Abstract
Context: Essential oils carry diverse antimicrobial and anti-enzymatic properties.
Objective: Matrix metalloproteinase (MMP) inhibition characteristics of Salvia fruticosa Miller (Labiatae), Myrtus communis Linnaeus (Myrtaceae), Juniperus communis Linnaeus (Cupressaceae), and Lavandula stoechas Linnaeus (Labiatae) essential oils were evaluated.
Materials and methods: Chemical compositions of the essential oils were analyzed by gas chromatography–mass spectrometry (GC–MS). Bioinformatical database analysis was performed by STRING 9.0 and STITCH 2.0 databases, and ViaComplex software. Antibacterial activity of essential oils against periodontopathogens was tested by the disc diffusion assay and the agar dilution method. Cellular proliferation and cytotoxicity were determined by commercial kits. MMP-2 and MMP-9 activities were measured by zymography.
Results: Bioinformatical database analyses, under a score of 0.4 (medium) and a prior correction of 0.0, gave rise to a model of protein (MMPs and tissue inhibitors of metalloproteinases) vs. chemical (essential oil components) interaction network; where MMPs and essential oil components interconnected through interaction with hydroxyl radicals, molecular oxygen, and hydrogen peroxide. Components from L. stoechas potentially displayed a higher grade of interaction with MMP-2 and -9. Although antibacterial and growth inhibitory effects of essential oils on the tested periodontopathogens were limited, all of them inhibited MMP-2 in vitro at concentrations of 1 and 5 µL/mL. Moreover, same concentrations of M. communis and L. stoechas also inhibited MMP-9. MMP-inhibiting concentrations of essential oils were not cytotoxic against keratinocytes.
Discussion and conclusion: We propose essential oils of being useful therapeutic agents as MMP inhibitors through a mechanism possibly based on their antioxidant potential.
Introduction
Bacteria-induced tissue degradation in periodontitis is driven by an inflammatory state, which activates tissue-degrading enzymes, mainly matrix metalloproteinases (MMPs), by the host (CitationSorsa et al., 2006). MMPs are necessary for tissue repair and cell migration; however, their over-expression or hyperactivity in periodontal tissues leads to degradation of tooth-supporting tissues (CitationMäkelä et al., 1999). Inhibition of excessively produced MMPs is the major goal in host-modulation therapy (CitationGolub et al., 1995). Although the inhibition of MMPs is thought to decrease tissue degradation, studies on MMP knock-out animal models indicated that MMP-deficient animals are more prone to periodontal degradation in comparison with non-deficient controls (CitationKuula et al., 2009). Instead of the total inhibition of MMP secretion and activity, bringing their levels down to similar ones as typically existing in healthy tissues could be the aim in periodontal therapy.
Essential oils of several plants have been used in ethno-medicine because of their antifungal, antibacterial, or anti-inflammatory activities (CitationKalemba & Kunicka, 2003). Traditionally prepared essential oils are common home remedies against cold, cough, fever, gastrointestinal irritation, and inflamed wounds (CitationYesilada et al., 1995; CitationHonda et al., 1996; CitationUzun et al., 2004). Essential oils are antibacterial or antifungal against several microorganisms (CitationFilipowicz et al., 2003; CitationPitarokili et al., 2003; CitationDadalioglu & Evrendilek, 2004), including periodontitis-associated bacteria (CitationGursoy et al., 2009), but their effects on the enzyme activity of the host have not extensively been studied. There is only one study available in the periodontal literature showing that an essential oil may inhibit neutrophilic MMP-9 (CitationAbraham et al., 2005), but still, the underlying mechanism for such an effect remains a question mark.
Bioinformatics represents a solid additional approach to traditional biological research. Strong efforts within the scientific community are made to develop novel computational tools to elaborate scaling hypothesis from large amounts of experimental knowledge and to test large data sets that would remain virtually impossible by other means. Computer modeling is able to perform efficient simulations from available experimental data, generating new hypotheses which could be later tested by other approaches in vivo or in vitro. Potential advantages of computer modeling over the wet laboratory work include relatively lower cost and faster execution, where different species can be monitored at the same time under a number of diverse conditions. For instance, some bioinformatic programs help the researcher to understand the function, regulation, and post-translational modifications of a gene of interest. Our group and others have successfully developed and applied computational tools in different areas of scientific research, such as redox biology, cancer biology and therapeutics, and evolutionary biology (CitationCastro et al., 2006, Citation2007; CitationBonatto, 2007; CitationBarea & Bonatto, 2009; CitationRybarczyk-Filho et al., 2011; CitationDalmolin et al., 2011; CitationPapp et al., 2011). We have utilized bioinformatical methods in order to evaluate the current experimental landscape of interaction between essential oil components, MMPs and tissue inhibitors of metalloproteinases (TIMPs), as well as the potential compounds or proteins crosslinking with them (if there were any), by using protein–protein, chemical-chemical, and protein–chemical database and experimental data. This can be represented in a novel protein–chemical network where the characteristics of potential interactions will additionally be analyzed.
In the present study, we first aimed to evaluate the potential in silico co-interaction between selected essential oils and MMPs through bioinformatical approaches and, afterwards, to analyze the anti-gelatinolytic, cytotoxic, proliferative, and antibacterial (activity against common periodontopathogens) effects of essential oils in vitro.
