2006). Studies on the non-alkaloid constituents of Sinomenium acutum. J Guiyang Med Coll, 31, 154–155.
- Li XJ, Wang PX, Liu L, Wang WJ, Zhou L, Liang RY, Cao LY. (2004). Anti-inflammatory and antirheumatic mechanism of sinomenine. J Guangzhou Univ TCM, 21, 34–36.
- Li XJ, Wang PX, Liu L, Chen GX, Zhao JX, Zeng YY, Chen JP. (2005). Effects of sinomenine on cell activation and intracellular Th1 type cytokines expression of T lymphocytes. Chin J Immunol, 20, 249–258.
- Li L. (2000). Study of prevention and control of primary hypertension. Zhonghua Liu Xing Bing Xue Za Zhi, 21, 165–166.
- Li JQ, Luo R, Ge XY, Li YP, Zhao XS. (2009). Modified Zhizhu decoction in the treatment of functional constipation. J Anhui TCM Coll, 28, 11–13.
- Liao DH, Chen Z, Luo ZG, Wang Y, Pan GH, Li GH, Fang JL, Xu L, Du YC. (2009). Effect of alkaloid sinomenine on interleukin-6 levels in venous blood of rat renal allograft models. CRTER, 13, 8693–8696.
- Lin XJ, Cai XY, Ye JH. (2009). The clinical observation on sinomenine for rheumatoid arthritis. J Hunan Univ TCM, 29, 52–54.
- Ling JJ, Wang Y, Xie B, Zhou LL. (2008). Bioequivalence study of sinomenine patch by microdialysis. China J Chin Mat Med, 33, 2482–2485.
- Ling JJ, Xie B, Wang Y, Zhou LL. (2008). Dynamic determination of sinomenine in skin by microdialysis. J Chin Med Mater, 31, 1062–1065.
- Liu L, Li XJ, Wang PX, Wang WJ, Zhou L, Liang RY, Cao LY. (2002). Effects of sinomenine on IL-1β, IL-8 gene expression of human peripheral blood. Chin J Immunol, 18, 241–244.
- Liu JH, Li WD, Teng HL, Lin ZB. (2005). Immunopharmacological action of sinomenine, an alkaloid isolated from Sinomenium acutum, and its mechanism of action in treating rheumatoid arthritis. Acta Pharm Sin, 40, 127–131.
- Liu Z, Zheng Jf Yang, LQ, Yi L, Hu B. (2007). Effects of sinomenine on NO/nNOS system in cerebellum and spinal cord of morphine dependent and withdrawal mice. Acta Physiolog Sin, 5, 285–292.
- Liu ZQ, Zhou H, Liu L, Jiang ZH, Wong YF, Xie Y, Cai X, Xu HX, Chan K. (2005a). Influence of co-administrated sinomenine on pharmacokinetic fate of paeoniflorin in unrestrained conscious rats. J Ethnopharmacol, 99, 61–67.
- Liu ZQ, Jiang ZH, Chan K, Zhou H, Wong YF, Bian ZX, Xu HX, Liu L. (2005). Pharmacokinetic interaction of paeoniflorin and sinomenine: pharmacokinetic parameters and tissue distribution characteristics in rats and protein binding ability in vitro. J Pharmacol Sci, 99, 381–391.
- Liu ZQ, Chan K, Zhou H, Jiang ZH, Wong YF, Xu HX, Liu L. (2005). The pharmacokinetics and tissue distribution of sinomenine in rats and its protein binding ability in vitro. Life Sci, 77, 3197–3209.
- Liu W, Liu XY, Liu B. (2006). Clinical observation on treatment of rheumatoid arthritis with Zhengqing Fengtongning retard tablets: A report of 60 cases. J Chin Integrative Med, 4, 201–202.
- Long LH, Wu PF, Chen XL, Zhang Z, Chen Y, Li YY, Jin Y, Chen JG, Wang F. (2010). HPLC and LC-MS analysis of sinomenine and its application in pharmacokinetic studies in rats. Acta Pharmacol Sin, 31, 1508–1514.
- Lu ZL, Chen WY, Viljoen AH. Hamman J. (2010). Effect of sinomenine on the in vitro intestinal epithelial transport of selected compounds. Phytother Res, 24, 211–218.
- Mark W, Schneeberger S, Seiler R, Stroka DM, Amberger A, Offner F, Candinas D, Margreiter R. (2003). Sinomenine blocks tissue remodeling in a rat model of chronic cardiac allograft rejection. Transplantation, 75, 940–945.
- Min YD, Choi SU, Lee KR. (2006). Aporphine alkaloids and their reversal activity of multidrug resistance (MDR) from the stems and rhizomes of Sinomenium acutum. Arch Pharm Res, 29, 627–632.
