Abstract
Context: Bombacaceae is a small family of the order Malvales and contains about 28 genera and 200 species. Members of this family are not only showy ornamentals but they possess significant economical and commercial reputation as well. In addition, various plant parts of several species are widely used as foods and traditional medicines in many parts of the world.
Objective: Chemical analyses of Bombacaceae species have recently yielded a number of important phytocompounds belonging to different classes. Hence, this work represents a comprehensive appraisal of the phytochemical studies conducted on Bombacaceae plants.
Materials and methods: Searches were conducted using electronic databases (e.g., Medline, Pubmed, Academic Journals, and Springer Link); general web searches were also undertaken using Google applying some related search terms "e.g., Bombacaceae, phytochemical studies of Bombacaeae plants, and chemical investigation of Bombacaeae", journals and scientific theses. The bibliographies of papers relating to the review subject were also searched for further relevant references.
Results: Chemical investigations were concentrated primarily on certain species leaving fertile fields for further phytopharmacological research.
Conclusion: The reviewed findings present Bombacaceae species as an untapped reservoir of phytocompounds which may play a supportive role in the pharmaceutical field and will be of high chemotaxonomic value within this recently separated family.
Keywords::
Introduction
Bombacaceae (Bombax, Baobab or Kapok family) is a small family of flowering plants which contains about 28 genera and 200 species (CitationJoly, 1991). Plants of this family are perennial, deciduous and woody trees. They occur naturally throughout the tropical and subtropical regions of the world especially in tropical America (CitationBenson, 1970). Many species grow to become large trees, with Ceiba pentandra L. Gaertn. the tallest, reaching a height of 70 m. Additionally, some of these plants have considerable girth, so called "bottle trees" and their trunks are usually with buttresses at the base (CitationFrankham et al., 1996). Besides the great significance of Bombacaceae plants as ornamentals due to their large branches and brightly colored flowers, several genera are economically and commercially important, producing timber, edible fruits, vegetable oils or useful fibers, e.g., silk floss trees (Chorisia spp.) and Kapok (fibers of Ceiba fruits) (CitationPerez-Arbelaez, 1956). The family is also noted for some of the softest hardwoods commercially traded, especially Balsa wood (Ochroma lagopus Swartz). The Baobabs (Adansonia spp.) are important icons in certain parts of Africa and Australia, noted for their immensely stout trunk development which is a mechanism for enhancing water storage (CitationPaula et al., 1997). Moreover, members of Bombacaceae found several folkloric medicinal uses in many countries due to their antipyretic, analgesic, anti-inflammatory, astringent, stimulant, diuretic, and antimicrobial properties (CitationPaula et al., 1997).
In old classical literature of taxonomy, Bombacaceae has been considered as a taxon or subfamily under Malvaceae. However, in the majority of the recent taxonomic works, Bombacaceae has been treated as an independent family of the order Malvales (CitationCronquist, 1981; CitationHeywood et al. 2007). In several anatomical and floral characters, Bombacaceae shows close affinities with Malvaceae. However, many of its genera show a close relationship with Dilleniaceae on the basis of their stamen morphology. Bombacaceae differs from Malvaceae in (i) being exclusively arborescent (woody trees), (ii) often possessing a prickly trunk, (iii) bearing dithecous anthers (bi-chambered) in some and monothecous in others, and (iv) always having smooth pollen grains (CitationSharma, 1993). Additionally, CitationCronquist (1981) shows a close relationship of Bombacaceae with Malvaceae, Sterculiaceae, and Tiliaceae.