Material and methods
Preparation of essential oils
Selection of essential oils was done according to their use in folk medicine and diversity in their chemical composition (CitationGursoy et al., 2009). Leaves of Myrtus communis Linnaeus (Myrtaceae) (myrtle), leaves and flowers of Salvia fruticosa Miller (Labiatae) (sage), and Lavandula stoechas Linnaeus (Labiatae) (lavender), and fruits of Juniperus communis Linnaeus (Cupressaceae) (juniper) were collected in the west-Mediterranean region of Turkey in May–July 2006. Samples were air-dried in shade for 2–4 days. Essential oils, obtained with the traditional water and steam distillation technique, were kept in dark at 4°C.
Chemical composition analysis
Chemical compositions of the essential oils were analyzed by using gas chromatography–mass spectrometry (GC–MS) as previously described (CitationGursoy et al., 2009). Briefly, the GC–MS analyses were carried out using a Shimadzu QP 5050 (Kyoto, Japan) GC–MS system operating in the EI mode at 70 eV, equipped with an Cp-Wax 52 CB column (50 m × 0.32 mm; film thickness 1.2 µm). The initial temperature of the column being 60°C and was raised to 220°C at a 2°C/min rate. The carrier gas was helium, flow rate was 10 psi. The identification of the chemical constituents was based on data obtained from authentic samples and/or the Wiley, Nist, and Tutor libraries spectra.
Keratinocyte cultures
HaCaT human skin keratinocytes (generously provided by Hubert Fusenig, German Cancer Center, Heidelberg, Germany) were maintained as frozen stocks and cultured in a 5% CO2 atmosphere at 37°C in Dulbecco’s Modified Eagle’s Medium containing 10% fetal calf serum, 1% l-glutamine, and 1% penicillin G.
Bacterial cultures
Bacterial species and strains used in this study came from the culture collection of anaerobic bacteria of the National Institute for Health and Welfare (THL) and were: Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans NTCC 9710 and AHN 24195; Porphyromonas gingivalis ATCC 33277, AHN 24155, and AHN 24135; Parvimonas micra (formerly Peptostreptococcus micros) ATCC 33270, AHC 15107, and AHC 15154; Fusobacterium nucleatum ATCC 25586 and AHN 9508; Prevotella intermedia ATCC 25611 and AHN 8290; Prevotella nigrescens ATCC 33563 and AHN 8293. The strains were revived from frozen (−70°C) stocks and subcultured for purity. Bacteria were grown on brucella blood agar enriched with hemin, and incubated at 37°C in an anaerobic chamber for 5 days.
In silico analysis
Development of an essential oil components-MMP interaction network model
By using a database resource search tool (STRING 9.0) for the retrieval of interacting genes (CitationSzklarczyk et al., 2011), protein–protein interaction database and experimental data on MMPs and TIMPs were screened under a score of 0.4 (medium) and a prior correction of 0.0. Thereafter, by using a search tool for chemicals vs. chemicals and chemicals vs. proteins interactions (STITCH 2.0) (CitationKuhn et al., 2008, Citation2010), interactions between essential oil components, as well as essential oil components vs. MMPs and TIMPs were investigated under a score of 0.4 (medium) and a prior correction of 0.0 (). The results obtained from both in silico screenings were cross-linked in order to generate a network model representing the interaction between essential oil components, MMPs, and TIMPs (Model for Essential Oil components and MMP Interactions, the EOMI network).
Table 1. Table listing both compound and ensemble protein identifiers (CID and Ensemble Protein IDs) for the in silico approach.
Connectivity and clustering coefficient within the network model
Connectivity and clustering coefficient studies within the network were performed by using the ViaComplex software (CitationCastro et al., 2009), which is an open-source application that builds landscape maps of gene expression networks.
Connectivity
Connectivity (ki) of one node i is defined as the number of neighbors directly connected to a node.
Clustering coefficient
Clustering coefficient represents the fraction of possible associations between neighbors of a single node tending to cluster together. If one node is connected to a pair of nodes, then these three nodes may build a “triple”. The number of triples represents the number of possible associations between the neighbors of one node. Clustering coefficient (Ci) is then calculated as:
In this equation, ni represents the number of links connecting the neighbors of node i (ki) one to each other.
Antimicrobial activity
The agar dilution method was used to determine the minimum inhibitory concentration (MIC) of the essential oils on periodontal bacteria according to the Clinical Laboratory Standards Institute guidelines (CitationCLSI, 2007). Brucella blood agars were prepared with serial two-fold dilutions of essential oils, ranging from 0.125 to 128 µL/mL. Bacterial inocula were prepared by adjusting their density to 0.5 McFarland standards with phosphate buffered saline (PBS) and inoculated to each agar plate with a multipoint inoculating device. The plates were incubated at 37°C in an anaerobic chamber for 5 days.
