Abstract
Context: Lagenaria siceraria (Molina) Standl. (Cucurbitacae) (LS) has been reported to possess cardioprotective, antihyperlipidemic, and diuretic activities.
Objective: To evaluate antihypertensive and cardioprotective effects of the Lagenaria siceraria fruit powder in NG-nitro-l-arginine methyl ester (L-NAME) induced hypertension in rats.
Materials and methods: Male Wistar rats were divided in four groups. Control 2% gum acacia p.o., L-NAME (40 mg/kg p.o.), LS (500 mg/kg p.o.) + L-NAME (40 mg/kg p.o.), L-arginine (100 mg/kg p.o.) + L-NAME (40 mg/kg p.o.). Treatment period was 4 weeks. On day 29 serum marker enzymes, cholesterol and heamodynamic parameters were measured. Histology of heart was performed. LS powder was characterized by HPLC.
Result: Systolic blood pressures were increased by L-NAME (p < 0.001). In both drug treated groups systolic and diastolic blood pressures were reduced significantly (p < 0.001) compared to L-NAME. In L-NAME group significantly (p < 0.01) elevated cholesterol which was reduced (p < 0.05) by LS treatment. In L-NAME group inflammation and necrosis (0–35%) was present in heart whereas there was no change in myocardium of LS and L-arginine treated rats. Vitexin, orientin and isoorientin were detected in methanol extract of LS powder.
Discussion and conclusion: L-NAME induced hypertension in rats was reduced by treatment with LS. The absence of necrosis, inflammation in the heart and significant reduction in serum cholesterol in LS and L-arginine treated rats indicated cardioprotective activity. Antioxidant activity of orientin and isoorientin appears to reduce the L-NAME induced damage. It is concluded that LS fruit possess antihypertensive and cardioprotective activity.
Keywords::
Introduction
Cardiovascular disease (CVD) will be the most important cause of mortality in India by the year 2015 (CitationGilski & Borkenhagen, 2005). It is worthwhile to mention that ischemic heart disease (IHD) continues to be the major cause of CVD. This disease would persist as the major and the most common threat to human life (CitationLopez & Murray, 1998). Hypertension is a major risk factor of IHD. NG-nitro-l-arginine methyl ester (L-NAME) has been used to induce hypertension in rats by CitationHropot et al. (1994).
Therapeutic effect of many plant extracts and herbal formulation such as Solanum torvum Sw. (Solanaceae) fruits (CitationNguelefack et al., 2009), Crataegus tanacetifolia (Lam.) Pers. (Rosaceae) leaf (CitationKoçyildiz et al., 2006), Solanum anguivi Lam. (Solanaceae) (CitationBahgat et al., 2008), Fritillaria cirrhosa D. Don (Liliaceae) (CitationKang et al., 2004), bark of Mammea africana Sabine (Guttiferae) (CitationNguelefack-Mbuyo et al., 2008) and herbal formulation Toki-shakuyaku-san (CitationTakei et al., 2004) in reducing L-NAME induced hypertension have been reported.
In the traditional system Lagenaria siceraria (Molina) Standley (Cucurbitacae) fruit (Bottle gourd) is used as a cardiotonic, in urinary infection, as an antihelmintic, hepatoprotective (CitationDvorkin & Whelan, 2007), diuretic (CitationGhule et al., 2007), antihyperlipidemic (CitationGhule et al., 2009), analgesic and anti-inflammatory (CitationGhule et al., 2006). Previous studies in our laboratory showed cardioprotective and antihypertensive effect of Lagenaria siceraria (LS) fruit powder in isoprenalin-induced cardiotoxicity and dexamethasone induced hypertension rats, respectively (Mali & Bodhankar, 2010 a, b). However, the cardioprotective and antihypertensive effect of LS in L-NAME induced hypertension model in rats have not been reported. Using chronic administration of L-NAME in vivo, we designed the present study with the following objectives. The first objective was to study the antihypertensive and cardioprotective effect of LS fruit powder in L-NAME induced hypertensive rats. The second objective was to characterize the methanol extract of LS fruit by HPLC.
Materials and methods
Authentication and preparation of samples
The mature fruit with seeds were collected in the month of April from local market of Pune, India and was identified and authenticated by Dr. A. S. Upadhye, Agharkar Research Institute (ARI), Pune, India (Auth.07-47 and Voucher no. F-174). LS fruits were processed at the manufacturing facilities of Ayush Wheatgrass Remedies Pvt. Ltd. (Pune, India). Whole fruits were cut into small flakes and dried on tray dryer at the temperature of 40°C. The dried flakes were processed in pulveriser to obtain the powder which was labeled as LS powder.
Chemicals
NG-Nitro-L-arginine methyl ester (L-NAME) manufactured by Fluka Analytical was procured for induction of hypertension. L-arginine was procured from Central Drug House (P) Ltd., New Delhi, India.