Phytochemical studies
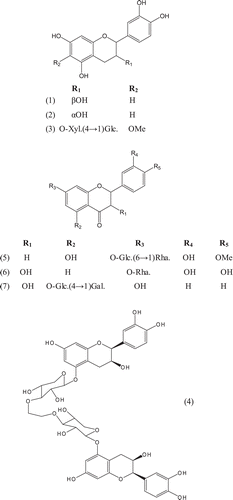
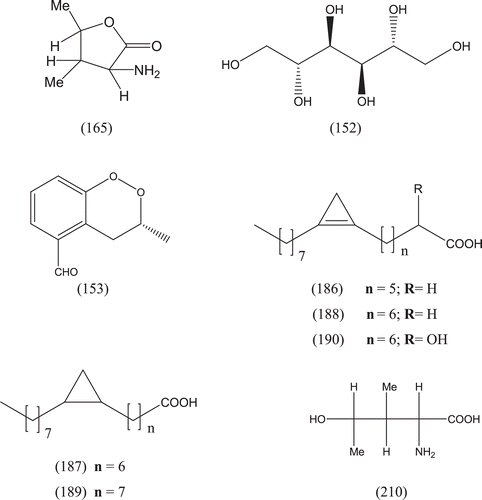
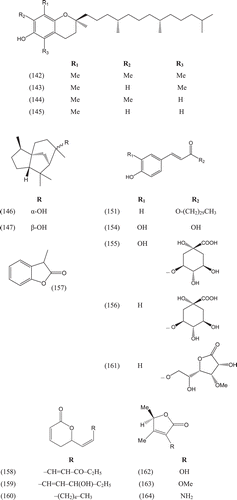
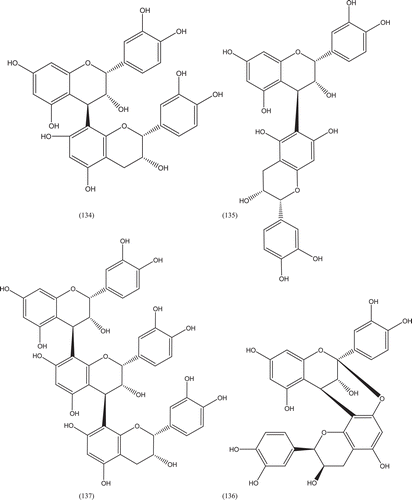
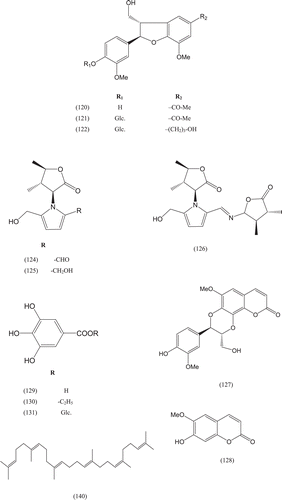
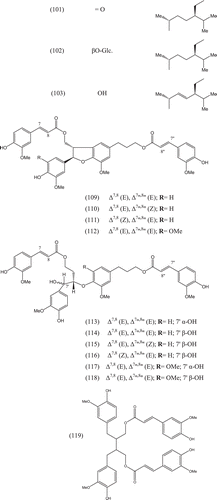
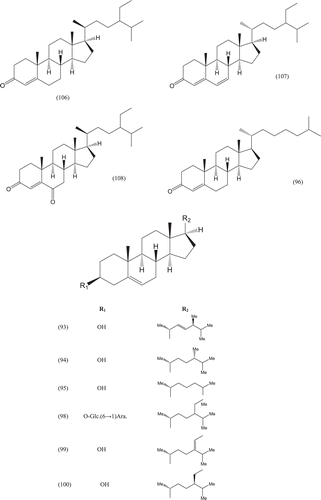
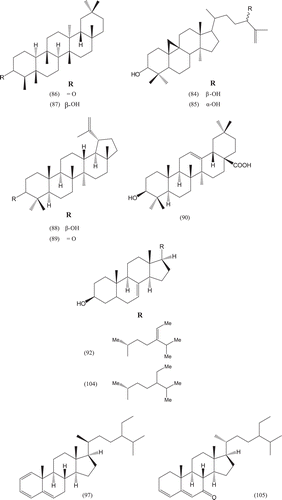
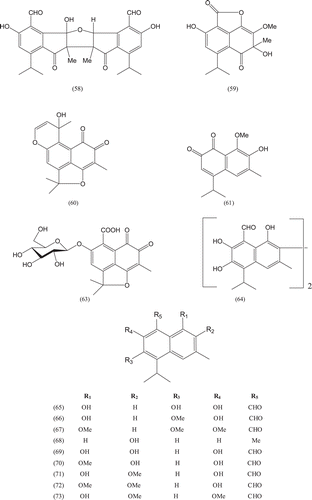
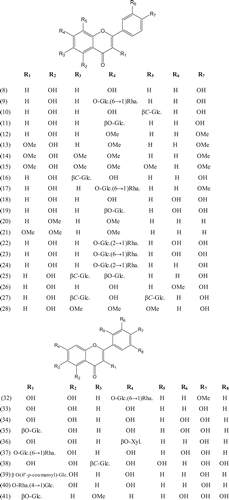
Phytochemical investigations of various parts of Bombacaceae plants resulted in the isolation of several diverse classes of compounds ( and ). From the data available in the literature, it can be observed there is apparently no relationship between the genera studied from the phytochemical point of view. However, this does not necessarily result in questioning of the botanical classification of the species of this family. This relationship cannot be observed among species of the same genus, mainly because from most of them, only few numbers were studied (CitationPaula et al., 1997). Adansonia, Bombax ceiba (syn. Bombax malabaricum, Bombax malabarica, Salmalia malabaricum, Gossampinus malabarica), Ceiba pentandra (syn. Bombax pentandrum), Chorisia, Ochroma, Pachira (syn. Bombacopsis), Pseudobombax and Quararibea are the most studied members. It is worth mentioning that the genus Durio (included in the review by CitationPaula et al., 1997) was excluded from Bombacaceae after CitationHeywood et al. (2007). Accordingly, this work represents an up-to- date comprehensive account on various classes of the isolated active principles from Bombacaceae plants together with their structural and stereochemical differences. In addition, their distribution in different plant parts of various species studied so far is also completely considered.