Antimicrobial activities of essential oils against the tested periodontal pathogens were analyzed with a disc diffusion assay with slight modifications (CitationMurray et al., 1995). One 5-day-old colony from the first passage of each bacterial strain was inoculated on brucella blood agar. The 6 mm diameter discs were impregnated with 15 µL of each essential oil at concentrations of 1, 5, 10, 25, and 50 µL/mL in dimethyl sulfoxide (DMSO) and placed on to the inoculated agar plates. Antibacterial activities were interpreted as follows: −, no antimicrobial activity and an inhibition zone <1 mm; +, a modest antimicrobial activity and an inhibition zone corresponding to 2–4 mm; ++, a clear antimicrobial activity and an inhibition zone corresponding to 5–10 mm; +++, a strong antimicrobial activity and an inhibition zone >10 mm (CitationOjala et al., 2000). A penicillin G (10 U) disc was used as a positive control and pure DMSO as a negative control. The agar plates were incubated at 37°C in an anaerobic chamber for 5 days. After incubation, the inhibition zones were measured and the effect was calculated. All tests were done in triplicates.
Cell cytotoxicity analysis and cellular proliferation
HaCaT cells were grown in 96-well plates. Cells were washed twice with PBS, and fresh medium was then placed to each well. Each essential oil was added into the culture medium at 1 and 5 µL/mL concentrations. After incubating for 24 h, the medium in the wells was collected and the cytotoxic effect of each essential oil was measured with a colorimetric cytotoxicity detection kit (Roche, Mannheim, Germany), measuring the lactate dehydrogenase (LDH) activity in the culture media. Cellular proliferation was analyzed by using the Cell Titer 96 assay (Promega, USA). All tests were done in triplicates.
Analysis of MMP-2 and MMP-9 activities
HaCaT cells were grown in 96-well plates as described above. Essential oils were added into the culture medium at 1 and 5 µL/mL concentrations, together with 10 ng/mL of phorbol 12-myristate 13-acetate (PMA) (Sigma), a well-known MMP-9 inducer (CitationShin et al., 2007). After incubation for 24 h, the medium in the wells was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, by using 8% gels containing 1 mg of gelatin (Sigma)/mL. After electrophoresis, the gels were washed four times in 0.05 M Tris–HCl (pH 7.5), 0.02% NaN3 buffer, 25% Tween 80. The second wash was supplemented with 10 mM ZnCl2 and 100 mM CaCl2. The incubation buffer consisted of 0.05 M Tris–HCl (pH 7.5), 0.02% NaN3 buffer, 10 mM ZnCl2, and 100 mM CaCl2. After incubation for 24 h at 37°C, the gels were fixed and stained with 0.1% Coomassie blue R-250 in 30% methanol and 10% acetic acid. Finally, the gels were analyzed by the Bio-Rad Model GS-700 Imaging Densitometer using Scion Image 4.0.2 for Windows (Scion Corporation, USA). All tests were done in triplicates.
Statistical analysis
Differences in the epithelial cell toxicity, proliferation, and PMA-induced MMP-2 and -9 activity inhibition were analyzed by using the Student t-test (p < 0.05).
Results
The major constituents of essential oils were as follows: 1,8-cineole/eucalyptol (49.5%), camphor (13.3%), β-pinene (7.2%), α-pinene (5.8%), and camphene (5.1%) for S. fruticosa, α-pinene (79.5%), β-pinene (4.0%), and 1,8-cineole/eucalyptol (3.9%) for J. communis, camphor (49.1%), fenchone (27.7%), and 1,8 cineol/eucalyptol (13.9%) for L. stoechas, and 1,8-cineole/eucalyptol (37.0%), α-pinene (30.2%), linalool (9.7%), terpineol (7.5%), and limonene (4.8%) for M. communis.
In silico analysis by using STRING 9.0 and STITCH 2.0 gave rise to the EOMI network for the interaction between the essential oil components, MMPs, and TIMPs (). The EOMI network shows the direct interconnection between essential oil components and MMP-2 and -9 through previous interactions with ·OH, O2, and H2O2. Analysis of the main characteristics of the network by the ViaComplex software revealed that both ·OH and O2 displayed the highest connectivity ( and ) to a highly clustered group of essential oil components ( and ).
Figure 1. EOMI network. An essential oil components-MMP interaction network model was developed by utilizing the STRING 9.0 and STITCH 2.0 database resource search tools, under a score of 0.4 (medium) and a prior correction of 0.0.
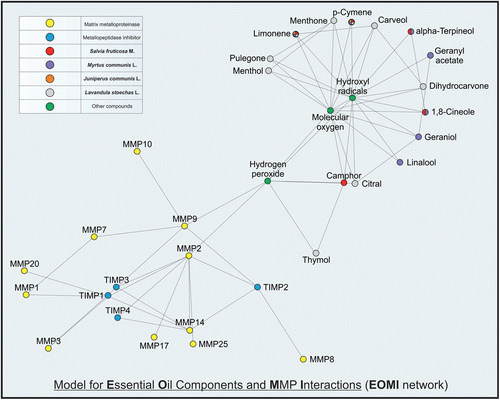
Figure 2. Software analysis (ViaComplex) of the properties within the EOMI network. Figure shows 2D and 3D representations of connectivity (a, b) and clustering coefficient (c, d) of each component in the network model.