Solution preparation
Weighed quantity of LS fruit powder (500 mg/kg, p.o., LS suspended in 2% gum acacia), L-arginine (100 mg/kg) and L-NAME (40 mg/kg) was administered by oral route with distilled water using oral feeding gavages.
Experimental animals
Male albino Wistar rats weighing between 200 and 230 g were used in the study. The research protocol was approved by the Institutional Animal Ethics Committee (IAEC) (CPCSEA/74/10). The animals were housed at an ambient temperature 25 ± 2°C and relative humidity 50 ± 2% and light and dark cycle (12 h light/dark). The animals had access to pellet diet (Chakan Oil Mills, Pune) and water ad libitum.
Acute oral toxicity
Acute oral toxicity study was performed by using OECD-423 guideline. Female Swiss albino mice weighing 22–24 g were divided in two groups each consisting of three animals. In first group LS powder was administered orally at the dose of 2,000 mg/kg while the second group received 5,000 mg/kg. The animals were observed for toxic symptoms and mortality for 72 h after LS administration.
Experimental design and protocol
A total number of 24 animals were randomly divided into four groups comprising of six animals per group. Once a week, the rat’s blood pressure was measured by tail-cuff (ADInstrument, Australia). Hypertension was induced in rats of Groups II, III and IV by administration of L-NAME (40 mg/kg) p.o. according to method reported by CitationNguelefack et al. (2009).
Group I. Control group—2% gum acacia in distilled water (vehicle) for 4 weeks
Group II. L-NAME group—L-NAME (40 mg/kg/day p.o.) for 4 weeks
Group III. L-arginine (100 mg/kg/day p.o. for 4 weeks) + L-NAME (40 mg/kg/day p.o. for 4 weeks)
Group IV. LS (500 mg/kg, p.o. LS suspended in 2% gum acacia, p.o. for 4 weeks) + L-NAME (40 mg/kg/day p.o. for 4 weeks).
Serum parameters
On day 29 the rats were lightly anaesthetized with anaesthetic ether and blood was withdrawn from the retro-orbital plexus of each rat. Serum was separated and lactate dehydrogenase (LDH), creatine phosphokinase-MB isoenzyme (CK-MB), aspartate transaminase (AST) and alanine transaminase (ALT) were measured by using standard kits (Merck Specialities Pvt. Ltd., India). The serum total cholesterol (CHOL), triglycerides (TG) was quantified using enzymatic kits (Accurex Biomedical limited Pvt. Ltd, India) as per manufacturer’s instruction manual.
Haemodynamic parameters
At the end of experiment (i.e., on the day 29) animals were anaesthetized by urethane (1.25 g/kg). The right carotid artery of each rat was cannulated for the measurement of heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean aterial blood pressure (MABP). The cannula was filled with heparinized saline and connected to pressure transducer. After 30 min of stabilization the haemodynamic parameters were recorded by eight channel Power lab recorder (ADInstruments, Australia) having LABCHART-6 pro software.
After recording haemodynamic parameters the animals were euthanized and heart from each animal was removed and placed in 10% formalin solution.
Histology
The organ specimens were subjected to dehydration with xylene (1 h) and for 2 h with strength of alcohol of 70, 90 and 100%, respectively. The infiltration and impregnation was carried out by treatment with paraffin wax twice, each time for 1 h. Parrafin wax was used to prepare paraffin L-molds. Specimens were cut into sections of 3–5 mm thickness and stained with haematoxylin and eosin (H & E). The sections were mounted by diestrene phthalate xylene (D.P.X.). The parameters of histopathology assessment of heart sections were inflammation, necrosis and congestion. The grading system used for assessment of parameter was [−−, absence of change; +, 0–35% area shows changes; ++, 35–70% area shows changes; +++, 70–100 area shows changes].
Characterization of methanol extract of LS powder
Standardization: LS powder was treated with methanol (cold) and the filtrate was concentrated and dried below 40°C to get a paste and the yield was 33%. The obtained viscous material was dissolved in methanol and subjected to HPLC analysis under the following conditions:
Column: Kromasil C-18 (5 micron RP) 250 mm length × 4.6 mm diameter
Detector: UV 210 nm
Mobile phase: water:methanol starting at 75/25 and after 20 min 65/35 under linear gradient of 1 mL/min flow rate.
Instrument: HPLC, JASCO—model binary-2000 series comparising of UV-2075 plus, PU-2080 plus, AS-2055 plus.
Reference standards (vitexin, orientin, isoorientin) from Chromadex were used and the flavonol C-glycosides were identified and estimated in the paste. The quantity present in the dried LS powder was derived.
Statistical analysis
Data were expressed as the mean ± SEM. Statistical analysis was carried out by one-way ANOVA followed by post hoc Bonferroni test using Graphpad prism-5.
Results
Acute toxicity
The acute oral toxicity test showed that LS powder 2,000 and 5,000 mg/kg was nontoxic to the mice.
Biochemical parameters
In L-NAME alone group there was increase in serum CK-MB and LDH on day 29, whereas concomitant treatment with LS + L-NAME for 28 days showed reduction in serum CK-MB and LDH compared to control group. Concomitantly administration of L-arginine + L-NAME for 28 days showed reduced CK-MB and LDH which was elevated after L-NAME alone treatment.
