Abstract
Context: In oral insulin delivery, blood glucose profiles of a subject can be a function of complicated transfer of water and insulin between gastrointestinal and blood compartments.
Objective: This study examined the effects of orally administered water on blood glucose profiles of oral insulin-fed diabetic rats. The blood glucose, insulin and red blood cell concentration profiles of streptozotocin-induced diabetic rats were determined following administration of deionized water and insulin solution (14 IU/kg) with control receiving none. The aqueous solution samples were subjected to X-ray diffractometry (XRD), fourier transform infra-red spectroscopy (FTIR), viscometry and osmolality tests to elucidate the state of water-insulin interaction.
Results and discussion: Deionized water reduced blood glucose concentration by more than 70% via dilution of systemic circulation. The addition of insulin into deionized water negated its blood glucose lowering property instead of no influence due to insulin had not shown marked oral bioavailability. The insulin interacted with water to form solution with higher viscosity and osmolality values.
Conclusion: Insulin-water interaction could be one reason where water migration from the gastrointestinal lumen into systemic circulation is reduced and blood glucose lowering capacity of deionized water is negated.
Introduction
Diabetes mellitus is an endocrine disease which is related to disorders of carbohydrate metabolism brought about by deficiency in insulin secretion, insulin resistance or both (CitationMalecki & Klupa, 2005; CitationWong, 2009, Citation2010). Epidemiology studies indicate that hyperglycemia is the primary cause of diabetes. Chronic hyperglycemia is responsible for long term sequelae of diabetes namely retinopathy, nephropathy, neuropathy, cardiovascular and peripheral vascular disorders. Diabetes is broadly classified as type 1 diabetes which requires exogenous insulin for survival and type 2 diabetes which may require exogenous insulin at the later cycle of the illness. Exogenous insulin is administered by the subcutaneous route. Long term administration of insulin by injection brings about various complications such as hypoglycemia, risk of infection, lipoatrophy or lipohypertrophy at the injection sites. It inflicts a pain sensation and lowers the quality of life in diabetic patients.
Oral insulin delivery has generated widespread interest among many pharmaceutical and medical researchers for its painless mode of drug administration (CitationWong, 2009, Citation2010). Among all oral dosage forms, nanoparticles are gaining a high research and commercialization attention in the scientific community, attributing to their potential in protection of insulin from the harsh environment of the gastrointestinal tract and facilitation of insulin absorption by paracellular and/or transcellular route (CitationWong, 2010). As an oral insulin carrier, the blood glucose lowering effects of nanoparticles is often evaluated against orally administered pure insulin solution (CitationChung et al., 2002; CitationLin et al., 2007). Perfusion of the gastrointestinal tract with hypotonic solution is known to increase its transepithelial permeability to solute, possibly through the distension of channels between adjacent epithelial cells (CitationLifschitz et al., 1973). The incorporation of solution with hydrocolloid and glycerol, on the other hand, has been reported to induce opposing influences on gastrointestinal fluid movement (CitationWapnir et al., 1996; CitationSharpe et al., 2007), with large molecular weight hydrocolloid reducing the propensity of water migration from the intestinal lumen to systemic circulation unlike low molecular weight glycerol. Having that both solute and water may experience varying oral absorption profiles as a function of the solution property, it is hypothesized that the blood glucose profile of a subject can be a resultant effect of complicated transfer of water and/or insulin between intestinal medium and the blood compartment. Using streptozotocin-induced diabetic rats as the animal model, this study aims to examine the blood glucose modulation profiles of deionized water in oral insulin-fed rats. Using glucose as the main marker of blood dilution or concentration, the possibility of insulin affecting the water movement in gastrointestinal tract will be discussed and its implication in drug and dosage form development will be addressed.
Materials and methods
Animal model
Streptozotocin-induced diabetic Sprague–Dawley male rats (250–300 g, 8–10 weeks old, blood glucose level >250 mg/dl; Genetic Improvement and Farm Technologies Sdn Bhd, Malaysia) were acclimatized for at least 7 days under 12 h light/dark cycle at 25 ± 2°C with deionized water and animal feed given ad libitum. These rats were subjected to 15 h of fasting prior to experiments. All experiments were conducted in accordance to the institutional ethics regulations adapting international guidelines on animal experimentation conduct.