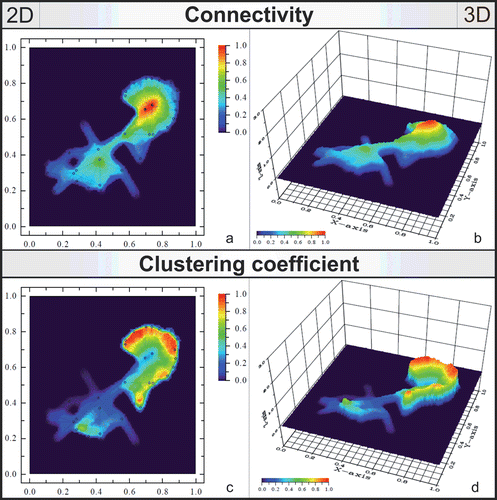
The MIC values for the essential oils ranged from 0.25 to 8 µL/mL (). None of the essential oils showed clear antimicrobial activity on the tested periodontopathogens (). There was a modest antibacterial activity at 50 µL/mL concentration of all essential oils against P. micra ATCC 33270 and AHC 15107, and at 50 µL/mL concentration of J. communis essential oil against P. intermedia ATCC 25611 and P. nigrescens AHN 8293. Penicillin G (the positive control) was strongly effective against all examined bacterial strains, while DMSO (the negative control) did not have any antibacterial effect.
Table 2. Antibacterial effects of essential oils against laboratory (NTCC and ATCC) and clinical (AHN and AHC) strains of six periodontal pathogens.
Figure 3. MIC of the tested essential oils against periodontal bacteria (A.a.: Aggregatibacter actinomycetemcomitans; F.n.: Fusobacterium nucleatum; P.g.: Porphyromonas gingivalis; P.m.: Parvimonas micra; P.i.: Prevotella intermedia; P.n.: Prevotella nigrescens).
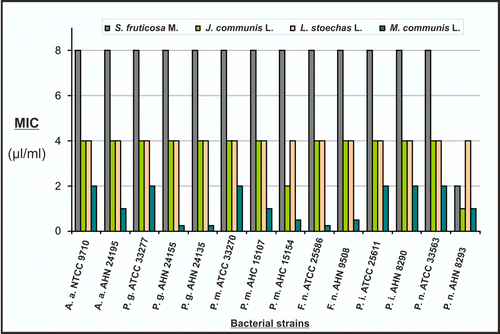
When MMP-9 (a 92 KDa band) and MMP-2 (a 72 KDa band) activities were measured by a gelatin-zymography technique, the concentrations of 1 and 5 µL/mL of essential oils from M. communis and L. stoechas significantly inhibited the MMP-9 activity, while all essential oils tested significantly reduced the MMP-2 activity when compared with the positive control (PMA) ().
Figure 4. Effect of essential oils on MMP-9 and MMP-2 activities of keratinocytes. HaCaT human skin keratinocytes were treated with essential oils at 1 and 5 µL/mL concentrations in the culture medium, together with 10 ng/mL of PMA, a well-known MMP-9 inducer. Untreated HaCaT and PMA-treated cells represent the negative and positive control, respectively. Gelatinolytic activities seen on 92 and 72 kDa areas represent the MMP-9 (a) and MMP-2 (b) activities, respectively (*Statistical difference with the positive control; p < 0.005).
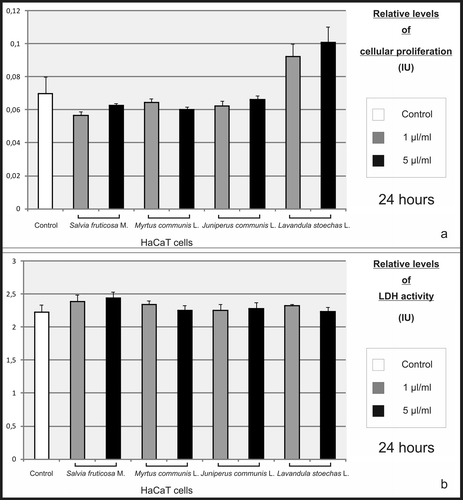
The treatment of HaCaT cells for 24 h with 1 and 5 µL/mL of essential oils from S. fruticosa, M. communis, J. communis, and L. stoechas did not change the cellular proliferation () and none of the essential oils showed any cytotoxic effect on MMP-inhibiting concentrations (). The concentrations of essential oils higher than 5 µL/mL were not analyzed by zymography, since those concentrations triggered the cellular detachment of keratinocytes.
Figure 5. Effect of essential oils on proliferation and viability of HaCaT cells. HaCaT keratinocytes were treated with essential oils at concentrations of 1 and 5 µL/mL. After incubating for 24 h, no significant differences were found in the proliferation (a) and LDH activity (b) of these cells, when compared to control.
Discussion
The present study provides significant highlights about the inhibitory effect of essential oils over MMP-2 and -9 activities, based on both in silico and in vitro data. Bioinformatical tools may represent a “bridge” interconnecting different areas of research, such as biochemistry, pharmacology, molecular biology, medicine, etc. This is essential for developing a new way to do science: a modern, multidisciplinary, and, therefore, integrative approach that allows the researcher to work with large amounts of data.