L-NAME alone increased AST and ALT, whereas in LS + L-NAME treated animals AST and ALT were not increased after 28 days. Similar concomitant treatment with L-arginine + L-NAME was also effective in reducing AST and ALT. In L-NAME alone group, there was significant increase in serum cholesterol but TG increased nonsignificantly when compared to control group. Whereas concomitant treatment with LS + L-NAME for 28 days showed significant reduction in cholesterol and nonsignificant decrease in TG levels as compared to L-NAME alone ().
Table 1. Effect of LS on serum biochemical parameters in rats after L-NAME induced hypertension.
Non-invasive blood pressure (Tail-cuff method)
L-NAME alone produced significant (p < 0.001) increase in the systolic BP (mmHg) compared to that of the control group on 2nd, 3rd and 4th week. In LS + L-NAME group the systolic BP significantly decreased compared to L-NAME alone on 3rd and 4th week. The result thus indicated that LS pretreatment for 28 days is required for revealing reduction of L-NAME induced hypertension. The decrease was significant p < 0.05 after 3 week and p < 0.001 after 4 weeks treatment. These results are similar to that of L-arginine + L-NAME treated rats in which significant reduction of systolic blood pressure was observed compared to L-NAME alone ().
Table 2. Effect of L-NAME on non-invasive blood pressure (Tail-cuff method) during 28 days treatment of LS (500 mg/kg).
Invasive blood pressure
The control rats showed mean blood pressure on day 29 as systolic BP (SBP) 123.3 ± 1.25 mmHg, diastolic BP (DBP) 92.67 ± 2.72 mmHg and the mean arterial blood pressure (MABP) 118.2 ± 2.35 mmHg. Rats treated with L-NAME showed SBP 167.2 ± 0.85 mmHg, DBP 144.6 ± 0.81 mm Hg, MABP 151.9 ± 0.74 mmHg. L-NAME produced significant increase in SBP, DBP and MABP in comparison to control. The result thus indicated the hypertensive effect of L-NAME. LS + L-NAME on the other hand showed SBP 146.1 ± 3.35 mmHg, DBP 124.3 ± 2.30 mmHg and MABP 131.9 ± 1.03 mmHg compared to L-NAME alone group. This indicated a significant reduction in SBP, DBP and MABP. Rats treated with L-NAME + L-arginine showed SBP 139.3 ± 1.68 mmHg, DBP 121.7 ± 1.11 mmHg and MABP 124.3 ± 1.20 mmHg. The results indicated further that LS as well as L-arginine treatment produced significant (p < 0.001) attenuation of hypertensive effect of L-NAME ().
Table 3. Effect of L-NAME alone and with LS and l-arginine on heamodynamic parameters recovered by invasive method in rats treated for 4 weeks.
Histopathology
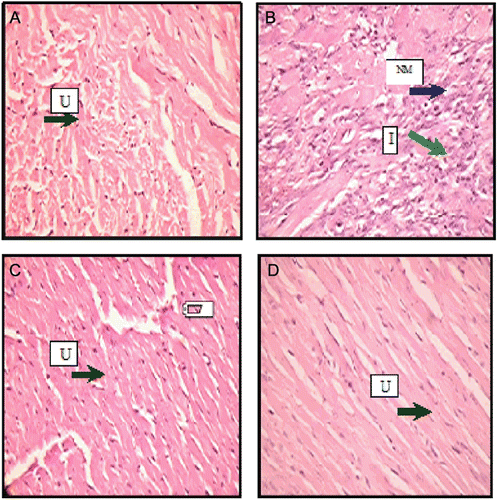
The heart of control group rat (A) showed absence of inflammation, necrosis and atrophy (graded as −−: absence of changes). L-NAME alone (40 mg/kg, p. o., 4 weeks) (B) administered group showed inflammation (+) and necrosis (+) which means that 0–35% area of the rat’s heart was inflamed and necrosis occurred in 0–35% area of heart. Inflammation and necrosis in large areas of heart confirmed cardiotoxicity in rats. On the other hand, inflammation and necrosis were absent in the heart of rat treated with LS and L-arginine. The results of histology thus confirmed that the rat’s heart was protected by concominant administration of LS (500 mg/kg) or L-arginine (100 mg/kg) with L-NAME ().
Figure 1. Photomicrographs of histological changes of rat heart. (A) (40×) (−−, absence of change), Control group; (B) (40×) (+, 0–35% necrosis, inflammation); L-NAME (40 mg/kg, p.o.) group; (C) (40×) LS (500 mg/kg, p.o.) + L-NAME (40 mg/kg, p.o.) (−−, absence of change); (D) (40×) l-arginine (100 mg/kg p. o.) + L-NAME (40 mg/kg, p. o.) U, Unremarkable; NM, Necrotic myocardial cells; I, Inflammatory cells.