- Mo ZX, Liang RN, Wang CY. (2004). Effects of Caulis sinomenii and sinomenine on morphine-induced place preference and cyclic AMP level in mice. Chin JMAP, 21, 87–90.
- Mo ZX, Zhou JY. (2006). Effects of Caulis sinomenii and sinomenine on brain histamine level in place preference mice. Pharmacol Clin Chin Mater Med, 22, 20–22.
- Mo ZX, An SL, Zhou JY. (2006). Effects of Caulis sinomenii and sinomenine on morphine-induced place preference and brain histamine level in mice. J Southern Med Univ, 26, 1709–1713.
- Nishida S, Satoh H. (2006). In vitro pharmacological actions of sinomenine on the smooth muscle and the endothelial cell activity in rat aorta. Life Sci, 79, 1203–1206.
- Oh YC, Kang OH, Choi JG, Brice OO, Lee YS, Keum JH, Kim SB, Shin DW, Ma J, Jeong GH, Kwon DY. (2011). Anti-allergic effects of sinomenine by inhibition of prostaglandin D2 and leukotriene C4 in mouse bone marrow-derived mast cells. Immunopharmacol Immunotoxicol, 33, 266–270.
- Ou YQ, Chen LH, Li XJ, Lin ZB, Li WD. (2009). Sinomenine influences capacity for invasion and migration in activated human monocytic THP-1 cells by inhibiting the expression of MMP-2, MMP-9, and CD147. Acta Pharmacol Sin, 30, 435–441.
- Qian L, Xu Z, Zhang W, Wilson B, Hong JS, Flood PM. (2007). Sinomenine, a natural dextrorotatory morphinan analog, is anti-inflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J Neuroinflammation, 4, 23.
- Shaw CY, Chen CH, Hsu CC, Chen CC, Tsai YC. (2003). Antioxidant properties of scopoletin isolated from Sinomonium acutum. Phytother Res, 17, 823–825.
- Song YB, Chen WM, Qu GX, Qiu F. (2007). Chemical constituents of Sinomenium acutum. Shenyang Pharm Univ, 24, 79–80.
- Song SH, Zhang YH, Huang ZY. (2011). Study on the alkaloids of Sinomenium acutum. Lishizhen Med Mat Med Res, 22, 327–328.
- Song SH, Shen XY, Tang Y, Wang ZX, Guo WY, Ding GS, Wang QX, Fu ZR. (2009). Sinomenine pretreatment attenuates cold ischemia/reperfusion injury in rats: The role of heme oxygenase-1. Int Immunopharmacol, 10, 679–684.
- Sun Y, Ou Yang HL, Zhang YL, Gao F, He Y, Ji WQ, Huang Z, Hu JL, Li NL, Xiao LB. (2009). Effect of low molecular weight sinomenine on collage induced arthritis model in rat. J Hunan Univ TCM, 29, 41–45.
- Sun J, Shi JM, Zhang TH, Gao K, Mao JJ, Li B, Sun YH, He ZG. (2005). Impact of release characteristics of sinomenine hydrochloride dosage forms on its pharmacokinetics in beagle dogs. World J Gastroenterol, 11, 4547–4551.
- Sun JP, Gao YX, Dong H. (2007). Clinical observation on treatment of glomerulonephritis with sinomenine preparation: A report of 28 cases. Clinical Focus, 22, 1194–1195.
- Tranter M, Jones WK. (2008). Anti-inflammatory effects of HO-1 activity in vascular endothelial cells, commentary on “Carbon monoxide donors or heme oxygenase (HO-1) overexpression blocks interleukin-18-mediated NF-kappaB-PTEN-dependent human cardiac endothelial cell death”. Free Radic Biol Med, 44, 261–263.
- Wang X, Jin H, Li Z, Qin G. (2007). 8-Demethoxyrunanine from Sinomenium acutum. Fitoterapia, 78, 593–595.
- Wang XL, Liu BR, Wang JR, Chen CK, Qin GW, Lee SS. (2011). Two new morphinane alkaloids from Sinomenium acutum. J Asian Nat Prod Res, 13, 523–528.
- Wang WJ, Wang PX, Li XJ. (2003). The effect of sinomenine on cyclooxygenase activity and the expression of COX-1 and COX-2 mRNA in human peripheral monocytes. China J Chin Mat Med, 28, 352–355.
- Wang Y, Chen Z, Xiong L, Luo ZG, Qin GQ, Li JJ. (2004). Effects of the alkaloid sinomenine on T cell proliferation and acute rejection in rat renal allografts. Chin J Exp Surg, 21, 573–574.