Periodontitis is an infectious disease, characterized by breakdown of the tooth-supporting tissues and triggered by inflammatory response mechanisms of the host. The inflammatory response is initiated as an immediate reaction to bacterial challenge in the subgingival area. However, both extension and exacerbation of the inflammatory response leads to the destruction of periodontal tissues. When periodontitis is treated with anti-infective strategies, the treatment outcome is not always optimal and predictable in the healing process (CitationPreshaw, 2008). Therefore, controlling an excessive host response and minimizing the tissue destruction can be a milestone for the treatment of periodontitis (CitationTonetti & Chapple 2011). Essential oils have been considered potential therapeutic agents in periodontal therapy, however, their beneficial effects as an adjunctive treatment option have been underestimated when limiting their use as antimicrobial agents solely (CitationFeng et al., 2011). According to our findings in the present study, some essential oils could be used for the modulation of the host response, since they were able to inhibit an up-regulated gelatinolytic activity at non-antibacterial concentrations against periodontal pathogens. An essential oil-based management of excessive tissue destruction may lead researchers to develop new treatment modalities in the area of periodontology. In addition, if the beneficial use of essential oils in host-modulation therapy could be demonstrated in vivo, their applications would not only be limited to periodontal diseases; in fact, those may be applied to other inflammatory diseases, such as inflammatory lung diseases or gastrointestinal diseases.
Our bioinformatical approach was based on the STRING (version 9.0) tool (CitationSzklarczyk et al., 2011), which covers more than 1100 completely sequenced organisms, the STITCH (version 2.0) tool (CitationKuhn et al., 2008, Citation2010), which links proteins from 630 organisms to over 74000 different chemicals (including 2200 drugs), and the ViaComplex tool for studying the characteristics within the network (CitationCastro et al., 2009). The characteristics of these tools let researchers to analyze a large amount of information coming from experiments and databases or even text mining. Hydroxyl radicals, molecular oxygen, and hydrogen peroxide were provided by the database for interconnecting the essential oils components, MMPs, and TIMPs in silico. In fact, one can already find in the literature various reports demonstrating the antioxidant potential of the essential oils used in our study (CitationEmami et al., 2007; CitationPapageorgiou et al., 2008; CitationAidi Wannes et al., 2010; CitationMessaoud et al., 2011). According to our knowledge, however, this is the first time when a bioinformatical approach is being used in such a study context. Our in silico approach took into consideration databases and experimental data on MMPs and TIMPs exclusively under a score of 0.4 (medium) and a prior correction of 0.0. Text mining or text analytics was avoided. This means that our software analysis did not gather information from comments, hypotheses and/or discussions in the literature, for developing the EOMI network model.
In the present study, HaCaT non-tumorigenic human keratinocytes were selected as cell lines. Although this cell line has a non-oral origin, it is a widely used model in oral epithelial cell studies (CitationUitto et al., 1992; CitationBoukamp et al., 1988; CitationGursoy et al., 2008a) and its MMP-2 and -9 secretion characteristics are well demonstrated (CitationGursoy et al., 2008b).
In periodontitis, specific pathogenic bacteria exert their virulent effect by inducing host cells to secrete destructive enzymes, such as MMPs (CitationDing et al., 1995) (). Among them, MMP-9 (a 92 kDa gelatinase) and MMP-2 (a 72 kDa gelatinase) are enzymes that are secreted by keratinocytes, fibroblasts, neutrophils, macrophages, and osteoclasts (CitationSorsa et al., 2006). Increase in the MMP-9 production initiates the degradation of the basement membrane and connective tissue, events that can be clinically detected as loss of periodontal attachment (CitationMäkelä et al., 1994; CitationDong et al., 2009). Therefore, there is a search for developing new, efficient, and safe therapeutic strategies to bring the excessive MMP expression and activity to physiological levels. In order to achieve this goal, a deeper comprehension about the molecular mechanisms of MMP production by the host cells would be necessary. In the present study, the EOMI network showed direct interconnections between essential oil components and MMP-2 and MMP-9 through previous interactions with ·OH, O2, and H2O2. Further analyses revealed that both ·OH and O2 displayed the highest connectivity to a highly clustered group of essential oil components. Further studies are warranted to explain these interactions in detail. If the mechanism of interaction between essential oils and MMPs, such as MMP-2 and -9, occurs through the interaction with ·OH and O2, then one could predict a possible synergism between these components over MMPs.
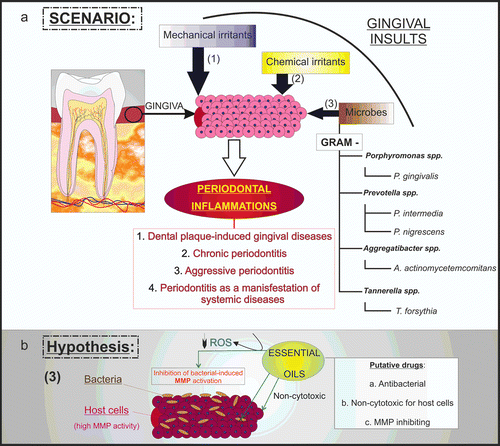
Figure 6. Hypothetical essential oil-induced inhibition in periodontitis. Mechanical and chemical irritants, as well as microbes can trigger periodontal inflammation (a). In such scenarios, specific bacteria exert their virulent effect by inducing host cells to secrete destructive enzymes, such as MMPs. In this work, we hypothesized that essential oil-induced MMP inhibition may occur thanks to the antioxidant properties of these compounds and ROS scavenging (b).
