Abstract
Context: Funtumia elastica (Preuss) Stapf. (Apocynaceae) has a long ethnopharmacological history for uses such as treatment of whooping cough, asthma, blennorhea, painful menstruation, fungal infections, and wounds.
Objective: To investigate the antimicrobial and anti-inflammatory properties of ethanol extracts from the leaves and stem bark of Funtumia elastica based on its ethnopharmacological uses and also determine the secondary metabolites present in the extracts.
Materials and methods: The antimicrobial activities of ethanol leaf and bark extracts of F. elastica were determined using the microdilution technique (MIC determination) and agar diffusion method using 10, 25, and 50 mg/mL concentrations against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, Candida albicans, Aspergillus flavus and Aspergillus niger as test organisms. Anti-inflammatory activities of the doses of extracts at 30, 100, and 300 mg/kg per body weight were determined by carrageenan-induced edema in the footpad of 7-day-old chicks and the foot volumes measured at hourly interval post-treatment for 5 h.
Results: The MIC ranges of both ethanol leaf and bark extracts against the test organisms were 125 (lowest MIC) to1550 µg/mL (highest MIC) and 125 (lowest MIC) to 1750 µg/mL (highest MIC), respectively. The ethanol leaf and bark extract of F. elastica showed significant anti-inflammatory activity (p ≤ 0.001) at 30, 100 and 300 mg/kg. Preliminary phytochemical screening revealed that F. elastica bark contains hydrolysable tannins, sapogenetic glycosides, steroids and saponins while the leaves contain hydrolysable tannins, flavonoids, starch and alkaloids. Tannin contents of the leaf and stem bark were 2.4 and 1.3% w/w (related to the dried material), respectively.
Discussion and conclusion: Both ethanol leaf and bark extracts of F. elastica showed antimicrobial and anti-inflammatory activities and these pharmacological properties may be responsible for the ethnomedicinal uses of the leaves and stem bark of the plant.
Introduction
There has been increase in antibiotic resistance and reports of treatment failures with antibiotic therapy in both humans and other animals over the past recent years (CitationZetola et al., 2005) especially the appearance of methicillin-resistant Staphylococcus aureus (MRSA) and multiple drug-resistant strains of Salmonella typhi. It has been estimated that in the United States of America alone, at least 18,000 people die every year from drug-resistant infections (CitationAlderman & Hastings, 2003). It is estimated that about 19,000 people in the United States die every year from MRSA (CitationKlevens et al., 2007) and in 2004, 63% of all reported Staphylococcus infections in the United States were caused by MRSA (CitationCDC, 2007). Methicillin was developed as an alternative treatment for the increasing rate of Staphylococcus infections already resistant to penicillin. At that time, about 60% of all Staphylococcus infections were resistant to penicillin. Hence, traditional antibiotics are not being used for the treatment of methicillin-resistant Staphylococcus infections (CitationMicet, 2007) but rather “last-resort” intravenous vancomycin and other newer antibiotics are being used or prescribed for the treatment of MRSA infections consequently intensive use of antibiotics often resulted in the development of resistant strains (CitationTenover, 2006). There is a need for a search for new antibiotics from other sources including medicinal plants.
Inflammation refers to an innate, nonspecific series of highly interrelated series of events that are set into motion in response to foreign invasion, tissue damage or both with the ultimate goal of bringing to the invaded tissue or infected area, phagocytes and plasma proteins that isolate, destroy or inactivate the invaders, remove debris, and prepare for subsequent healing and repair (CitationSherwood, 2006). Inflammation and infection are frequently accompanied by imbalance in the intestinal microflora. Inflammation is one of the responses of the individual to pathogenic invasion and occurs in most bacterial infections and disorders of the cardiovascular, respiratory, lymphatic, genitourinary, nervous and dermatological systems. A strong inflammatory response may then be mounted to microfloral bacteria, leading to perpetuation of the inflammation and gut barrier dysfunction (CitationIsolauri et al., 2002). For instance, Helicobacter pylori resides in the gastric mucus layer of humans and induces a chronic inflammatory response that can result in both peptic ulceration and gastric neoplasms (CitationBaser, 1993) and chronic colonization of the lower respiratory tract by bacterial pathogens induces a chronic inflammatory response with lung damage (CitationSethi, 2000). Hence, any bioactive agent with antimicrobial and anti-inflammatory properties may be helpful in the above disease conditions. A survey conducted by United Nations Conference on Trade and Development (UNCTAD)-General Agreement on Tariffs and Trade (GATT) showed that 33% of total drugs produced by pharmaceutical industries are derived from plants and microbes, 60% of medicinal products are natural origin (CitationSrinivasan et al., 2001). Some traditional orthodox anti-inflammatory agents have been demonstrated to exhibit antibacterial activity (CitationAnnadurai et al., 1998; CitationDutta et al., 2004) and hence any bioactive agent possessing both properties may be beneficial to patients especially those suffering from acute inflammation and bacterial infections at the same time. Some medicinal and pharmacological values have been observed in different species of Cola (CitationDaels-Rakotoarison et al., 2003; CitationSteinegger & Hansel, 1992).