Blood glucose test
The blood glucose concentration of rats was determined using the glucometer (Ascensia Elite, Bayer Diagnostic Europe Ltd, Ireland) over a period of 24 h following oral administration of a) 6 ml/kg of deionized water, b) 6 ml/kg of deionized water with 14 IU/kg bovine insulin (Sigma Aldrich, St. Louis, MO, USA) added, and c) none. The reduction extent of blood glucose concentration was defined as a quotient of the difference between the blood glucose concentration at a specified time and at 0 h to that of blood glucose concentration at 0 h, expressed in percentage.
Blood insulin test
The blood insulin concentration of rats was determined by first clotting and centrifuging the blood at 5000 rpm for 20 min at 25 ± 2°C (Microfuge 18 centrifuge, Beckman Coulter, Brea, California, USA) to obtain the serum sample. The serum sample was preserved at −20°C until further analysis for its insulin level using the rat insulin enzyme immunoassay kit (SPI-BIO, France).
Hemocytometry
The red blood cells of rats were counted using a hemocytometer (Assistent, Germany). Blood samples were withdrawn from the rat tail vein and diluted with Toisson’s fluid prior counting.
The state of insulin-water interaction was assessed using X-ray diffractometry (XRD), fourier transform infra-red spectroscopy (FTIR), viscometry and osmolality measurement techniques with at least triplicates for each sample and the results were averaged.
XRD
The sample was subjected to X-ray diffractometry test (Ultima IV, Rigaku Corporation, Japan) with Cu-Kα radiation generated at 40 kV and 40 mA. The X-ray diffraction was operated at a scanning speed of 3°/min, ranging from 3° to 70° (2θ).
FTIR
Dry potassium bromide (KBr FTIR grade, Aldrich, Germany) was ground into a fine powder before compressing into a disc. Each disc was added with 1 mg of sample and scanned at a resolution of 4 cm−1 over a wavenumber region of 400–4000 cm−1 using a FTIR spectrometer (Spectrum RX1 FTIR system, Perkin Elmer, Norwalk, Connecticut, USA) at 25 ± 1°C.
Viscometry
A U-tube (Size A, Poulten Selfe & Lee Ltd, UK) was used to examine the specific viscosity of sample at 37 ± 0.5°C.
Osmolality
Sample (50 µl) was pipetted into a 1 ml Eppendorf tube and subjected to osmolality measurement using a osmometer (Osmomat 010, Ganotec GmBH, Germany).
Results
Three isolated studies on the effects of orally administered deionized water on blood glucose profiles of diabetic rats were conducted by different researchers using different batches of animals at different times over a period of 2 years (2008–2010 by Nurjaya Sumiran, Mohammad Tarmizi Mohd Mokhtar, and Aminah Kadir). Dosing of rats with deionized water and insulin solution showed blood glucose lowering effects when compared to control which received no aqueous sample (). The extent of blood glucose lowering progressed in the following order: deionized water > insulin solution > none (ANOVA, p < 0.050). Apparently, the administration of rats with samples of deionized water gave rise to a lower red blood cell count in a unit volume of blood (deionised water: 6.85 ± 0.28 million/ml, none: 8.03 ± 0.31 million/ml; Student’s t-test, p < 0.050). The treatment of rats with insulin solution had no marked tendency to lead to a rise in their blood insulin level against those of receiving deionized water (). The addition of insulin into deionized water was accompanied by a reduction in water cluster crystallinity and and a rise in O-H bond strength of water/insulin as well as water viscosity and osmolality as indicated by XRD, FTIR, viscometry and osmolality analysis respectively ().
Figure 1. Blood glucose lowering profiles of diabetic rats receiving deionized water (♦), insulin solution (▪) and none (▴) in three independent studies (n = 5/group).
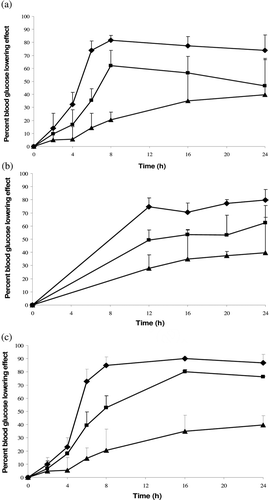
Table 1. FTIR, XRD, viscosity and osmolality profiles of deionized water and insulin solution, and blood insulin concentration of diabetic rats following administration of these aqueous samples.
