Abstract
Context: Antidepressant effects of various plants are generally attributed to their anti-inflammation and antioxidant activities. Cynanchum auriculatum Royle ex Wight (Asclepiadaceae) is a traditional medicinal plant in China and India used for immunological regulation, anti-inflammation, and antioxidant purposes. However knowledge about its antidepressant activity has been poorly investigated.
Objective:To investigate the antidepressant activities of the total glycosides of C. auriculatum (TGC) and its CHCl3/MeOH (10:1) fractions (TGC-D and TGC-E) in mice.
Materials and methods: TGC, TGC-D and TGC-E (20, 40 and 80 mg/kg) were intragastrically administered to mice twice a day for 5 days. The tail suspension test, forced swimming test, and locomotor activity test in mice were used to evaluate the effect of C. auriculatum. The inhibition of [3H]-serotonin reuptake in rat brain synaptosomes was detected to investigate their mechanism.
Results:TGC, TGC-D and TGC-E (80 mg/kg) decreased the immobility time by 61.7, 64.5, and 61.9% in tail suspension test. TGC (80 mg/kg), TGC-D (80 mg/kg) and TGC-E (20 mg/kg) decreased the immobility time by 32.6, 47.3, and 48.7% in forced swimming test. TGC (80 mg/kg) and TGC-E (20 and 40 mg/kg) decreased the crossing distances by 28.8, 29.5, and 36.2% in locomotor activity test. TGC, TGC-D and TGC-E (10 mg/L) inhibited serotonin reuptake by 7.4, 4.5, and 71.1% in rat brain synaptosomes, and IC50 value of TGC-E was 5.2 mg/L.
Discussion and Conclusion:TGC, TGC-D and TGC-E have potential antidepressant activities. The antidepressive effect of TGC-E maybe attributed partly by the inhibiting effect on serotonin reuptake.
Introduction
Cynanchum auriculatum Royle ex Wight (Asclepiadaceae) is mainly distributed in China and India. Its root, known as ‘Baishouwu’ in traditional Chinese medicine, has been widely used as a beneficial and tonic agent in clinics since ancient times (CitationPeng et al., 2011; CitationShan et al., 2006, Citation2005). It has multiple pharmacological actions for the treatment of geriatric diseases, nourishing the blood, replenishing the liver and kidney, strengthening the bones and muscles, clearing away toxins, enriching vital essence, astringing primordial energy, enhancing immunity, and prolonging life (CitationLi et al., 2008; CitationLiu et al., 2010; CitationPeng et al., 2011; CitationShan et al., 2006, Citation2005). C. auriculatum contains many essential nutrients, such as sugars, starch, protein, lipid, amino acids, vitamins, macronutrients, and 30 microelements (CitationGong, 1988; CitationGong et al., 1988). Modern pharmacological studies have shown that extracts and fractions of C. auriculatum have a variety of pharmacological actions, including antitumor, immunological regulation, clearing away free radicals, antioxidant, anti-aging, antimicrobial, anti-inflammation, hepatoprotection, reducing high serum cholesterols, trichogenous activity, stomach protective effect, and attenuating atrophy of spleen and thymus (CitationLiu et al., 2010; CitationPeng et al., 2011; CitationShan et al., 2006, Citation2005; CitationYin et al., 2004).
Although no study has reported on the antidepressant activity of C. auriculatum, multiple considerations suggest that C. auriculatum might have antidepressant activity. The reasons are as follows. Firstly, C. auriculatum has enhancing immunity function, clearing away free radicals, anti-inflammation and antioxidant effects (CitationLiu et al., 2010; CitationYin et al., 2004). Some evidence has shown that the activation of immune-inflammatory process, increase of monoamines catabolism, and abnormalities in oxidative/antioxidative status related to the pathophysiology of major depression (CitationBilici et al., 2001; CitationTsuboi et al., 2006; CitationTunez et al., 2010). Secondly, both Cynanchum otophyllum Schneid (Asclepiadaceae) and C. auriculatum belong to Asclepiadaceae family, and C. otophyllum has an antidepressant activity (CitationYang, 2007). C21-steroidal glycoside was considered as the major active component in antidepressant activity of C. otophyllum (CitationYang, 2007; CitationYang et al., 2005), and many C21-steroidal glycosides are also isolated from the root tuber of C. auriculatum (CitationChen et al., 1990; CitationLi et al., 2008). Therefore, this study was designed to evaluate the possible antidepressant effect of C. auriculatum and the effect on serotonin reuptake, which could possibly lead to the production of new effective antidepressant drug.