Table 1. A list of compounds isolated from family Bombacaceae.
In addition to the compounds mentioned in , the volatile constituents of some Bombacaceae plants were analyzed using GC/MS technique and several compounds belonging to different structural types were identified as follows.
The volatile oil of Adansonia digitata L. flowers was found to contain isoprenoids including: monoterpene hydrocarbons (0.6%) [e.g. (E)-ocimene (0.6%)], oxygenated monoterpenes (0.2%) [e.g. linalool (0.2%)], and irregular terpenes (21.1 %) [e.g. 6-methyl-5-hepten-2-ol (0.7%), 2-methyl-butanal (1%), 3-methylbutanal (16.3%), (E)-2-methyl-2-butenal (2.1%), 3-methyl-1-butanol (1.8%)], fatty acid derivatives (13%) including: [nonane (1.2%), 3-pentanone (1.4%), decane (0.5%), 3-pentanol (3.6%), butanol (1.7%), 1-penten-3-ol (2.2%), 2-heptanone (0.2%), 2-hexenal (1.7%), 3-hydroxy-2-butanone (0.6%)], benzenoids (7.8%) including: [benzaldehyde (7.8%)], and sulphur compounds (15.3%) including: [methyl thioacetate (1.4%), dimethyl disulphide (10.3%), methyl thiobutanoate (0.8%), methyl 3-methylbutan-ethioate (1.9%), dimethyl trisulphide (0.4%), methyl thiohexanoate (0.6%)]. On the other hand, the oil was free from sesquiterpene hydrocarbons and nitrogenous compounds (CitationPettersson et al., 2004).
Similarly, the volatile oil of Ceiba pentandra L. flowers was found to contain isoprenoids including: monoterpene hydrocarbons (34%) [e.g. α-pinene (7.1%), β-pinene (3.4%), sabinene (20.8%), α-phellandrene (0.9%), limonene (0.7%), p-cymene (1.2%)], oxygenated monoterpenes (8.4%) [e.g. 1,8-cineole (5.3%), trans-sabinene hydrate (1%), cis-sabinene hydrate (0.6%), terpinen-4-ol (0.5%), verbenone (0.9%)], and sesquiterpene hydrocarbons (26.9%) [e.g. α-copaene (5.5%), (E,E)-α-farnesene (20.3%)], fatty acid derivatives (18.1%) including: [3-pentanol (1%), 1-penten-3-ol (0.5%), 2-hexenal (8.9%), (Z)-3-hexenol (1.1%), 1-octen-3-ol (4.1%), pentanoic acid (2.5%)], benzenoids (7.8%) including: [benzaldehyde (1.9%), methyl benzoate (1.6%), 1-methoxy-4-(2-propenyl)-benzene (0.4%), methyl salicylate (1.8%), benzyl alcohol (1.8%), 2-phenyl ethanol (0.3%), 4-methoxy benzaldehyde (0.4%)], and miscellaneous compounds (2%) [e.g. 5-ethyl-2(5H)-furanone (2%)]. On the other hand, the oil was free from sulphur and nitrogen-containing compounds (CitationPettersson et al., 2004).