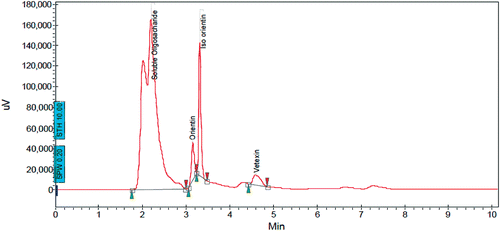
Characterization of the methanol extract of LS fruit by HPLC
The quantities of flavonol C-glycosides in milligram per 100 g of the LS powder were Vitexin: 28 mg/100 g (0.028%), Isoorientin: 95 mg/100 g (0.095%), Orientin: 18 mg/100 g (0.018%) and other constituents were identified as water soluble oligosaccharides ().
Discussion
In the present study L-NAME was used to induce hypertension in rats. L-NAME has been reported to induce arterial hypertension associated with deficiency of nitric oxide (NO). It has been also reported that in L-NAME treated animals, cardiac hypertrophy is present which is compensatory to chronic increase in blood pressure (CitationDevlin et al., 1998). A number of reports have analyzed the reason for L-NAME induced arterial hypertension, which is said to be due to deficiency of NO that has been reported to control coronary vascular tone (CitationKelm & Schrader, 1990). This decrease in endothelium dependent NO arterial dilatation is related to the risk of coronary ischemia and infarction (CitationCelermajer et al., 1994). The chronic elevation of NO synthase also caused myocardial infarction in rats. The blockade of NO synthase by L-NAME results in increased serum cholesterol level in rats (CitationKhedara et al., 1996). In view of these reports, it is hypothesized that decreased NO levels may be a risk factor for coronary disease and myocardial infarction. In this study, chronic administration of L-NAME increased systolic BP in rats which was recorded by tail cuff method. Our results are thus in agreement with the earlier reports by CitationVogel & Vogel, (2008). It has been reported that NO inhibition by L-NAME accelerates hypertension and induces perivascular inflammation (CitationHsieh et al., 2004). L-NAME induced hypertension and cardiac damage were selected for evaluating the antihypertensive and cardioprotective activity of LS.
It is well established that the biological markers like endogenous enzymes are organ specific and leak from the damaged organ during necrosis (CitationHearse, 1979). The endogenous cardiac biomarkers such as LDH and CK-MB are released into the perfusate or serum during damage to myocardium (CitationDumoulin et al., 2005).
In the present study L-arginine and L-NAME were concomitantly administered. Nitric oxide (NO) is produced from L-arginine by a family of enzymes known as NO synthases (NOS). Cardiac muscle fibers and coronary vascular beds express the enzyme endothelial NOS (CitationBalligand et al., 1995). Numerous studies have shown that hypertension is accompanied by reduced endothelial function, which can be rescued to varying degrees by L-arginine supplementation (CitationSchlaich et al., 2004; CitationHigashi et al., 1995; CitationQuyyumi et al., 1997; CitationLerman et al., 1998; CitationLekakis et al., 2002). L-Arginine is conditionally essential amino acid in the human diet. L-Arginine deficiency is linked to a variety of inflammatory and oxidative processes in the vascular endothelium and may be crucial in the development of atherosclerosis. The most common dietary sources of L-arginine are meat, poultry, fish, dairy products and nuts (CitationVisek, 1986; CitationFeldman, 2002; CitationHu et al., 1998). Lower intake of L-arginine rich foods such as fish and nuts has been consistently shown to be associated with future cardiovascular risk (CitationJärvinen et al., 2006). The metabolic effects of antihypertensives are important because hypertension does not occur in isolation but most often is accompanied by obesity, hyperlipidemia and hyperinsulinemia. This cluster is known as metabolic syndrome which leads to increased morbidity and mortality from cardiovascular disease (CitationRevan, 1993). The result of the present study showed attenuation of L-NAME induced hypertension by concomitant L-arginine treatment. Numerous studies (CitationVijayakumara et al., 2011) including work from our group (CitationHassanpour et al., 2008; CitationMali & Bodhankar, 2010a) have shown that LS posses cardioprotective activity.
This investigation confirmed the beneficial effect of LS powder on regression of hypertension and the prevention of myocardial damages associated with L-NAME treatment. LS also showed antihyperlipidemic (CitationGhule et al., 2009) and antihypertensive activity (CitationMali & Bodhankar, 2010b) in high fat diet induced hyperlipedimic and dexamethasone induced hypertension, respectively. Reduction in cholesterol in LS + L-NAME treated rats endorses the antihyperlipidemic effect of LS in the present L-NAME model of hypertension. Furthermore in the present study L-NAME produced inflammation and necrosis which confirmed cardiotoxicity. The histology of heart of rat treated with LS or L-arginine did not show inflammation and necrosis. This observation correlated with the cardioprotective effect of LS or L-arginine. In this investigation LS and L-arginine showed similarity in exhibiting antihypertensive, antihyperlipidemic and cardioprotective effects.