Materials and methods
Plant material and extraction
The whole plant of C. auriculatum was collected in Duyun, Guizhou province, China, in September 2007, and identified by Qing-Fu Chen (School of Life Sciences, Guizhou Normal University, Guiyang, China). A voucher specimen (No. 0709-08) was deposited at the Herbarium of School of Life Sciences, Guizhou Normal University, Guiyang, China. Air-dried rhizomes of C. auriculatum (2.0 kg) were powdered and extracted with 95% EtOH at room temperature for three times (72, 72 and 48 h, 5 L × 3). After removal of the organic solvent in vacuo, the extracts were suspended in water (1 L), then diluted with water (2 L) and filtered. The filtrate was applied on the AB-8 macroporous absorbent resin column (10 cm × 120 cm) with H2O as elution for three column volumes. The column was further eluted with 80% EtOH for three column volumes, and the 80% EtOH fraction was concentrated and spray-dried to afford light yellow powder, the total glycosides of C. auriculatum (TGC, 71.1 g).
A part of the TGC (30.0 g) was subjected to silica gel chromatography column (11 cm × 120 cm) and eluted with CHCl3/MeOH (10:1) to produce five fractions (Fr.1–Fr.5), including Fr.4 (TGC-D, 6.6 g) and Fr.5 (TGC-E, 10.8 g), the most active fractions in our previous screening for antidepressant activity.
Animals
Male ICR mice (23 ± 1 g) obtained from the Vital River Laboratories (Beijing, China) were used in the tail suspension test, forced swimming test, locomotor activity test and the acute toxicity test. Adult male Sprague-Dawley rats (220 g), supplied by the Institute of Laboratory Animal Sciences (CAMS&PUMC, Beijing, China) were used in the [3H]5-HT reuptake test. Animals were housed in a room with temperature (25 ± 1°C) and humidity (50 ± 10%) for a week before the experiment. They were kept under a 12 h light/12 h dark cycle (08:00–20:00, light) and free access to food and water. The animals were deprived of food for 12 h before drug administration, with free access to drinking water. Each animal was used only once in the experiment. All animals were treated humanely according to the “Principles of Laboratory Animal Care” and the “Guide for the Care and Use of Laboratory Animals of Institute of Materia Medica, CAMS&PUMC”. All experimental protocols were approved by the Animal Care and Use Committee of the College.
Drug
Duloxetine hydrochloride (Lilly S.A., Indiana, USA), a double norepinephrine (NE) and serotonin (5-HT) reuptake inhibitor antidepressant, was used as a positive control.
Treatment
TGC, TGC-D and TGC-E were tested at doses of 20, 40, and 80 mg/kg, respectively. Duloxetine hydrochloride was tested at a dose of 30 mg/kg. All tested samples and duloxetine were dissolved in 0.5% carboxymethylcellulose sodium (CMC-Na) each day prior to administration. Animals were administrated TGC, TGC-D, TGC-E, duloxetine or 0.5% CMC-Na intragastrically (i.g.) twice a day for 5 consecutive days, respectively. Tail suspension test, forced swimming test and locomotor activity test were carried out 1 h after last administration on the 5th day.
Tail suspension test (TST)
The procedure was performed based on the method of CitationSteru et al. (1985) and was conducted between 13:00–16:00 in a quiet room (lower than 60 dB). Mice were suspended by the tail from a level (50 cm high) for 6 min and their movement was recorded by a video system. The observer was unaware of drug treatment. Animals were considered to be immobile when they showed no body movement, and were hanging passively. The duration of immobility in the last 4 min was recorded as the “behavior despair” status.