Moreover, the volatile oil of Eriotheca longitubulosa A. Robyns flowers was found to contain monoterpenes including [trans-ocimene (9.87%), limonene (1.67%), linalool (0.52%), camphor (0.48%), δ-cadinene (0.11%)]; sesquiterpenes including [α-farnesene (28.03%), germacrene-D (25.68%), caryophyllene (0.69%), germacrene-A (0.37%)]; irregular terpenes including [4,8,12-trime-1,3(E),7(E),11-tridecatetraene (1.2%)]; fatty acid derivatives including [cis-3-hexenol (0.63%), n-nonanal (1.85%), 6-methyl-3-heptanol (1.60%), 1-hepten-3-ol (1.13%), n-hexanal (0.64%), n-heptanal (0.36%), heptadecane (0.29%), undecane (0.16%), dodecane (0.15%), n-octadecane (0.14%)], benzenoids including: [toluene (6.92%), salicylaldehyde (2.24%), benzaldehyde (0.55%), naphthalene (0.48%), O-xylol (0.25%), phenol (0.93%), methyl salicylate (0.15%), acetophenone (0.15%)]; N-containing compounds including: [pyridine (0.94%)]; other miscellaneous compounds including cyclopentanone (0.27%), methyl senecioate (2.10%), hexenyl valerate (1.03%), 2-methyl-2-butensre-methyl (0.90%), methyl-2-methyl-butyrate (0.76%), cis-3-hexenyl-butyrate (0.59%), ethyl n-amyl ketone (3.17%), δ-3-carene (0.3%), (E,E)-2,6-Dime-1,3,5,7-octatetraene (0.26%), cis-3-hexenyl senecioate (0.25%), cis-geranyl-acetone (0.25%), β-cydrane (0.2%), β-jonylcrotonate (0.2%), precyclemone B (0.19%), 2-ethyl-hex-propionate (0.14%), α-muurolen (0.12%)] (CitationMacfarlane et al., 2003).
In another study, the volatile oil of Pachira dolichocalyx A. Robyns bark and leaves were found to contain (Z)-2-hexenol, octanone, hexenyl butanoate, allo-ocimene, trans-linalool-oxide, mentha-1-7(8)-diene, p-cymenene, verbenene, cadina-1(10),6-diene and epi-α-muurolol (CitationCourtois et al., 2009).
Conclusion
The overview of phytochemical studies on Bombacaceae has revealed a variety of chemical constituents produced by these plants. Flavonoids, anthocyanins, oxidized naphthalenes, sesquiterpenes, sesquiterpene lactones, triterpenes, steroids, lignans, alkaloids, amino acids, coumarins, long chain fatty acids as well as their esters, cyclopropenoid fatty acids and carbohydrates are the most significant isolated substances. Moreover, analysis of the volatile oils prepared from some Bombacaceae species demonstrated their richness in various compounds belonging to different structural types. As a result, the above reviewed findings doubtlessly present members of Bombacaceae as an untapped reservoir of chemical principles. This fact can also be substantiated by two evidences. Firstly, despite the large number of isolated compounds–especially after the great advance in isolation and spectral techniques–chemical investigations have focused predominantly on certain few species, with Adansonia, Bombax, Ceiba, Chorisia, Ochroma and Pachira, being relatively the most phytochemically visited genera leaving fertile fields for further phytochemical and pharmacological researches. Secondly, hybridization among different species is considered a common phenomenon among these plants. Both the unstudied species and new hybrids open the gate towards isolation of further new compounds. Furthermore, the chemical investigations of these untouched species will be of high chemotaxonomic value within this recently separated family.
Declaration of interest
The authors report no declarations of interest.
References
- Agrawal GD, Rizvi SA, Gupta PC, Tewari JD. (1972). Study of a polysaccharide from the stamens of Bombax malabaricum flowers. Planta Med, 21, 293–303.
- Al-Qarawi AA, Al-Damegh MA, El-Mougy SA. (2003). Hepatoprotective influence of Adansonia digitata pulp. J Herbs Spices Med Plants. 10, 1–6.
- Beleski-Carneiro EB, Ganter JL, Reicher F. (1999). Structural aspects of the exudate from the fruit of Chorisia speciosa St. Hil. Int J Biol Macromol, 26, 219–224.
- Beleski-Carneiro E, Sierakowski, MR, Ganter JL, Zawadzki-Baggio SF, Reichera F. (1996). Polysaccharides from Chorisia speciosa St. Hil. Prog Biotechnol, 14, 549–559.
- Beleski-Carneiro E, Sugui J, Reicher F. (2002). Structural and biological features of a hydrogel from seed coats of Chorisia speciosa. Phytochemistry. 61, 157–163.
- Bell AA, Stipanovic RD, O′Brien DH, Fryxell PA. (1978). Sesquiterpenoid aldehyde quinones and derivatives in pigment glands of Gossypium. Phytochemistry. 17, 1297–1305.