It is well known that natural products show antioxidant activity. The correlation between antioxidant and cardioproctetive activities of phenolic extracts of fruits of Aristotelia chilensis (Molina) Stuntz (Elaeocarpaceae) have been demonstrated (CitationCespedes et al., 2008). The antihypertensive activity of Phyllanthus urinaria L. (Euphorbiaceae) (CitationLin et al., 2008), Trigonella foenum-greacum L. (Leguminosae) seeds (CitationBalaraman et al., 2006), Viscum triflorum DC. (Santalaceae) (CitationAdsersen et al., 2011) and Moringa oleifera Lam. (Moringaceae) (CitationDangi et al., 2002) has been reported.
Hypertension is an important risk factor for coronary heart disease and stroke. Flavonoids have been considered as active principles of several antihypertensive plant extracts like leaves of Diospyros virginiana L. (Ebenaceae) (CitationKameda et al., 1987), Capparis cuatrecasana Dugand (Capparaceae) (CitationGilani & Khalid, 1994), rhoifolin, luteolin, and apiin (CitationOcchiuto & Limardi, 1994), Rhamnus lycioides L. (Rhamnaceae) (CitationVillar et al., 1986) and Crataegus oxyyacantha L. (Rosaceae) (CitationWalker et al., 2002). Flavonoids affect the inflammatory process of the mammalian system and possess antiinflammatory as well as immunomodulatory activities in vitro and in vivo. As nitric oxide (NO) produced by inducible nitric oxide synthase (iNOS) is one of the inflammatory mediators, the effects of various naturally occurring flavonoids on NO production in LPS-activated RAW 264.7 cells were evaluated in vitro (CitationKim et al., 1999). Flavonoid complexes occurring in the medicinal plants thus showed variation. Chromatographic analysis of the methanol extract of LS fruit powder showed presence of vitexin, isoorientin, and orientin. Our results indicated that vitexin is present in the Indian variety of the fruits. The effects of plant flavonoids on mammalian cells have been of substantial recent interest with attention focused on the implications of these agents for cardiovascular disease and for cancer. Some epidemiological studies designed to examine a possible protective effect of flavonoids in cardiovascular disease have reported inverse associations (CitationBayard et al., 2007).
CitationKrauze and Cisowiski (1994) reported the separation of complex of flavone C-glycosides of LS. Vitexin was absent in flowering herbs, whereas saponarin, isoorientin and saponnarin 4-O-glucoside were present. CitationChen et al. (2008) reported isolation of four new D: C-friedooleanane triterpenes from the methanol extract of the stems of LS and reported anticancer activity against SK-Hep1 cell lines.
CitationOcchiuto et al. (1990) examined the effect of rhoifolin and vitexin on haemodynamic effects in the dog. Vitexin reduced aortic pressure (8%), significant (p < 0.01) reduction in mean arterial pulmonary pressure and in pulmonary capillary wedge pressure. Vitexin did not depress left ventricular function. In the hybrid of LS two triterpenoid namely 22-deoxocucurbitacin and 22-deoxoisocucurbitacin D were identified (CitationEslin et al., 1967). However, pharmacological actions of these compounds have not been reported.
Isoorientin and vitexin were isolated from Arum palaestinum Boiss. (Araceae). Isoorientin produced dose- dependent inhibition, amplitude and frequency of the phasic contractions of rat and guinea pig uterus but not of isolated aorta, ileum or trachea (CitationAfifi et al., 1999). Isolation of orientin from Gentiana olivieri Griseb. (Gentianaceae) and its hepatoprotective effect has been reported by CitationOrhan et al. (2003). CitationKo et al. (1998) demonstrated antioxidant activity of isoorientin-6-O-glycoside obtained from Gentiana arisanensis Hayata (Gentianaceae) and correlated it to the prevention of LDL oxidation. CitationBudzianowski et al. (1991) reported antioxidant activity of orientin and vitexin. CitationLin et al. (2002) observed weak antioxidant activity of isovitexin. On the other hand CitationHoffmann-Bohm et al. (1992) could not establish a link between antioxidant activity and hepatoprotective action of orientin. It has been reported that cardioprotective effect of Ilex paraguariensis A.St.-Hil. (Aquifoliaceae) (CitationSchinella et al., 2009), Salvia miltiorrhiza var. miltiorrhiza (Lamiaceae) (CitationTang et al., 2011) may be due to antioxidant activity. In this context, the antioxidant property of orientin and isoorentin may contribute to cardioprotecive effect of LS observed in the present study.
Conclusion
It is concluded that concomitant administration of LS fruit powder or L-arginine with L-NAME significantly reduced L-NAME induced hypertension in rats. The flavonol vitexin detected in LS appear to contribute to this antihypertensive activity. L-NAME damaged myocardium which was confirmed by presence of inflammation and necrosis. The absence of myocardial necrosis and inflammation in LS treated group suggested cardioprotective activity. The antioxidants orientin and isoorentin detected in LS fruit powder appear to reduce L-NAME induced myocardial inflammation and necrosis. It is thus concluded that LS fruit posses antihypertensive and cardioprotective activity.