Forced swimming test (FST)
The procedure was performed based on the method of CitationPorsolt et al. (1978) and was conducted between 13:00–16:00 in a quiet room (lower than 60 dB). Mice were placed individually and forced to swim in a plexiglass cylinder (20 cm in height and 14 cm in diameter), which was filled with water (25 ± 1°C) to a height of 10 cm. The test was performed over a period of 2 days. A preliminary experiment was performed 24 h before the official test. On the 4th day, 13:00–16:00, mice were placed into water to swim for a habituation period for 15 min, with no measurements (CitationHall et al., 2010), and given drugs immediately after swimming. On the 5th afternoon, 1 h after administration, mice were dropped into water and observed for 6 min. During this period, mice were assessed for diving, attempts to climb the wall of the cylinder, escape attempts, and struggling. All these behaviors were classified as escape behavior. Mice were assessed for immobility when they had no movement for 3 s. After the first 2 min of vigorous activity, mouse may assume a typical immobile posture; the immobility time was recorded during the next 4 min by a video system (CitationShen et al., 2009). After each session, mice were dried by a terry-cloth towel and placed under a warm-lamp for a period of 10 min. The cylinder was cleaned after each session.
Locomotor activity test (LAT)
The activity level of animals was monitored via mice locomotor activity recorder apparatus (RD1413, Shanghai Mobile Datum Company, China). The mice were placed in the apparatus for 5 min. The distance traveled in the open field (50 cm × 50 cm × 40 cm) was automatically recorded by recorder apparatus. Each animal was tested individually and used only once. The apparatus was cleaned after each test session in order to prevent the influence of the urine and feces left by the previous mouse.
Inhibition of [3H]serotonin reuptake in rat brain synaptosomes
Serotonin reuptake inhibitor may be potential antidepressant. The test was used to detect the samples that inhibit serotonin reuptake in rat brain synaptosomes (CitationVogel et al., 2002). One male SD rat was decapitated and the brain was rapidly removed. The whole brain minus cerebellum was weighed and homogenized in 19 volumes of ice-cold 0.32 mol/L sucrose solution by a Potter-Elvejhem homogenizer (Institute of Materia Medica, Beijing, China). The homogenate was centrifuged at 1000g at 4°C for 10 min. The supernatant was decanted and used for reuptake experiments. One hundred of microliter suspension was mixed with 50 µL drug/sample solution or krebs buffer. The total binding tubes and sample tested tubes were preincubated at 37°C for 20 min, then added 400 µL 40.1 nmol/L [3H]5-HT solution in krebs buffer and incubated at 37°C for 20 min. The nonspecific binding tubes were preincubated in 0°C ice water for 20 min, then added 400 µL 40.1 nmol/L [3H]5HT solution in krebs buffer and incubated in 0°C ice water for 20 min. All tubes were filtrated by a cell harvester immediately after incubation. The fiberglass filter membrane was washed 5-times by ice-cold saline, then put into scintillation vials, and counted in 4 mL of liquid scintillation cocktail. The percent inhibition of sample on [3H]5-HT reuptake = (cpm of total binding tubes – cpm of sample tubes)/(cpm of total binding tubes – cpm of nonspecific binding tubes) × 100%. The percent inhibition at each sample concentration was the mean of three determinations. IC50 values were calculated by log-probit analysis.
Acute toxicity test in mice
The acute toxicity test for C. auriculatum evaluated any possible toxicity. ICR mice were tested by orally administered different doses of the extract. All animals were observed for any gross effect or mortality within 7 days (CitationMa et al., 2011). The given doses of TGC, TGC-D and TGC-E were up to 2 g/kg, while the control mice were only administered 0.5% CMC-Na at 0.1 mL/10 mg body weight.
Statistical analysis
The data were presented as means ± SEM. The results of FST, TST and LAT were analyzed by one-way ANOVA (Graph Pad Prism 5), followed by Dunnett’s t-test. Difference was considered statistically significant when the p < 0.05.
Results
Effects of TGC, TGC-D and TGC-E on the tail suspension behavior of mice
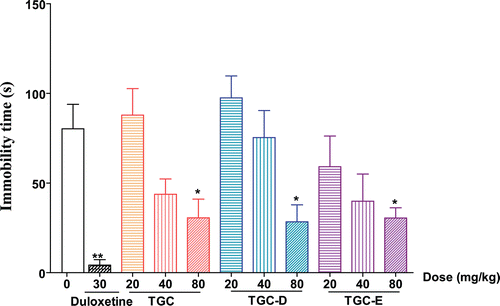
The antidepressant effects of TGC, TGC-D and TGC-E were examined in tail suspension test. As shown in , compared with vehicle control group, duloxetine (30 mg/kg) significantly decreased the immobility time by 94.7% during tail suspension session. TGC, TGC-D and TGC-E dose-dependently reduced the immobility time on tail suspension behavior in mice. At the dose of 80 mg/kg, TGC, TGC-D and TGC-E significantly reduced the immobility time by 61.7, 64.5, and 61.9%, respectively.