Oxidative environments are necessary for the activation of these MMPs, since oxidants stimulate proteolytic cleavage, a crucial mechanism of zymogen activation (CitationMorgunova et al., 1999; CitationNelson & Melendez, 2004). When cells consume oxygen, superoxide anion (−O2) is produced by electron leakage at mitochondrial complexes I and III (CitationFinkel & Holbrook, 2000). −O2, H2O2, and ·OH (the latter can be generated by Fenton or Harber–Weis reactions from −O2 and H2O2) are commonly generated by reactive oxygen species (ROS) in the cells (CitationKamata & Hirata, 1999; CitationDalmolin et al., 2007). This is in line with the observation that periodontitis is associated with a cumulative increase in ROS and MMP productions (CitationAsman et al., 1984; CitationShapira et al., 1991).
Interestingly, essential oils have been found to exert antioxidant or anti-inflammatory activities, which can provide beneficial effects in protecting the tissues from exacerbated host response (CitationMiguel et al., 2011). Plants have evolved antioxidant mechanisms, such as catalase and peroxidase systems, that can remove ROS from the cells (CitationNoctor & Foyer, 1998). Plant monoterpenes can also scavenge O3 in experimental conditions (CitationFares et al., 2008). Since MMPs can be activated through a ROS-dependent pathway (CitationMeli et al., 2003), scavenging of ROS may inhibit the MMP activity. This could be the mechanism by which essential oils are able to inhibit MMPs ().
Antimicrobial effect against microorganisms is a common characteristic of several essential oils. In order to simulate the pathogenic microbial community present at infected subgingival sites, we selected six bacterial species that have been associated with periodontitis (CitationSocransky & Haffajee, 2005). It is known that there are strain-dependent variations within a bacterial species, therefore, we also included more than one strain of each species, representing both clinical and laboratory strains, in the study. The four essential oils selected for the present study had either a weak antibacterial effect or no effect. Even though possible antibacterial effects could be found upon treatment with higher concentrations of essential oils, those concentrations could also be cytotoxic against host cells (CitationPrashar et al., 2004). Microbes are able to generate defense mechanisms against natural antimicrobial compounds, for example, by degrading monoterpenes in anaerobic conditions (CitationHarder & Probian, 1995). The degradation of active essential oil compounds by periodontopathogenic bacteria was not in the scope of the present study. However, in a clinical use, anti-enzymatic monoterpenes can be utilized with strong antibacterial phenolic components, such as carvacrol, for improving the success of periodontal therapy (CitationBotelho et al., 2008).
In the present study, none of the essential oils displayed cytotoxic effects against keratinocytes. However, at concentrations higher than 5 µL/mL, all essential oils induced cellular detachment on our in vitro model. Detachment of cells after essential oil treatment has already been described (CitationTakarada et al., 2004; CitationGursoy et al., 2009), but the underlying mechanism for such an effect is still unknown.
Conclusions
To the best of our knowledge, this is the first study integrating bioinformatics in the context of essential oils vs. MMP inhibition. Although the essential oils tested had no clear antibacterial activity against any of the examined periodontal pathogens, essential oils, even at non-antibacterial concentrations, act as potential agents for the inhibition of excessive MMP-2 and MMP-9 secretions.
In summary, based on our in silico and in vitro results, it can be postulated that some essential oils could be used as functional MMP inhibitors and, as such, as potential therapeutic agents in diseases where excessive MMP activity causes tissue destruction, such as periodontal diseases.
Declaration of interest
This study was supported by the National Council for Scientific and Technological Development (CNPq) of the Federal Republic of Brazil, CAPES (PNPD/SUS), and FAPERGS (F.Z.-C, J.L.R-F, and J.C.F-M), and the University of Helsinki (HUS-EVO grant for V-J.U). F. Z.-C. is grateful to the Marie Curie Early Stage Research Training (EST) program for his previous funding at the University of Helsinki, Finland.
References
- Abraham S, Kumar MS, Sehgal PK, Nitish S, Jayakumar ND. (2005). Evaluation of the inhibitory effect of triphala on PMN-type matrix metalloproteinase (MMP-9). J Periodontol, 76, 497–502.
- Aidi Wannes W, Mhamdi B, Sriti J, Ben Jemia M, Ouchikh O, Hamdaoui G, Kchouk ME, Marzouk B. (2010). Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem Toxicol, 48, 1362–1370.
- Asman B, Engström PE, Olsson T, Bergström K. (1984). Increased luminol enhanced chemiluminescence from peripheral granulocytes in juvenile periodontitis. Scand J Dent Res, 92, 218–223.
- Barea F, Bonatto D. (2009). Aging defined by a chronologic-replicative protein network in Saccharomyces cerevisiae: An interactome analysis. Mech Ageing Dev, 130, 444–460.
- Bonatto D. (2007). A systems biology analysis of protein-protein interactions between yeast superoxide dismutases and DNA repair pathways. Free Radic Biol Med, 43, 557–567.
- Botelho MA, Rao VS, Montenegro D, Bandeira MA, Fonseca SG, Nogueira NA, Ribeiro RA, Brito GA. (2008). Effects of a herbal gel containing carvacrol and chalcones on alveolar bone resorption in rats on experimental periodontitis. Phytother Res, 22, 442–449.
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. (1988). Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol, 106, 761–771.
- Castro MA, Onsten TG, Moreira JC, de Almeida RM. (2006). Chromosome aberrations in solid tumors have a stochastic nature. Mutat Res, 600, 150–164.
- Castro MA, Mombach JC, de Almeida RM, Moreira JC. (2007). Impaired expression of NER gene network in sporadic solid tumors. Nucleic Acids Res, 35, 1859–1867.