- Benson L. (1970). Plant Classification. New Delhi, Bombay:Oxford and IBH publishing Co., 793–797.
- Bianchini JP, Ralaimanarivo A, Gaydou EM, Waegell B. (1982). Hydrocarbons, sterols and tocopherols in the seeds of six Adansonia species. Phytochemistry. 21, 1981–1987.
- Bohannon MB, Kleiman R.. (1978). Cyclopropene fatty acids of selected seed oils from Bombacaceae, Malvaceae, and Sterculiaceae. Lipids. 13, 270–273.
- Bose S, Dutta AS. (1963). Structure of Salmalia malabarica gum, Part-I: Nature of sugars present and the structure of aldobiuronic acids. J Indian Chem Soc. 40, 257–262.
- Bravo JA, Lavaud C, Bourdy G, Giménez A, Sauvainc M. (2002). First bioguided phytochemical approach to Cavanillesia Aff. hylogeiton. Rev Bol Quim. 19, 18–24.
- Caffini NO, Lufrano NS. (1978). Mucílagos de Malvales. I- Análisis fitoquímico del mucílago de hojas de Chorisia speciosa St.Hil. (Bombacaceae). Rev Farm. 120, 75–80.
- Chauhan JS, Chaturvedi R, Kumar S. (1982). A new flavanol glycosoide from the stems of Adansonia digitata. Indian J Chem B. 21, 254–255.
- Chauhan JS, Kumar S, Chaturvedi R. (1984). A new flavanonol glycoside from Adansonia digitata roots. Planta Med, 50, 113.
- Chauhan JS, Kumar S, Cahturvedi R. (1987). A new flavanone glycoside from the roots of Adansonia digitata. Nat Acad Sci Lett. 10, 77–79.
- Chauhan JS, Sultam M, Srivastava SK. (1980). Constituents from Salmalia malabaricum. Can J Chem 58, 328–330.
- Courtois EA, Paine CE, Blandinieres PA, Stien D, Bessiere JM, Houel E, Baraloto C, Chave J. (2009). Diversity of the volatile organic compounds emitted by 55 species of tropical trees: A survey in French Guiana. J Chem Ecol, 35, 1349–1362.
- Coussio JD. (1964). Isolation of rhoifolin from Chorisia species (Bombacaceae). Experientia, 20, 562.
- Cronquist A. (1981). An Integrated System of Classification of Flowering plants. New York:Columbia University Press.
- Das S, Ghosal PK, Ray B. (1990). Structural studies of a polysaccharide from the seeds of Salmalia malabarica. Carbohydr Res. 207, 336–340.
- De Caluwé E, Halamova K, Van Damme P. (2010). Adansonia digitata L. A review of traditional uses, phytochemistry and pharmacology. Afrika focus. 23, 11–51.
- Dhar DN, Munjal RC. (1976). Chemical examination of the seeds of Bombax malabaricum. Planta Med, 29, 148–150.
- Di Fabio JL, Dutton GG, Moyna P. (1982). The structure of Chorisia speciosa gum. Carbohydr Res. 99, 41–50.
- El-Alfy TS, El-Sawi SA, Sleem A, Moawad DM. (2010). Investigation of flavonoidal content and biological activities of Chorisia insignis H.B.K. leaves. Aust J Basic Appl Sci. 4, 1334–1348.
- EL-Hagrassi AM, Ali MM, Osman AF, Shaaban M. (2011). Phytochemical investigation and biological studies of Bombax malabricum flowers. Nat Prod Res. 25, 141–151.
- Faizi S, Ali M. (1999). Shamimin: A new flavonol C-glycoside from leaves of Bombax ceiba. Planta Med, 65, 383–385.
- Frankham R, Ballou JD, Briscoe DA. (1996). A Primer of Conservation Genetics. 2nd edition, Cambridge, UK:The Press Syndicate Of The University Of Cambridge, p. 82.
- Gaydou EM, Bianchini J-P, Ralaimanarivo A. (1981). Cyclopropenoid fatty acids in Malagasy baobab : Adansonia grandidieri (Bombacaceae) seed oil. Fette Seifen Anstrichmittel. 84, 468–472.
- Gibbs RD. (1974). Chemotaxonomy of Flowering Plants. Montreal:McGill-Queens University Press, V.1, p. 516; V.2, p. 1450.
- Gopal H, Gupta RK. (1972). Chemical constituents of Salmalia malabarica Schott and Endl. flowers. J Pharm Sci, 61, 807–808.