Medicinal plants have been used in the management of various bacterial infections and inflammatory conditions and some of them have been found to possess both antimicrobial and anti-inflammatory properties. For example, crude extracts of Dodonaea viscosa (L.) Jacq. (Sapindaceae), Rumex nervosus Vahl. (Polygonaceae) and Rumex abyssinicus Jacq. (Polygonaceae) (CitationGetie et al., 2003) and Mallotus peltatus (Geist) Muell. Arg. (Euphorbiaceae) leaf extract (CitationChattopadhyay et al., 2002) have been shown to exhibit both antimicrobial and anti-inflammatory activities and an ethanol extract of Desmodium caudatum (Thunb.) DC. (Leguminosae), traditionally used for the treatment of various forms of pains, has been found to possess analgesic, anti-inflammatory and antipyretic properties (CitationKe-Jia et al., 2011). Funtumia elastica (Preuss) Stapf. (Apocynaceae), known commonly as silkrubber (local Asante-Twi name is “Frumtum/Ofruntum”), is traditionally used to treat whooping cough (CitationBurkill, 1995), inflammatory diseases such as asthma, blennorhea, and painful menstruation (CitationOlaniyi, 1989), cutaneous fungal infections, hemorrhoids, syphilis, gonorrhea (CitationOdugbemi, 2006; CitationBurkill, 1995) and wounds (CitationAdekunle & Ikumapayi, 2006). Some steroidal alkaloids (holarrhetine, conessine, holarrhesine and isoconessimine) have been isolated from the stem bark and conanine group, namely, irehdiamine A and D, irehamine, conkuchine and irehine from leaves of F. elastica (CitationZirihi et al., 2005). This study investigated the antimicrobial and anti-inflammatory activities of ethanol stem bark and leaf extracts of F. elastica based on its ethnobotanical uses. Preliminary phytochemical screening for secondary metabolites was also performed.
Materials and methods
Plant material and chemicals
Stem bark and leaves of Funtumia elastica were collected in July, 2007 from the Bobiri Forest Reserves of the Forestry Research Institute of Ghana (FORIG) near Kubease, Ashanti Region, Ghana, and identified by Dr. A. Asase, Department of Botany, University of Ghana, Ghana. A voucher specimen of the plant (number AA 113) has been deposited at Ghana Herbarium, University of Ghana, Ghana. Unless stated otherwise, all the chemicals were purchased from Sigma (Deisenhofen, Germany).
Preparation of extracts
The plant materials were air-dried and powdered. Powdered materials (200 g) of Funtumia elastica were extracted with 70% ethanol using a Soxhlet apparatus. The ethanol extracts obtained were evaporated to dryness under reduced pressure and kept in a desiccator. The yield of the stem extracts were 5% w/w while leaves yielded 19.8% w/w. Quantities of the leaf extract (FLE) and stem bark extract (FBE) were dissolved in normal saline and dimethyl sulfoxide (DMSO) for acute anti-inflammatory and antimicrobial determinations, respectively.