Figure 1. Effects of TGC, TGC-D and TGC-E on the immobility time of mice in the tail suspension test. Mice were orally given TGC, TGC-D and TGC-E (20, 40 and 80 mg/kg) or vehicle (0.5% CMC-Na) for 5 consecutive days, respectively. Tail suspension test were carried out 1 h after last administration on the 5th day. Data are presented as mean ± SEM, n = 10. *p< 0.05, significance versus vehicle control.
Effects of TGC, TGC-D and TGC-E on the forced swimming behavior of mice
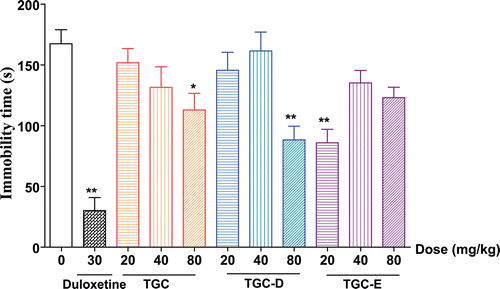
In order to further examine the antidepressant effects of TGC, TGC-D and TGC-E, the immobility time of mice were detected in forced swimming test. As shown in , compared with vehicle control group, duloxetine (30 mg/kg) significantly decreased the immobility time by 82.0% during forced swimming session. TGC (80 mg/kg), TGC-D (80 mg/kg) and TGC-E (20 mg/kg) significantly reduced the immobility time by 32.6, 47.3, and 48.7%, respectively. No significant effect was observed at the other doses of TGC, TGC-D and TGC-E.
Figure 2. Effects of TGC, TGC-D and TGC-E on the immobility time of mice in the forced swimming test. Mice were orally given TGC, TGC-D and TGC-E (20, 40 and 80 mg/kg) or vehicle (0.5% CMC-Na) for 5 consecutive days, respectively. Forced swimming test were carried out 1 h after last administration on the 5th day. Data are presented as mean ± SEM, n = 10. *p < 0.05 and **p < 0.01, significance versus vehicle control.
Effects of TGC, TGC-D and TGC-E on the locomotor activity behavior of mice
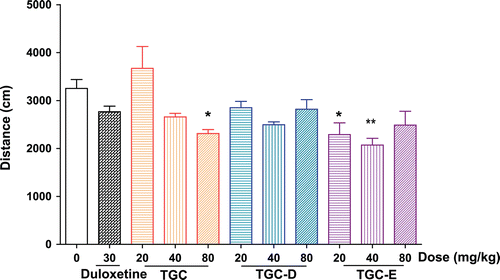
In order to detect whether the decline in immobility was mediated by stimulation of the overall motor activity, the activity level of animals were evaluated in the locomotor activity test. As shown in , compared with the vehicle control group, the treatment with TGC, TGC-D and TGC-E (20, 40, 80 mg/kg) did not increase the traveled distances in mouse locomotor activity test. Instead, TGC (80 mg/kg) and TGC-E (20 and 40 mg/kg) decreased the distance of crossings by 28.8, 29.5, and 36.2%.
Figure 3. Effects of TGC, TGC-D and TGC-E on the crossing distances of mice in the locomotor activity test. Mice were orally given TGC, TGC-D and TGC-E (20, 40 and 80 mg/kg) or vehicle (0.5% CMC-Na) for 5 consecutive days, respectively. Locomotor activity test were carried out 1 h after last administration on the 5th day. Data are presented as mean ± SEM, n = 10. *p < 0.05 and **p < 0.01, significance versus vehicle control.
Inhibition of [3H]-serotonin reuptake in rat brain synaptosomes
In order to investigate the antidepressant mechanism of C. auriculatum, the dose-related results of the functional inhibition of serotonin reuptake transporter (SERT) were presented in . TGC, TGC-D and TGC-E (10 mg/L) inhibited SERT by 7.4, 4.5, and 71.1% in rat brain synaptosomes, and IC50 value of TGC-E was 5.2 mg/L.