- Hafez SS, Abdel-Ghani AE, El-Shazly AM. (2003). Pharmacognostical and antibacterial studies of Chorisia speciosa St. Hill. flower (Bombacaeae). Mans J Pharm Sci. 19, 40–43.
- Haq QN, Gomes J. (1973). Studies on water soluble polysaccharide from the roots of Salmalia malabarica. Bangladesh J Sci Ind Res. 8, 16–20.
- Hassan AA. (2009). Phytochemical and Biological Investigation of Certain Plants Containing Pigments. A Thesis for the Doctor Degree submitted to Faculty of Pharmacy, Egypt: Mansoura University.
- Heywood VH, Brummitt RK, Culham A, Seberg O. (2007). Flowering Plant Families of the World. Richmond Hill, Ontario, Canada: Firefly Books.
- Joly AB. (1991). Botany: An Introduction to Plant Taxonomy. 10th ed., São Paulo: National Publishing Company, p. 462.
- Kaimal TN, Gollamudi L. (1972). Changes in lipids of maturing Ceiba pentandra seeds. Phytochemistry. 11, 1617–1622.
- Kishore PH, Reddy MV, Gunasekar D, Caux C, Bodo B. (2003). A new naphthoquinone from Ceiba pentandra. J Asian Nat Prod Res, 5, 227–230.
- Lockett CT, Calvert CC, Grivetti LE. (2000). Energy and micronutrient composition of dietary and medicinal wild plants consumed during drought. Study of rural Fulani, northeastern Nigeria. Int J Food Sci Nutr, 51, 195–208.
- Lufrano NS, Caffini NO. (1981). Mucilagos foliares de Chorisia H.B.K. (Bombacaceae): Analisis fitoquimico y enfoque quimiotaxonomico. Phyton. 40, 13–20.
- Macfarlane AF, Mori SA, Purzycki K. (2003). Notes on Eriotheca longitubulosa (Bombacaceae), a rare, putatively hawkmoth-pollinated species new to the Guianas. Brittonia. 55, 305–316.
- Matsuda M, Endo Y, Fushiya S, Endo T, Nozoe S.. (1994). Cytotoxic 6-substituted 5,6-dihydro-2H-pyran-2-ones from a Brazilian medicinal plant, Chorisia crispiflora. Heterocycles. 38, 1229–1232.
- Mukherjee J, Roy B. (1971). Chemical examination of Salmalia malabarica-D Synonym Bombax malabaricum-D. J Ind Chem Soc, 48, 769–770.
- Ngounou FN, Meli AL, Lontsi D, Sondengam BL, Atta-ur-Rahman, Choudhary MI, Malik S, Akhtar F. (2000). New isoflavones from Ceiba pentandra. Phytochemistry, 54, 107–110.
- Niranjan GS, Gupta PC. (1973). Anthocyanins from the flowers of Bombax malabaricum. Planta Med, 24, 196–199.
- Noreen Y, el-Seedi H, Perera P, Bohlin L. (1998). Two new isoflavones from Ceiba pentandra and their effect on cyclooxygenase-catalyzed prostaglandin biosynthesis. J Nat Prod, 61, 8–12.
- Ogbobe O, Ezeukwu AO, Ozoh NG. (1996). Physicochemical properties of seed and fatty acid composition of Bombax costatum seed oil. Riv Ital Sostanze Grasse. 73, 271–272.
- Osman MA. (2004). Chemical and nutrient analysis of baobab (Adansonia digitata) fruit and seed protein solubility. Plant Foods Hum Nutr, 59, 29–33.
- Paula VF. (1995). Study of Chemical and Plant-insect Interaction Ochroma lagopus Swartz (Bombacaceae). A Thesis for Master Degree submitted to Universidade Federalde Viçosa, Brazil.
- Paula VF, Barbosa LC, Erringto W, Howarth OW, Cruz MP. (2002). Chemical constituents from Bombacopsis glabra (Pasq.) A. Robyns: Complete 1H and 13C NMR assignments and X-Ray structure of 5-hydroxy-3,6,7,8,4'-pentamethoxyflavone. J Braz Chem Soc. 13, 276–280.
- Paula VF, Barbosa LC, Demuner AJ. (1997). The chemistry of the Bombacaceae family. Quim Nova. 20, 627–630.
- Paula VF, Barbosa LC, Demuner AJ, Campos LA, Pinheiro AL. (1997). Entomotoxicity of the nectar from Ochroma lagopus Swartz (Bombacaceae). Ciência e Cultura. 49, 274–277.