- Castro MA, Filho JL, Dalmolin RJ, Sinigaglia M, Moreira JC, Mombach JC, de Almeida RM. (2009). ViaComplex: Software for landscape analysis of gene expression networks in genomic context. Bioinformatics, 25, 1468–1469.
- CLSI (Clinical and Laboratory Standards Institute). (2007). Performance of Standards for Antimicrobial Susceptibility Testing, approved standard M100-S17. 17th Informational Supplement. Pennsylvania, Wayne: Clinical and Laboratory Standards Institute.
- Dadalioglu I, Evrendilek GA. (2004). Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J Agric Food Chem, 52, 8255–8260.
- Dalmolin RJ, Zanotto-Filho A, De Oliveira RB, Duarte RF, Pasquali MA, Moreira JC. (2007). Retinol and retinoic acid increase MMP-2 activity by different pathways in cultured Sertoli cells. Free Radic Res, 41, 1338–1347.
- Dalmolin RJ, Castro MA, Rybarczyk Filho JL, Souza LH, de Almeida RM, Moreira JC. (2011). Evolutionary plasticity determination by orthologous groups distribution. Biol Direct, 6, 22.
- Ding Y, Uitto VJ, Firth J, Salo T, Haapasalo M, Konttinen YT, Sorsa T. (1995). Modulation of host matrix metalloproteinases by bacterial virulence factors relevant in human periodontal diseases. Oral Dis, 1, 279–286.
- Dong W, Xiang J, Li C, Cao Z, Huang Z. (2009). Increased expression of extracellular matrix metalloproteinase inducer is associated with matrix metalloproteinase-1 and -2 in gingival tissues from patients with periodontitis. J Periodont Res, 44, 125–132.
- Emami SA, Javadi B, Hassanzadeh MK. (2007). Antioxidant activity of the essential oils of different parts of Juniperus communis. subsp. hemisphaerica. and Juniperus oblonga. Pharm Biol, 45, 769–776.
- Fares S, Loreto F, Kleist E, Wildt J. (2008). Stomatal uptake and stomatal deposition of ozone in isoprene and monoterpene emitting plants. Plant Biol (Stuttg), 10, 44–54.
- Feng HS, Bernardo CC, Sonoda LL, Hayashi F, Romito GA, De Lima LA, Lotufo RF, Pannuti CM. (2011). Subgingival ultrasonic instrumentation of residual pockets irrigated with essential oils: A randomized controlled trial. J Clin Periodontol, 38, 637–643.
- Filipowicz N, Kaminski M, Kurlenda J, Asztemborska M, Ochocka JR. (2003). Antibacterial and antifungal activity of juniper berry oil and its selected components. Phytother Res, 17, 227–231.
- Finkel T, Holbrook NJ. (2000). Oxidants, oxidative stress and the biology of ageing. Nature, 408, 239–247.
- Golub LM, Sorsa T, Lee HM, Ciancio S, Sorbi D, Ramamurthy NS, Gruber B, Salo T, Konttinen YT. (1995). Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis gingiva. J Clin Periodontol, 22, 100–109.
- Gursoy UK, Könönen E, Uitto VJ. (2008a). Intracellular replication of fusobacteria requires new actin filament formation of epithelial cells. APMIS, 116, 1063–1070.
- Gursoy UK, Könönen E, Uitto VJ. (2008b). Stimulation of epithelial cell matrix metalloproteinase (MMP-2, -9, -13) and interleukin-8 secretion by fusobacteria. Oral Microbiol Immunol, 23, 432–434.
- Gursoy UK, Gursoy M, Gursoy OV, Cakmakci L, Könönen E, Uitto VJ. (2009). Anti-biofilm properties of Satureja hortensis L. essential oil against periodontal pathogens. Anaerobe, 15, 164–167.
- Harder J, Probian C. (1995). Microbial degradation of monoterpenes in the absence of molecular oxygen. Appl Environ Microbiol, 61, 3804–3808.
- Honda G, Yesilada E, Tabata M, Sezik E, Fujita T, Takeda Y, Takaishi Y, Tanaka T. (1996). Traditional medicine in Turkey. VI. Folk medicine in west Anatolia: Afyon, Kütahya, Denizli, Mugla, Aydin provinces. J Ethnopharmacol, 53, 75–87.
- Kalemba D, Kunicka A. (2003). Antibacterial and antifungal properties of essential oils. Curr Med Chem, 10, 813–829.
- Kamata H, Hirata H. (1999). Redox regulation of cellular signalling. Cell Signal, 11, 1–14.
- Kuhn M, von Mering C, Campillos M, Jensen LJ, Bork P. (2008). STITCH: Interaction networks of chemicals and proteins. Nucleic Acids Res, 36, D684–D688.
- Kuhn M, Szklarczyk D, Franceschini A, Campillos M, von Mering C, Jensen LJ, Beyer A, Bork P. (2010). STITCH 2: An interaction network database for small molecules and proteins. Nucleic Acids Res, 38, D552–D556.
- Kuula H, Salo T, Pirilä E, Tuomainen AM, Jauhiainen M, Uitto VJ, Tjäderhane L, Pussinen PJ, Sorsa T. (2009). Local and systemic responses in matrix metalloproteinase 8-deficient mice during Porphyromonas gingivalis-induced periodontitis. Infect Immun, 77, 850–859.