Preliminary phytochemical screening
Phytochemical screening was conducted on both leaf and stem bark extracts of F. elastica to ascertain the presence of starch, tannins, glycosides (sapogenetic, anthracene, and cyanogenetic), flavonoids, steroids and alkaloids (CitationWagner & Bladt, 1996; CitationHarborne, 1998). The presence of flavonoids, alkaloids and carbohydrates in the extracts were detected by thin-layer chromatography (TLC) as described by CitationWagner and Bladt (1996). The presence of flavonoids, glycosides, tannins and carbohydrates in the extracts (FLE and FBE) were determined using a TLC method with mobile phase (EtOAc/HCOOH/H20, 90/5/5) detected with 1% Naturstoff, 1% ferric chloride and thymol-H2SO4 reagents, respectively. The TLC plate (silica gel 60 F254) used in the detection of carbohydrates were heated at 105°C for 5 min after spraying with thymol-H2SO4. The alkaloids were extracted with H2SO4 and made basic (alkaline) with the addition of NH3 before using a TLC method with mobile phase (EtOAc/HCOOH/H20, 90/5/5) to detect the presence of alkaloids with Dragendorff’s reagent. The presence of saponins in the extracts was determined by the frothing method. In addition to the TLC method for the detection of glycosides, the extracts were also added to dilute H2SO4 and boiled for 5 min. They were then filtered, cooled and 20% of NaOH was added to the extracts. Fehling solutions A&B were also added and the result observed.
The tannin content was determined according to the method of CitationGlasl (1983) using pyrogallol (Merck, Darmstadt, Germany, purity 99.5%, HPLC) as reference compound. The powdered plant material, 750 mg (m1) was weighed into a round bottom flask with 150 mL of distilled water and heated at 90°C for 30 min and then cooled under running water. The aqueous extract was transferred into a 250 mL narrow necked volumetric flask and the volume made up to 250 mL with distilled water. The extract (30 mL) was centrifuged (3000 × g, 10 min). The clear solution was labeled as the stock solution. The filtrate (5 mL) (stock solution) was pipetted into a 25 mL volumetric flask and then diluted with distilled water to 25 mL (Total Phenol Solution, TPS). The filtrate (10 mL) was added to 100 mg of hide powder for determination of tannic acid, slightly chromated (Merck Darmstadt, Germany), shaken for 60 min, and filtered. The filtrate (5 mL) was diluted to 25 mL with distilled water (Remaining Phenol Solution, RPS). Pyrogallol (0.05 g) (m2) was added to 100 mL distilled water and 5 mL of the resultant solution was diluted to 100 mL with distilled water (Reference Solution, RS). One mL Folin-Ciocalteus phenol reagent (Sigma) was added to 2 mL of TPS and 10 mL of distilled water were added. The resultant solution was diluted to 25 mL with 14.06% w/v sodium carbonate solution (Merck, Darmstadt, Germany). The above procedure was repeated for RPS and RS. After 30 min, the absorbances (A) of the solutions were measured at 760 nm after the absorbance of distilled water was zeroed. The percentage of tannin content was determined by the following formula:
where, A, measured absorbance at 760 nm; m1, mass of plant material; m2, mass of pyrogallol (reference material). The average of three separate measurements (A) was used in the calculation.
Determination of antimicrobial activity
Agar diffusion method
The antimicrobial activities of the extracts (FLE and FBE) and reference drugs (chloramphenicol and clotrimazole) (Sigma, Deisenhofen, Germany) were determined using the agar diffusion method. Nutrient agar (Oxoid Limited, United Kingdom) and Sabouraud agar (Oxoid Limited, United Kingdom) media were used for both determinations of antibacterial and antifungal activities, respectively. The test organisms (1x106 cfu/mL) (100 µL) (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, Bacillus subtilis NCTC 10073 and clinical fungal agents Candida albicans, Aspergillus niger, and Aspergillus flavus) were used to seed nutrient agar and Sabouraud agar plates, respectively. In each of these plates, 4 equidistant wells (8 mm) were cut out using sterile cork borer and wells filled with different concentrations of extracts and reference drugs dissolved in DMSO and allowed to diffuse at room temperature (28–30°C) for 1 h. The zones of growth inhibition measured after 24 h incubation at 37°C (for bacteria) and 3 days at 30°C (for fungi). The activity of solvent alone (DMSO) was determined and was found to exhibit no antimicrobial activity against the test organisms.