Declaration of interest
The authors report no declarations of interest.
Acknowledgements
The authors would like to acknowledge Dr. S. S. Kadam, Vice-Chancellor and Dr. K. R. Mahadik, Principal, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Pune, India, for providing necessary facilities to carry out the study. We are also thankful to Mr. Redekar (Ayush Wheatgrass Remedies Pvt. Ltd. Pune, India) for providing us Lagenaria siceraria fruit powder. We are also thankful to Dr. Amit Nisal and Dr. Sujit Joshi (Pathologist, Deenanath Mangeshkar Hospital, Pune) for evaluating biochemical study and histopathological parameter.
References
- Adsersen A, Mogensen P, Adsersen H. (2011). Angiotensin-converting enzyme inhibitory activity of Viscum triflorum is host plant-dependent. Pharm Biol, 49, 302–305.
- Afifi FU, Khalil E, Abdalla S. (1999). Effect of isoorientin isolated from Arum palaestinum on uterine smooth muscle of rats and guinea pigs. J Ethnopharmacol, 65, 173–177.
- Bahgat A, Abdel-Aziz H, Raafat M, Mahdy A, El-Khatib AS, Ismail A, Khayyal MT. (2008). Solanum indicum ssp. distichum extract is effective against L-NAME-induced hypertension in rats. Fundam Clin Pharmacol, 22, 693–699.
- Balligand JL, Kobzik L, Han X, Kaye DM, Belhassen L, O’Hara DS, Kelly RA, Smith TW, Michel T. (1995). Nitric oxide-dependent parasympathetic signaling is due to activation of constitutive endothelial (type III) nitric oxide synthase in cardiac myocytes. J Biol Chem, 270, 14582–14586.
- Balaraman R, Dangwal S, Mohan M. (2006). Antihypertensive effect of Trigonella foenum-greacum seeds in experimentally induced hypertension in rats. Pharm Biol, 44, 568–575.
- Bayard V, Chamorro F, Motta J, Hollenberg NK. (2007). Does flavanol intake influence mortality from nitric oxide-dependent processes? Ischemic heart disease, stroke, diabetes mellitus, and cancer in Panama. Int J Med Sci, 4, 53–58.
- Budzianowski J, Pakulski G, Robak J. (1991). Studies on antioxidative activity of some C-glycosylflavones. Pol J Pharmacol Pharm, 43, 395–401.
- Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. (1994). Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol, 24, 1468–1474.
- Cespedes CL, El-Hafidi M, Pavon N, Alarcon J. (2008). Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem, 107, 820–829.
- Chen CR, Chen HW, Chang CI. (2008). D:C-Friedooleanane-type triterpenoids from Lagenaria siceraria and their cytotoxic activity. Chem Pharm Bull, 56, 385–388.
- Dangi SY, Jolly CI, Narayanan S. (2002).Antihypertensive activity of the total alkaloids from the leaves of Moringa oleifera. Pharm Biol, 40, 144–148.
- Devlin AM, Brosnan JM, Graham D, Morton JJ, McPhaden AR, McIntyre M, Hamilton AC, Reid JL, Dominiczak AF. (1998). Vascular smooth muscle cell polyploidy and cardiomyocyte hypertrophy due to chronic NOS inhibition in vivo. Am J Heart Cir Physiol, 247, 52–59.
- Dumoulin MJ, Adam A, Burnett J, Heublein D, Yamaguchi N, Lamontagne D. (2005). The cardioprotective effect of dual metallopeptidase inhibition: Respective roles of endogenous kinins and natriuretic peptides. Can J Physiol Pharmacol, 83, 166–173.
- Dvorkin L, Whelan J. (2007). Boston healing landscape project. 1-3. Clinical, Herbal index, A detailed index of the various herbs used in traditional medicine. Lagenaria siceraria (online). Available at: http://www.bu.edu/bhlp/Clinical/crosscultural/herbal_index/herbs/Lagenaria%20Siceraria.html. Accessed on 14 August 2011.
- Eslin PR, Holzapfel CW, Barbara K. (1967). Bitter principles of Cucurbitaceae Part XV. Cucurbitacins from a hybrid of Lagenaria siceraria. J Chem Soc, 10, 964–972.
- Feldman EB. (2002). The scientific evidence for a beneficial health relationship between walnuts and coronary heart disease. J Nutr, 132, 1062S–1101S.
- Ghule BV, Ghante MH, Upaganlawar A, Yeole PG. (2006). Analgesic and anti-inflammatory activity of Lagenaria siceraria fruit juice extract in rats and mice. Pharm Mag, 2, 232–238.
- Ghule BV, Ghante MH, Yeole PG, Saoji AN. (2007). Diuretic activity of Lagenaria siceraria fruits extract in rats. Ind J Pharm Sci, 69, 817–819.