- Mäkelä M, Salo T, Uitto VJ, Larjava H. (1994). Matrix metalloproteinases (MMP-2 and MMP-9) of the oral cavity: Cellular origin and relationship to periodontal status. J Dent Res, 73, 1397–1406.
- Mäkelä M, Larjava H, Pirilä E, Maisi P, Salo T, Sorsa T, Uitto VJ. (1999). Matrix metalloproteinase 2 (gelatinase A) is related to migration of keratinocytes. Exp Cell Res, 251, 67–78.
- Meli DN, Christen S, Leib SL. (2003). Matrix metalloproteinase-9 in pneumococcal meningitis: Activation via an oxidative pathway. J Infect Dis, 187, 1411–1415.
- Messaoud C, Chograni H, Boussaid M. (2011). Chemical composition and antioxidant activities of essential oils and methanol extracts of three wild Lavandula L. species. Nat Prod Res. DOI: 10.1080/14786419.2011.635343.
- Miguel G, Cruz C, Faleiro ML, Simões MT, Figueiredo AC, Barroso JG, Pedro LG. (2011). Salvia officinalis L. essential oils: Effect of hydrodistillation time on the chemical composition, antioxidant and antimicrobial activities. Nat Prod Res, 25, 526–541.
- Morgunova E, Tuuttila A, Bergmann U, Isupov M, Lindqvist Y, Schneider G, Tryggvason K. (1999). Structure of human pro-matrix metalloproteinase-2: Activation mechanism revealed. Science, 284, 1667–1670.
- Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolke RH. (1995). Manual of Clinical Microbiology. 6th edition. American Society for Microbiology. Washington (DC): ASM.
- Nelson KK, Melendez JA. (2004). Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med, 37, 768–784.
- Noctor G, Foyer CH. (1998). Ascorbate and glutathione: Keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol, 49, 249–279.
- Ojala T, Remes S, Haansuu P, Vuorela H, Hiltunen R, Haahtela K, Vuorela P. (2000). Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J Ethnopharmacol, 73, 299–305.
- Papageorgiou V, Gardeli C, Mallouchos A, Papaioannou M, Komaitis M. (2008). Variation of the chemical profile and antioxidant behavior of Rosmarinus officinalis L. and Salvia fruticosa Miller grown in Greece. J Agric Food Chem, 56, 7254–7264.
- Papp B, Notebaart RA, Pál C. (2011). Systems-biology approaches for predicting genomic evolution. Nat Rev Genet, 12, 591–602.
- Pitarokili D, Tzakou O, Loukis A, Harvala C. (2003). Volatile metabolites from Salvia fruticosa as antifungal agents in soilborne pathogens. J Agric Food Chem, 51, 3294–3301.
- Prashar A, Locke IC, Evans CS. (2004). Cytotoxicity of lavender oil and its major components to human skin cells. Cell Prolif, 37, 221–229.
- Preshaw PM. (2008). Host response modulation in periodontics. Periodontol 2000, 48, 92–110.
- Rybarczyk-Filho JL, Castro MA, Dalmolin RJ, Moreira JC, Brunnet LG, de Almeida RM. (2011). Towards a genome-wide transcriptogram: The Saccharomyces cerevisiae case. Nucleic Acids Res, 39, 3005–3016.
- Shapira L, Borinski R, Sela MN, Soskolne A. (1991). Superoxide formation and chemiluminescence of peripheral polymorphonuclear leukocytes in rapidly progressive periodontitis patients. J Clin Periodontol, 18, 44–48.
- Shin Y, Yoon SH, Choe EY, Cho SH, Woo CH, Rho JY, Kim JH. (2007). PMA-induced up-regulation of MMP-9 is regulated by a PKCα-NF-βB cascade in human lung epithelial cells. Exp Mol Med, 39, 97–105.
- Socransky SS, Haffajee AD. (2005). Periodontal microbial ecology. Periodontol 2000, 38, 135–187.
- Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, Golub LM, Brown DL, Mäntylä P. (2006). Matrix metalloproteinases: Contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med, 38, 306–321.
- Szklarczyk D, Franceschini A, Kuhn M, Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork P, Jensen LJ, von Mering C. (2011). The STRING database in 2011: Functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res, 39, D561–D568.
- Takarada K, Kimizuka R, Takahashi N, Honma K, Okuda K, Kato T. (2004). A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol Immunol, 19, 61–64.
- Tonetti MS, Chapple IL; Working Group 3 of Seventh European Workshop on Periodontology. (2011). Biological approaches to the development of novel periodontal therapies–consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol, 38 Suppl 11, 114–118.
- Uitto VJ, Larjava H, Peltonen J, Brunette DM. (1992). Expression of fibronectin and integrins in cultured periodontal ligament epithelial cells. J Dent Res, 71, 1203–1211.
- Uzun E, Sariyar G, Adsersen A, Karakoc B, Otük G, Oktayoglu E, Pirildar S. (2004). Traditional medicine in Sakarya province (Turkey) and antimicrobial activities of selected species. J Ethnopharmacol, 95, 287–296.
- Yesilada E, Honda G, Sezik E, Tabata M, Fujita T, Tanaka T, Takeda Y, Takaishi Y. (1995). Traditional medicine in Turkey. V. Folk medicine in the inner Taurus Mountains. J Ethnopharmacol, 46, 133–152.