- Wang CY, Mo ZX, Zhu QS, Wen L. (2002). Effects of sinomenine on withdrawal syndrome and neurotransmitter of morphine-dependent rats. J Chin Med Mater, 25, 337–339.
- Wang ND, Xue LQ, Xu DJ, Yuan AW, Deng ZB, Cui SL. (2006). Cloning and analysis of phage Fab antibodies of mouse male specific antigen. Sheng Wu Gong Cheng Xue Bao, 22, 727–732.
- Wang MH, Chang CK, Cheng JH, Wu HT, Li YX, Cheng JT. (2008). Activation of opioid mu-receptor by sinomenine in cell and mice. Neurosci Lett, 443, 209–212.
- Xu DH, Zhou CH. (2007). Effect of different doses of sinomenine on CD80 and CD86 expressions and extracellular interleukin-1 2 secretion in dendritic cells. CRTER, 11, 5654–5656.
- Xu M, Liu L, Qi C, Deng B, Cai X. (2008). Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Planta Med, 74, 1423–1429.
- Yao YM, Cao W, Cao YJ, Cheng ZN, Ou-Yang DS, Liu ZQ, Zhou HH. (2007). Effect of sinomenine on human cytochrome P450 activity. Clin Chim Acta, 379, 113–118.
- Yang CY, Cheng YW, Fu XL, Fei L, Jin NS, Xie CY, Tang YY, Wu YZ. (2007). Immunological J, 23, 265–269.
- Yang XH, Yang JY. (2003). Clinical observation on treatment of rheumatoid arthritis with Zhengqing Fengtongning Retard Tablets: A report of 60 cases. Chin J Int Med, 40, 297–298.
- Yu KQ, Luo R. (2009). Effect of sinomenine on the expression of chemokines and chemokine receptors in dendritic cells from patients with rheumatoid arthritis. Nan Fang Yi Ke Da Xue Xue Bao, 29, 635–637.
- Zeng Y, Gu B, Ji X, Ding X, Song C, Wu F. (2007). Sinomenine, an antirheumatic alkaloid, ameliorates clinical signs of disease in the Lewis rat model of acute experimental autoimmune encephalolmyelitis. Biol Pharm Bull, 30, 1438–1444.
- Zhang GM, Mo ZX, Wang CY. (2009). Study on the detoxification of alcohol extracts from Orient vine and its effective component on withdrawal syndromes of morphine. J Chin Med Mat, 32, 1414–1418.
- Zhao Y, Yu KQ, Li J. (2006). The efect of sinomenine on the activity of nuclear transcription factor kappa B in dendritic cells in rheurmyoid arthritis. Guangdong Med J, 27, 55–57.
- Zhao Y, Li J, Yu K, Liu Y, Chen X. (2007). Sinomenine inhibits maturation of monocyte-derived dendritic cells through blocking activation of NF-kappa B. Int Immunopharmacol, 7, 637–645.
- Zhao G, Bi C, Qin GW, Guo LH. (2009). Caulis Sinomenii extracts activate DA/NE transporter and inhibit 5HT transporter. Exp Biol Med (Maywood), 234, 976–985.
- Zheng JF, Liu Z, Zhu YH, Liu J, Hu B. (2008). Effect of sinomenine on the expression of HO2, sGC alpha 1 and sGC alpha 2 genes of spinal cord and cerebellum in morphine dependence mice. Chin Pharmacol Bull, 24, 1044–1048.
- Zheng H, Shi LF, Hu JH. (2007). Pharmacokinetic study of free-form sinomenine in rat skin by microdialysis coupled with liquid chromatography-electrospray mass spectrometry. Biomed Chromatogr, 21, 101–106.
- Zhou QX, Li YB, Jiang JQ. (2009). Triterpene constituents from Sinomenium acutum. Pharm Clin Res, 17, 36–38.
- Zhou SX, Su KYJ Peng Y, Zhou Y, Lin F, Li H. (2009). Effect of sinomenine on expression of P-selectin and ICAM-1 following cerebral ischemic reperfusion injury in diabetic rats. Lishizhen Med Mat Med Res, 20, 1593–1595.
- Zhou H, Wong YF, Wang J, Cai X, Liu L. (2008). Sinomenine ameliorates arthritis via MMPs, TIMPs, and cytokines in rats. Biochem Biophys Res Commun, 376, 352–357.
- Zhu QS, Liu MY, Liu L, Zhang MY. (2009). Effects of Caulis sinomenii on the learning and memory capacity of morphine addicted mice. Heilongjiang Med Pharm, 32, 8–10.
- Zou TX, Zhang YG, Zhang HG. (2008). Effects of sinomenine on phagocytosis ability of macrophage. Pharmacol Clin Chin Mater Med, 24, 15–17.