- Ghule BV, Ghante MH, Saoji AN, Yeole PG. (2009). Antihyperlipidemic effect of the methanolic extract from Lagenaria siceraria Stand. fruit in hyperlipidemic rats. J Ethnopharmacol, 124, 333–337.
- Gilani AH, Khalid A. (1994). Hypotensive and spasmolytic activities of ethanolic extract of Capparis curtilaginea Phyto Res, 8, 145–148.
- Gilski DJ, Borkenhagen B. (2005). Risk evaluation in action for cardiovascular health. Crit Care Nurse, 25, 26–8, 30.
- Hassanpour F, Bodhankar SL, Dikshit M. (2008). Cardioprotective activity of fruit of Lagenaria siceraria (Molina) Standley on doxorubicine induced cardiotoxicity in rats. Int J Pharmacol, 4, 466–471.
- Hearse D. (1979). Cellular Damage During Myocardial Ischaemia: Metabolic Changes Leading to Enzyme Leakage. In Enzymes in Cardiology. New York: John Wiley and Sons Ltd, 1–21.
- Higashi Y, Oshima T, Ozono R, Watanabe M, Matsuura H, Kajiyama G. (1995). Effects of l-arginine infusion on renal hemodynamics in patients with mild essential hypertension. Hypertension, 25, 898–902.
- Hoffmann-Bohm K, Lotter H, Seligmann O, Wagner H. (1992). Antihepatotoxic C-glycosylflavones from the leaves of Allophyllus edulis var. edulis and gracilis. Planta Med, 58, 544–548.
- Hropot M, Grötsch H, Klaus E, Langer KH, Linz W, Wiemer G, Schölkens BA. (1994). Ramipril prevents the detrimental sequels of chronic NO synthase inhibition in rats: hypertension, cardiac hypertrophy and renal insufficiency. Naunyn Schmiedebergs Arch Pharmacol, 350, 646–652.
- Hsieh NK, Wang JY, Liu JC, Wang SD, Chen HI. (2004). Nitric oxide inhibition accelerates hypertension and induces perivascular inflammation in rats. Clin Exp Pharmacol Physiol, 31, 212–218.
- Hu FB, Stampfer MJ, Manson JE, Rimm EB, Colditz GA, Rosner BA, Speizer FE, Hennekens CH, Willett WC. (1998). Frequent nut consumption and risk of coronary heart disease in women: Prospective cohort study. BMJ, 317, 1341–1345.
- Järvinen R, Knekt P, Rissanen H, Reunanen A. (2006). Intake of fish and long-chain n-3 fatty acids and the risk of coronary heart mortality in men and women. Br J Nutr, 95, 824–829.
- Kameda K, Takaku T, Okuda H, Kimura Y, Okuda T, Hatano T, Agata I, Arichi S. (1987). Inhibitory effects of various flavonoids isolated from leaves of persimmon on angiotensin-converting enzyme activity. J Nat Prod, 50, 680–683.
- Kang DG, Sohn EJ, Lee YM, Lee AS, Han JH, Kim TY, Lee HS. (2004). Effects of bulbus Fritillaria water extract on blood pressure and renal functions in the L-NAME-induced hypertensive rats. J Ethnopharmacol, 91, 51–56.
- Kelm M, Schrader J. (1990). Control of coronary vascular tone by nitric oxide. Circ Res, 66, 1561–1575.
- Khedara A, Kawai Y, Kayashita J, Kato N. (1996). Feeding rats the nitric oxide synthase inhibitor, l-N(omega)nitroarginine, elevates serum triglyceride and cholesterol and lowers hepatic fatty acid oxidation. J Nutr, 126, 2563–2567.
- Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP. (1999). Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem Pharmacol, 58, 759–765.
- Ko FN, Chu CC, Lin CN, Chang CC, Teng CM. (1998). Isoorientin-6”-O-glucoside, a water-soluble antioxidant isolated from Gentiana arisanensis. Biochim Biophys Acta, 1389, 81–90.
- Koçyildiz ZC, Birman H, Olgaç V, Akgün-Dar K, Melikoglu G, Meriçli AH. (2006). Crataegus tanacetifolia leaf extract prevents L-NAME-induced hypertension in rats: a morphological study. Phytother Res, 20, 66–70.
- Krauze BM, Cisowiski W. (1994). High-performance liquid chromatographic determination of flavone C-glycosides in some species of the Cucurbitaceae family. J Chromatography, 67, 240–243.
- Lekakis JP, Papathanassiou S, Papaioannou TG, Papamichael CM, Zakopoulos N, Kotsis V, Dagre AG, Stamatelopoulos K, Protogerou A, Stamatelopoulos SF. (2002). Oral l-arginine improves endothelial dysfunction in patients with essential hypertension. Int J Cardiol, 86, 317–323.
- Lerman A, Burnett JC Jr, Higano ST, McKinley LJ, Holmes DR Jr (1998). Long-term l-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation, 97, 2123–2128.
- Lin CM, Chen CS, Chen CT, Liang YC, Lin JK. (2002). Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem Biophys Res Commun, 294, 167–172.