Figure 4. The inhibitory effects of TGC, TGC-D and TGC-E on [3H]-serotonin reuptake in rat brain synaptosomes. TGC, TGC-D and TGC-E were tested at dose of 0.001, 0.01, 0.1, 1.0, and 10 mg/L; while duloxetine were tested at dose of 0.00033 mg/L (10−9 mol/L), 0.0033 mg/L (10−8 mol/L), 0.033 mg/L (10−7 mol/L), 0.33 mg/L (10−6 mol/L), and 3.3 mg/L (10−5 mol/L).
![Figure 4. The inhibitory effects of TGC, TGC-D and TGC-E on [3H]-serotonin reuptake in rat brain synaptosomes. TGC, TGC-D and TGC-E were tested at dose of 0.001, 0.01, 0.1, 1.0, and 10 mg/L; while duloxetine were tested at dose of 0.00033 mg/L (10−9 mol/L), 0.0033 mg/L (10−8 mol/L), 0.033 mg/L (10−7 mol/L), 0.33 mg/L (10−6 mol/L), and 3.3 mg/L (10−5 mol/L).](/cms/asset/a53f525d-bc14-429d-bfe7-6194c93c1aa6/iphb_a_656848_f0004_b.gif)
Acute toxicity
Acute toxicity test result showed LD50values for TGC and TGC-D were higher than 1 g/kg. Although the mice were given orally up to 2 g/kg of TGC-E, no mortality was observed during the assessment period.
Discussion
Depression constitutes the second most common chronic condition in clinical practice and will become the second leading cause of disability in the world by the year 2020 (CitationBhattamisra et al., 2008). The current status of therapy is still not satisfying with a variety of side effects, such as psychomotor impairment and dependence liability in about one third of all subjects treated with these antidepressants (CitationBhattamisra et al., 2008; CitationDai et al., 2010). Thus, there is an unmet need for safer, better tolerated and more powerful antidepressants (CitationMao et al., 2008). In some developing countries, herbal preparations have been widely used to treat diseases, some of them have been considered as effective alternatives in the treatment of depression, such as Saint John’s wort. Traditional Chinese medicine has also recorded many herbal formulae with psychotropic potential, and evaluated in animal models of depression (CitationMao et al., 2008; CitationZhang, 2004).
Both of the tail suspension test (TST) and forced swimming test (FST) represented the behavioral despair model, and were reported to reproduce a condition similar to human depression (CitationBhattamisra et al., 2008; CitationWillner & Mitchell, 2002). The decreased immobility duration in animals of TST and FST may predict the efficacy of antidepressants (CitationLi et al., 2007), as they are sensitive and selective for clinically used antidepressant drugs. In this study, we examined the antidepressant effects of the total glycosides of C. auriculatum (TGC) and its fractions (TGC-D and TGC-E) by using these behavioral despair models. After being treated with the TGC, TGC-D and TGC-E at 80 mg/kg, respectively, for 5 days, the mice showed a significant reduction of immobility time in TST. TGC (80 mg/kg), TGC-D (80 mg/kg) and TGC-E (20 mg/kg) significantly reduced the immobility time in FST. These results indicated the antidepressant effects of C. auriculatum. The findings that only the lower dose (20 mg/kg) of TGC-E, but not the higher dose (40 and 80 mg/kg), was effective in the FST, whereas higher doses were effective in the TST indicated the antidepressant effects of TGCand its fractions (TGC-D and TGC-E) have a dose-dependent characteristic, which needs more study to explain. However, different behaviors can be affected by different drug doses as many factors are interacting in the current tests for affective-like behaviors, so the fact that a lower dose might be more effective than a higher one is not rare. For example, in a study that examined the antidepressant effects of the acidic polysaccharide portion of ginseng WGPA fraction, WGPA 100 mg/kg but not 200 mg/kg reduced immobility time in FST in mice, whereas both doses were effective in the social interaction test (CitationWang et al., 2010).
On the other hand, because of agents that stimulate locomotor activity in these behavioral tests (TST and FST), false-positive results can be obtained (CitationBourin et al., 2001; CitationMao et al., 2008). Therefore, we also evaluated the effect of C. auriculatum on locomotion by the locomotor activity behavior test. The results showed that TGC and its fractions (TGC-D and TGC-E) treatment did not increase the mouse traveled distances in LAT. Instead, TGC (80 mg/kg), TGC-E (20 and 40 mg/kg) and duloxetine (30 mg/kg) decreased the traveled distances. It is not rare in the study of medical plants, for example, CitationLee et al. (2010) showed that the water extracts of Allium macrostemon Bunge (Liliaceae) significantly reduced the immobility duration in the FST and TST, while slightly decreased the total distance moved in spontaneous locomotor behavior test. These findings indicated that the reduction of immobility time elicited byTGC, TGC-D and TGC-E treatment in FST and TST was unlikely due to a psychomotor-stimulant effect, but rather an antidepressant-like effect. According to the results of acute toxicity, we speculated the inhibition effect of TGC, TGC-D, and TGC-E (20, 40 and 80 mg/kg) on mice immobility time was the pharmacology effect, but not toxicity effect.