- Paula VF, Barbosa LC, Demuner AJ, Howarth OW, Pilo-Veloso D. (1996). Chemical constituents of Ochroma lagopus Swartz. Quim Nova. 19, 225–229.
- Paula VF, Barbosa LC, Howarth OW, Demuner AJ, Cass QB, Vieira IJ. (1995). Lignans from Ochroma lagopus Swartz. Tetrahedron. 51, 12453–12462.
- Paula VF, Barbosa LC, Pilo-Veloso D, Demuner AJ, Howarth OW. (1998). Chemical constituents of the bark from Ochroma lagopus Swartz (Bombacaceae). Eclet Quim. 23, 45–57.
- Paula VF, Cruz MP. (2006). Chemical constituents of Bombacopsis glabra (Bombacaceae). Quim Nova. 29, 213–215.
- Paula VF, Rocha ME, Barbosa LC, Howarth OW. (2006). Aquatidial, a new bis-norsesquiterpenoid from Pachira aquatica Aubl. J Braz Chem Soc. 17, 1443–1446.
- Perez-Arbelaez E. (1956). Plantas utiles de Colômbia. 3rd edition, Bogotá: Camacho Roldan, p. 226.
- Petronici C, Bazan E, Panno M, Averna V. (1974). Compozicione acidica e struttura gliceridica dell’olio dei semi di Chorisia speciosa St. Hil. Riv Ital Sostanze Grasse. 51, 11–15.
- Pettersson S, Ervik F, Knudsen JT. (2004). Floral scent of bat-pollinated species: West Africa vs. The New World. Biol J Linn Soc. 82, 161–168.
- Puckhaber LS, Stipanovic RD. (2001). Revised structure for a sesquiterpene lactone from Bombax malbaricum. J Nat Prod, 64, 260–261.
- Qi Y, Guo S, Xia Z, Xie D. (1996). Chemical constituents of Gossampinus malabarica (L.) Merr. (II). Zhongguo Zhong Yao Za Zhi, 21, 234–5, 256.
- Raffauf RF, Zennie TM, Onan KD, Le Quesne PW. (1984). Funebrine, a structurally novel pyrrole alkaloid, and other γ-hydroxyisoleucine-related metabolites of Quararibea funebris (Llave) Vischer (Bombacaceae). J Org Chem. 49, 2714–2718.
- Rajua TS, Gowda DC, Anjaneyalu VY. (1989). Structural features of alkali-soluble acidic xylans isolated from the bark of Ceiba pentandra var. indica. Carbohydr Res. 191, 331–341.
- Ralaimanarivo A, Gaydou EM, Bianchini J-P. (1982). Fatty acid composition of seed oils from six Adansonia species with particular reference to cyclopropane and cyclopropene acids. Lipids. 17, 1–10.
- Ramesh D, Dennis TJ, Shingare MS. (1992). Constituents of Adansonia digitata root bark. Fitoterapia. 63, 278–279.
- Rao KV, Sreeramulu K, Gunasekar D, Ramesh D. (1993). Two new sesquiterpene lactones from Ceiba pentandra. J Nat Prod, 56, 2041–2045.
- Reddy MV, Reddy MK, Gunasekar D, Murthy MM, Caux C, Bodo B. (2003). A new sesquiterpene lactone from Bombax malabaricum. Chem Pharm Bull. 51, 458–459.
- Rizk AM, Al-Nowaihi AS. (1989). The Phytochemistry of the Horticultural Plants of Qatar. Oxford:Alden Press, p. 29.
- Rizvi SA, Saxena OC. (1974). New glycosides, terpenoids, colouring matters, sugars and fatty compounds from the flowers of Salmalia malabarica. Arzneimittelforschung, 24, 285–287.
- Saleem R, Ahmad SI, Ahmed M, Faizi Z, Zikr-ur-Rehman S, Ali M, Faizi S. (2003). Hypotensive activity and toxicology of constituents from Bombax ceiba stem bark. Biol Pharm Bull, 26, 41–46.
- Saleem R, Ahmad M, Hussain SA, Qazi AM, Ahmad SI, Qazi MH, Ali M, Faizi S, Akhtar S, Husnain SN. (1999). Hypotensive, hypoglycaemic and toxicological studies on the flavonol C-glycoside shamimin from Bombax ceiba. Planta Med, 65, 331–334.
- Saleh NA, El-Sherbeiny AE, El-Sissi HI. (1969). Local plants as potential sources of tannins in Egypt, Part. IV, Aceraceae to Flacourtiaceae. Qual Plant Mater Veg XVII. 4, 384–394.