- Lin SY, Wang CC, Lu YL, Wu WC, Hou WC. (2008). Antioxidant, anti-semicarbazide-sensitive amine oxidase, and anti-hypertensive activities of geraniin isolated from Phyllanthus urinaria. Food Chem Toxicol, 46, 2485–2492.
- Lopez AD, Murray CC. (1998). The global burden of disease, 1990-2020. Nat Med, 4, 1241–1243.
- Mali VR, Bodhankar SL. (2010a). Cardioprotective effect of Lagenaria siceraria (LS) fruit powder in isoprenalin-induced cardiotoxicity in rats. Eur J Integ Med, 2, 143–149.
- Mali VR, Bodhankar SL. (2010b).Effect of Lagenaria siceraria (LS) powder on dexamethasone induced hypertension in rats.Int J Adv Pharm Sci, 1, 50–53.
- Nguelefack-Mbuyo PE, Nguelefack TB, Dongmo AB, Afkir S, Azebaze AG, Dimo T, Legssyer A, Kamanyi A, Ziyyat A. (2008). Anti-hypertensive effects of the methanol/methylene chloride stem bark extract of Mammea africana in L-NAME-induced hypertensive rats. J Ethnopharmacol, 117, 446–450.
- Nguelefack TB, Mekhfib H, Dongmoc AB, Dimod T, Watchoa P, Zoheire J, Legssyerb A, Kamanyi A, Ziyyat A. (2009). Hypertensive effects of oral administration of the aqueous extract of Solanum torvum fruits in L-NAME treated rats: Evidence from in vivo and in vitro studies. J Ethnopharmacol, 124, 592–599.
- Occhiuto F, Limardi F. (1994). Comparative effects of the flavonoids luteolin, apiin and rhoifolin on experimental pulmonary hypertension in the dog. Phyto Res, 8, 153–156.
- Occhiuto F, Pasquale A, Briguglio F. (1990). Comparative haemodynamic effects of the flavonoids rhoifolin and vitexin in the dog. Phyto Res, 4, 118–120.
- Orhan DD, Aslan M, Aktay G, Ergun E, Yesilada E, Ergun F. (2003). Evaluation of hepatoprotective effect of Gentiana olivieri herbs on subacute administration and isolation of active principle. Life Sci, 72, 2273–2283.
- Quyyumi AA, Dakak N, Diodati JG, Gilligan DM, Panza JA, Cannon RO 3rd. (1997). Effect of l-arginine on human coronary endothelium-dependent and physiologic vasodilation. J Am Coll Cardiol, 30, 1220–1227.
- Revan GM. (1993). Role of insulin resistant in human disease (syndrome X): An expanded definition. Ann Rev Med, 44, 121–131.
- Schinella G, Fantinelli JC, Tournier H, Prieto JM, Spegazzini E, Debenedetti S, Mosca SM. (2009). Antioxidant and cardioprotective effects of Ilex brasiliensis: A comparative study with Ilex paraguariensis (yerba mate). Food Res Int, 42, 1403–1409.
- Schlaich MP, Parnell MM, Ahlers BA, Finch S, Marshall T, Zhang WZ, Kaye DM. (2004). Impaired l-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation, 110, 3680–3686.
- Takei H, Nakai Y, Hattori N, Yamamoto M, Kurauchi K, Sasaki H, Aburada M. (2004). The herbal medicine Toki-shakuyaku-san improves the hypertension and intrauterine growth retardation in preeclampsia rats induced by N-omega-nitro-l-arginine methyl ester. Phytomedicine, 11, 43–50.
- Tang Y, Wang M, Le X, Meng J, Huang L, Yu P, Chen J, Wu P. (2011). Antioxidant and cardioprotective effects of danshensu (3-(3, 4-dihydroxyphenyl)-2-hydroxy-propanoic acid from Salvia miltiorrhiza) on isoproterenol-induced myocardial hypertrophy in rats. Phytomedicine, 18, 1024–1030.
- Vijayakumara M, Selvia V, Krishnakumari S. (2011). Cardioprotective effect of Lagenaria siceraria (Mol) ameliorates isoproterenol-induced cardiac toxicity in rats by stabilizing cardiac mitochondrial and lysosomal enzymes. Toxicol Environ Chem, 93, 171–176.
- Villar A, Terencio MC, Paya M. (1986). Hypotensive effect of Rhamnus lycioides extracts. J Ethnopharmacol, 16, 269–273.
- Visek WJ. (1986). Arginine needs, physiological state and usual diets. A reevaluation. J Nutr, 116, 36–46.
- Vogel GH, Vogel VH. (2008). Drug Discovery and Evaluation, Pharmacological Assay. Second ed. Berlin, Heidelberg, New York: Springer-Verlag, 246–247.
- Walker AF, Marakis G, Morris AP, Robinson PA. (2002). Promising hypotensive effect of hawthorn extract: a randomized double-blind pilot study of mild, essential hypertension. Phytother Res, 16, 48–54.