So next, we then tried to explore the antidepressive mechanism of TGC and its fractions (TGC-D and TGC-E). The mechanism of depression is quite complex (CitationDai et al., 2010). Previous studies have implicated that serotonin plays an important role in the development, maintenance, prevention, and reversal of learned helplessness behavior; serotonin may be seen as a crucial ‘fine tuner’ of normal and pathological processes (CitationBhattamisra et al., 2008). Serotonin reuptake inhibitor may be potential antidepressant. It is not contradictory that TGC-E significantly inhibited SERT, while TGC and TGC-D had no effect on this transporter at 10 mg/L. Firstly, antidepressant effect of TGC-E may be mediated by the inhibition of serotonin reuptake, while antidepressant effect of TGC and TGC-D were mediated by other pathway which needed further research. Secondly, TGC contains five CHCl3/MeOH fractions, there may exist interactions among components on inhibition of SERT, and leading to the result that TGC had no effect on SERT, while one of its CHCl3/MeOH fractions, TGC-E, significantly inhibited SERT.
In our preliminary test, TGC-D was further subjected to reversed-phase silica gel RP-18 CC using a mobile phase of MeOH/H2O (80:20) and afforded two known compounds, auriculoside A and wilfoside K1N; TGC-E was further separated by reversed-phase silica gel CC repeatedly to give two known compounds, CynanauriculosideA and B, these four glycosides were all C21-steroidal glycoside, so we speculated C21-steroidal glycosides may be major active components of C. auriculatum, but it need further research in future.
In summary, we demonstrated that total glycosides of C. auriculatum (TGC) and its fractions (TGC-D and TGC-E) act as antidepressant agents. The antidepressive effect of TGC-E maybe attributed at least partly by the inhibition of serotonin reuptake. These results revealed that C. auriculatum has the potential for the treatment of depression.
Acknowledgements
This research work was supported by Natural Science Foundation of China (30760032), Natural Science Foundation of Beijing (7102116), and Major National Science and Technology Projects’ Creation of Major New Drugs’ from China (2012ZX09301-002-001).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bhattamisra SK, Khanna VK, Agrawal AK, Singh PN, Singh SK. (2008). Antidepressant activity of standardised extract of Marsilea minuta Linn. J Ethnopharmacol, 117, 51–57.
- Bilici M, Efe H, Köroglu MA, Uydu HA, Bekaroglu M, Deger O. (2001). Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments. J Affect Disord, 64, 43–51.
- Bourin M, Fiocco AJ, Clenet F. (2001). How valuable are animal models in defining antidepressant activity? Hum Psychopharmacol, 16, 9–21.
- Chen LL, Zhang ZX, Zhou J. (1990). Cynauricuosides A, B and C, steroid glycosides from the root of Cynanchum auriculatum. Acta Bot Yunnan, 12, 197–210.
- Dai Y, Li Z, Xue L, Dou C, Zhou Y, Zhang L, Qin X. (2010). Metabolomics study on the anti-depression effect of xiaoyaosan on rat model of chronic unpredictable mild stress. J Ethnopharmacol, 128, 482–489.
- Gong SS. (1988). [Determination of nutrient constituents in various processed samples of Cynanchum auriculatum Royle ex Wight]. Zhong Yao Tong Bao, 13, 17–8, 62.
- Gong SS, Liu CD, Liu SL, DuYR, Kang W, Dong XQ. (1988). Studies on constituents of the Chinese traditional drug baishouwu (Cynanchum auriculatum Royle ex Wight). Acta Pharmacol Sin, 23, 276–280.
- Hall BJ, Pearson LS, Buccafusco JJ. (2010). Effect of the use-dependent, nicotinic receptor antagonist BTMPS in the forced swim test and elevated plus maze after cocaine discontinuation in rats. Neurosci Lett, 474, 84–87.