Discussion
Previous studies highlighted that oral administration of water gave no substantial blood glucose lowering effect in animal except a recent investigation suggested that electrolysis of aqueous solution containing sodium or potassium salt could provide a moderate reduction in blood glucose level by 25–35% (CitationKim & Kim, 2006; CitationLin et al., 2007). The present independent studies nonetheless found that deionized water exerted blood glucose lowering effect via dilution of systemic circulation by absorbed water as reflected by the reduction in concentration of marker red blood cells and glucose itself. Deionized water, admixed with insulin, had its blood glucose lowering property negated instead of having insulin as an inactive compound as it was envisaged that unencapsulated insulin would degrade in gastrointestinal tract with time and had shown no significant oral bioavailability. XRD and FTIR studies indicated that insulin molecules interacted with the network of water clusters through reducing water-water affinity and increasing the association of insulin with the water molecules. The summative effects promoted a rise in viscosity and osmolality of insulin solution which then suggested to be able to reduce the migration of water from gastrointestinal lumen to systemic circulation as well as blood glucose lowering capacity of deionized water. With reference to deionized water, the unexpectedly low blood glucose lowering capacity of insulin solution might also be associated with insulin’s ability to accelerate small intestinal transit (CitationPeddyreddy & Rao, 2006; CitationReddy et al., 2006), through reducing the extent of water absorption. The orally administered insulin had not demonstrated a significant gastrointestinal absorption. Its intestinal transit effect might be ascribed to local pharmacological influences at gastrointestinal tract, different from previously reported observations where intestinal transit effect was initiated by peripheral or central insulin injection (CitationPeddyreddy & Rao, 2006; CitationReddy et al., 2006). Further investigation on predominant mechanism of insulin affecting the water movement between the gastrointestinal tract and systemic circulation, and its outcome on blood glucose level is required.
Conclusion
Deionised water demonstrates blood glucose lowering properties. The current findings imply that assessment of blood glucose lowering properties of a drug and dosage form development can be complicated by water movement between the gastrointestinal tract and blood compartment. The scope of this study limits the results to insulin only. Future investigations shall focus on other drugs and excipients, and their effects on blood glucose lowering of water.
Declaration of interest
The authors are grateful to the Ministry of Science, Technology and innovation (Nanofund), and the Ministry of Higher Education (0191403, FRGS) Malaysia for financial and facility support.
The authors report no declarations of interest.
References
- Chung H, Kim J, Um JY, Kwon IC, Jeong SY. (2002). Self-assembled “nanocubicle” as a carrier for peroral insulin delivery. Diabetologia, 45, 448–451.
- Kim MJ, Kim HK. (2006). Anti-diabetic effects of electrolyzed reduced water in streptozotocin-induced and genetic diabetic mice. Life Sci, 79, 2288–2292.
- Lifschitz MD, Garcia JA, Earley LE. (1973). Effect of passive water absorption on transepithelial movement of extracellular solutes in rat intestine. Kidney Int, 4, 362–368.
- Lin Y-H, Chen C-T, Liang H-F, Kulkarni AR, Lee PW, Chen CH, Sung HW. (2007). Novel nanoparticles for oral insulin delivery via the paracellular pathway. Nanotechnology, 18, 1–11.
- Malecki MT, Klupa T. (2005). Type 2 diabetes mellitus: from genes to disease. Pharmacol Rep, 57 Suppl, 20–32.
- Peddyreddy MK, Rao KR. (2006). Peripheral and centrally mediated effects of insulin on small intestinal transit in healthy mice. Clin Exp Pharmacol Physiol, 33, 633–636.
- Reddy PM, Dkhar SA, Subramanian R. (2006). Effect of insulin on small intestinal transit in normal mice is independent of blood glucose level. BMC Pharmacol, 6, 4.
- Sharpe K, Ward L, Cichero J, Sopade P, Halley P. (2007). Thickened fluids and water absorption in rats and humans. Dysphagia, 22, 193–203.
- Wapnir RA, Sia MC, Fisher SE. (1996). Enhancement of intestinal water absorption and sodium transport by glycerol in rats. J Appl Physiol, 81, 2523–2527.
- Wong TW. (2009). Chitosan and its use in design of insulin delivery system. Recent Pat Drug Deliv Formul, 3, 8–25.
- Wong TW. (2010). Design of oral insulin delivery systems. J Drug Target, 18, 79–92.