Microdilution method
The MIC of both extracts (FLE and FBE) against the test bacteria was determined using the modified microdilution technique as described by CitationEloff (1998). Test solutions (100 mg/mL) of both extracts were prepared with DMSO and test solution (25–100 µL) was serially diluted to 100 µg/mL and 100 µL (1x106 cfu/mL) of the test bacteria grown in nutrient broth (Oxoid Limited, United Kingdom) added to each well in the microplates. The covered microplates were incubated at 37°C for 24 h. To indicate growth, 30 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide [MTT, thiazolyl blue] dissolved in water was added to the microplate wells and incubated at 37°C for 30 min. Test fungal agents were cultivated in Sabouraud dextrose broth (Oxoid Limited, United Kingdom) and then incubated for 3 days at 30°C. The MICs of FLE and FBE against the test fungi were determined according to the guidelines described in the National Committee for CitationClinical Laboratory Standards (1998) for filamentous fungi. The minimum inhibitory concentration of the extract against the test organisms was detected as the minimum concentration of extracts that did not exhibit microbial growth after the addition MTT to the medium and incubation at 37°C for 20 min (CitationBerridge et al., 2005). The growth or nonsurvival of the test organisms with various concentrations of the extracts after the addition of the MTT were compared to the untreated cells (organism without extracts) and the MIC determined as described by CitationEloff (1998). The above experiments were repeated three times.
Determination of acute anti-inflammatory activity
The carrageenan-induced inflammatory model in 7-day-old chicks (CitationRoach & Sufka, 2003) was employed and the responsiveness of these chicks to anti-inflammatory drugs was determined.
Experimental animals
Day old cockerels Gallus gallus (Strain: Shaver 579) purchased from Darko Farms Company Limited, Kumasi, Ghana, were maintained in the Animal House of the Department of Pharmacology, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana. The chicks were housed in stainless steel cages and fed with normal commercial poultry diet (GAFCO, Tema, Ghana), given water ad libitum and maintained under laboratory conditions (temperature 28–30°C, relative humidity 60–70%, and normal light-dark cycle). A day before the experiment, the chicks were brought to the laboratory and habituated to experimenter handling and the apparatus to minimize the effect of stress and novelty. The chicks were used on day 7. All procedures and techniques used in these studies were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH, Department of Health Services publication No. 83–23, revised 1985). The protocols for the study were approved by the Departmental Ethics Committee.
Carrageenan-induced edema model
The initial foot volumes of the chicks were measured using a plethysmometer (IITC Life Science Inc., California, USA) after which 0.01 ml of 2% carrageenan was injected into the plantar of the right foot to induce inflammation. The inflammation produced was measured, the increase in foot volume calculated and those with an increase between 15–40% were selected and put into thirteen groups of five after which they were injected intraperitoneally with either diclofenac (Sigma, purity 98% HPLC) (10, 30 and 100 mg/kg) or dexamethasone (Sigma, purity 98% HPLC) (0.25, 0.5 and 1.0 mg/kg) based on recommended effective human doses per body weight, and FLE (30, 100 and 300 mg/kg), or FBE (30, 100 and 300 mg/kg) given orally based on preliminary investigation and the oral route of administration for the traditional uses of the plant for management of pain. One group, the no treatment group, did not receive any drug (control). Foot volumes were measured again at hourly intervals post-treatment for 5 h. A period of 1 h for oral and 30 min for intraperitoneal route were allowed for absorption of the drugs and extracts before first measurements. The percentage change in foot volume after induction, and treatment of inflammation was calculated and recorded for analysis.
In order to simulate tradition usage of the herbs and the difficulty in administering herbal preparations through the parenteral route, the oral route was chosen. However, for most studies, the parenteral route is preferred for the reference drugs. The intraperitoneal was therefore chosen for the reference drug because of its closeness to the oral as both routes of administration are subject to absorption and hepatic metabolism. The difference between the two routes will be in the time of absorption which is compensated for by 1 h delay for oral and 30 min for intraperitoneal route before measurements were taken.
Statistical analysis
GraphPad Prism Version 5.0 for Windows (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses. p < 0.05 was considered statistically significant in all analysis. The graphs were plotted using Sigma Plot for Windows Version 11.0 (Systat Software Inc., Germany).
Results
Preliminary phytochemical screening
FLE has hydrolysable tannins, flavonoids, alkaloids, steroids, saponins and carbohydrate while FBE contains hydrolysable tannins, sapogenetic glycosides, cyanogenetic glycosides, steroids, and saponins. The tannin content of leaf and stem bark were 2.4 and 1.3% w/w (related to the dried material), respectively ().