- Lee S, Kim DH, Lee CH, Jung JW, Seo YT, Jang YP, Ryu JH. (2010). Antidepressant-like activity of the aqueous extract of Allium macrostemon in mice. J Ethnopharmacol, 131, 386–395.
- Li S, Wang C, Li W, Koike K, Nikaido T, Wang MW. (2007). Antidepressant-like effects of piperine and its derivative, antiepilepsirine. J Asian Nat Prod Res, 9, 421–430.
- Li Y, Zhang J, Gu X, Peng Y, Huang W, Qian S. (2008). Two new cytotoxic pregnane glycosides from Cynanchum auriculatum. Planta Med, 74, 551–554.
- Liu K, Chen F, Zhang H. (2010). Antitumor effects by wilfoside C3N treatment in ECA109 cells. Anticancer Drugs, 21, 625–631.
- Ma KJ, Zhu ZZ, Yu CH, Zhang H, Liu J, Qin LP. (2011). Analgesic, anti-inflammatory, and antipyretic activities of the ethanol extract from Desmodium caudatum. Pharm Biol, 49, 403–407.
- Mao QQ, Ip SP, Tsai SH, Che CT. (2008). Antidepressant-like effect of peony glycosides in mice. J Ethnopharmacol, 119, 272–275.
- Peng YR, Ding YF, Wei YJ, Shu B, Li YB, Liu XD. (2011). Caudatin-2,6-dideoxy-3-O-methy-β-d-cymaropyranoside 1 induced apoptosis through caspase 3-dependent pathway in human hepatoma cell line SMMC7721. Phytother Res, 25, 631–637.
- Porsolt RD, Anton G, Blavet N, Jalfre M. (1978). Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur J Pharmacol, 47, 379–391.
- Shan L, Liu RH, Shen YH, Zhang WD, Zhang C, Wu DZ, Min L, Su J, Xu XK. (2006). Gastroprotective effect of a traditional Chinese herbal drug “Baishouwu” on experimental gastric lesions in rats. J Ethnopharmacol, 107, 389–394.
- Shan L, Zhang WD, Zhang C, Liu RH, Su J, Zhou Y. (2005). Antitumor activity of crude extract and fractions from root tuber of Cynanchum auriculatum Royle ex Wight. Phytother Res, 19, 259–261.
- Shen YH, Zhou Y, Zhang C, Liu RH, Su J, Liu XH, Zhang WD. (2009). Antidepressant effects of methanol extract and fractions of Bacopa monnieri. Pharm Biol, 47, 340–343.
- Steru L, Chermat R, Thierry B, Simon P. (1985). The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl), 85, 367–370.
- Tsuboi H, Tatsumi A, Yamamoto K, Kobayashi F, Shimoi K, Kinae N. (2006). Possible connections among job stress, depressive symptoms, lipid modulation and antioxidants. J Affect Disord, 91, 63–70.
- Túnez I, Drucker-Colín R, Montilla P, Peña J, Jimena I, Medina FJ, Tasset I. (2010). Protective effect of nicotine on oxidative and cell damage in rats with depression induced by olfactory bulbectomy. Eur J Pharmacol, 627, 115–118.
- Vogel HG, Wolfgang HV, Bernward AS, Sandow J, Müller G, Vogel WF. (2002). Inhibition of [3H]-serotonin uptake in synaptosomes. Drug Discovery and Evaluation: Pharmacological Assays. New York, 548–549
- Wang J, Flaisher-Grinberg S, Li S, Liu H, Sun L, Zhou Y, Einat H. (2010). Antidepressant-like effects of the active acidic polysaccharide portion of ginseng in mice. J Ethnopharmacol, 132, 65–69.
- Willner P, Mitchell PJ. (2002). The validity of animal models of predisposition to depression. Behav Pharmacol, 13, 169–188.
- Yang QX. (2007). The application of total glycoside of Baishouwu. Chinese Patent, CN 101002836A.
- Yang QX, Jin HK, Yang CR. (2005). Application of C21 steroid glycoside as antidepressants.CN 1634097A.
- Yin M, Feng X, Dong YF, Yuan CQ. (2004). Advances in research of chemistry and pharmacology of baishouwu. Chin Wild Plant Resour, 23, 8–11.
- Zhang ZJ. (2004). Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders. Life Sci, 75, 1659–1699.