- Sankaram AV, Reddy NS, Shoolerya JN. (1981). New sesquiterpenoids of Bombax malabaricum. Phytochemistry. 20, 1877–1881.
- Schuch R, Ahmad F, Mukherjee KD. (1986). Composition of triacylglycerols containing cyclopropene fatty acids in seed lipids of Munguba (Bombax munguba Mart.). J Am Oil Chem Soc. 63, 778–780.
- Scogin R. (1986). Reproductive phytochemistry of Bombacaceae: Floral anthocyanins and nectar constituents. Aliso. 11, 377–385.
- Seshadri V, Bhatta AK, Rangaswami S. (1971). Phenolic compounds of Bombax malabaricum (root-bark). Curr Sci. 40, 630–631.
- Seshadri V, Bhatta AK, Rangaswami S. (1973). Phenolic compounds of Bombax malabaricum. Indian J Chem. 11, 825–827.
- Seshadri V, Bhatta AK, Rangaswami S. (1976). A new crystalline lactone from Bombax malabaricum. Indian J Chem B, 14, 616–617.
- Shahat AA. (2006). Procyanidins from Adansonia digitata. Pharm Biol. 44, 445–450.
- Sharma OP. (1993). Plant Taxonomy. 1st ed., West Patel Nagar, New Delhi: Tata McGraw Hill Company Limited, p. 236–238.
- Shibatani M, Hashidoko Y, Tahara, S. (1999a). A major fungitoxin from Pachira aquatica and its accumulation in outer bark. J Chem Ecol. 25, 347–353.
- Shibatani M, Hashidoko Y, Tahara S. (1999b). Accumulation of isohemigossypolone and its related compounds in the inner bark and heartwood of diseased Pachira aquatica. Biosci Biotech Bioch 63, 1777–1780.
- Shukla YN, Dubey S, Jain SP, Kumar S. (2001). Chemistry, biology and uses of Adansonia digitata-A review. J Med Aromatic Pl Sci. 23, 429–434.
- Sichaem J, Siripong P, Khumkratok S, Tip-Pyang S. (2010). Chemical constituents from the roots of Bombax anceps. J Chil Chem Soc. 55, 325–327.
- Sipra-Dan S, Dan S. (1986). Phytochemical study of Adansonia digitata, Coccoloba excoriata, Psycotria adenophylla and Schleichera oleosa. Fitoterapia. 57, 445–446.
- Sood RP, Suri KA, Suri OP, Dhar KL, Atal CK. (1982). Sesquiterpene lactone from Salmalia malbarica. Phytochemistry. 21, 2125–2126.
- Ueda H, Kaneda N, Kawanishi K, Alves SM, Moriyasu M. (2002). A new isoflavone glycoside from Ceiba pentandra (L.) Gaertner. Chem Pharm Bull, 50, 403–404.
- Vazquez E, Martinez EM, Cogordan JA, Delgado G. (2001). Triterpenes, phenols, and other constituents from the leaves of Ochroma pyramidale (Balsa Wood, Bombacaceae). Preferred conformations of 8-C-β-d-glucopyranosyl-apigenin (vitexin). Rev Soc Quím Méx. 45, 254–258.
- Versiani MA. (2004). Studies in the Chemical Constituents of Bombax ceiba and Cuscuta reflexa. A Thesis for the Doctor Degree submitted to H.E.J Research Institute of Chemistry, Karachi University, Pakistan.
- Wu J, Zhang X, Zhang S, Xuan L. (2008). Three novel compounds from the flowers of Bombax malabaricum. Helv Chim Acta. 91, 136–143.
- Yazzie D, VanderJagt DJ, Pastuszyn A, Okolo A, Glew RH. (1994). The amino acid and mineral content of baobab (Adansonia digitata L.) leaves. J Food Compos Anal. 7, 189–193.
- Zennie TM, Cassady JM. (1990). Funebradiol, a new pyrrole lactone alkaloid from Quararibea funebris flowers. J Nat Prod, 53, 1611–1614.
- Zennie TM, Cassady JM, Raffauf RF. (1986). Funebral, a new pyrrole lactone alkaloid from Quararibea funebris. J Nat Prod, 49, 695–698.
- Zhang X, Zhu H, Zhang S, Yu Q, Xuan L. (2007). Sesquiterpenoids from Bombax malabaricum. J Nat Prod, 70, 1526–1528.