Table 1. Preliminary phytochemical screening of ethanol leaf (FLE) and stem bark (FBE) extracts of F. elastica.
Antimicrobial activity
Both ethanol extracts (FLE and FBE) were found to be active against the test bacteria (E. coli, P. aeruginosa, S. aureus, and B. subtilis) and C. albicans. A. niger, and A. flavus were found to be less susceptible to both extracts (). The minimum inhibitory concentration ranges of FLE and FBE against the test organisms were 125–1550 and 125–1750 µg/mL, respectively ().
Table 2. Antimicrobial activity of ethanol leaf extract (FLE) and ethanol stem bark extract (FBE) of F. elastica by agar diffusion method.
Table 3. Minimum inhibitory concentration (MIC) (mg/mL) of ethanol leaf extract (FLE) and ethanol stem bark extract (FBE) of F. elastica determined by microdilution method.
Acute anti-inflammatory activity
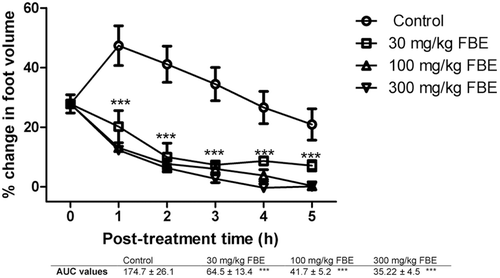
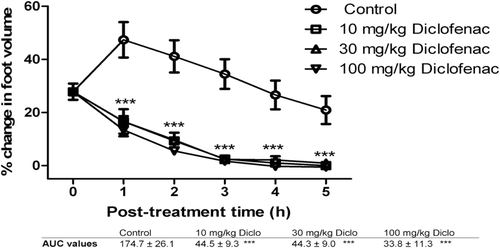
It was found that all doses of FLE, FBE, dexamethasone and diclofenac caused significant (p ≤ 0.001) dose-dependent reduction in foot volume of the chicks from the first to the fifth hour post-treatment (, , , and 4, respectively). AUC values reveal that diclofenac reduced the carrageenan-induced inflammation better than dexamethasone. FBE (30, 100 and 300 mg/kg) was found to exhibit higher anti-inflammatory effect than FLE (30, 100 and 300 mg/kg) ( and 2) and these effects were comparable to the effects of the reference anti-inflammatory agents.
Figure 1. The effects of 30, 100, and 300 mg/kg of ethanolic stem bark extract (FBE) on carrageenan-induced inflammation in chicks. ***implies p < 0.001 which signifies a significant reduction in foot volume at all dose levels. Data are presented as mean ± SEM (n = 5), analyzed by one-way ANOVA followed by Newman-Keuls test for column graphs. Control is the untreated group.
Discussion
Both ethanol extracts (FLE and FBE) of F. elastica exhibited inhibitory activity against the test organisms, namely, E. coli, P. aeruginosa, S. aureus, B. subtilis, C. albicans, A. flavus and A. niger (), and these test organisms were used to determine the spectrum of activity (Gram negative and positive bacteria and fungal pathogens) of the extracts and also their involvement in various microbial infections including serious systemic infections and opportunistic infections caused by C. albicans. In persons infected with HIV, S. aureus is responsible for invasive skin diseases including superficial and follicular lesions such as boils, carbuncles, etc. The MIC range of FLE against both bacterial and fungal test organisms from 125 to 1550 µg/mL and that of FBE was from 125 to 1750 µg/mL (). This may indicate that the bioactive agent(s) responsible for the antimicrobial activity are present in both the leaves and stem bark.
With the agar diffusion method, concentrations of both FLE and FBE equal or less than 10 mg/mL was found to exhibit no inhibitory against the test pathogens except E. coli and this may be attributed to the fact that the extracts were not fully diffusible into the agar medium (CitationCos et al., 2006; CitationEloff, 1998). P. aeruginosa was found to be the least susceptible bacterium to extracts with higher MICs for both extracts () compared to the other test bacteria and this was not surprising since P. aeruginosa has been found to resistant to most antibiotics (CitationLambert, 2002). S. aureus, B. subtilis and E. coli were more susceptible to both extracts with a MIC range of 125 to 250 µg/mL. Even with the agar diffusion technique, high concentrations of extract (50 mg/mL) exhibited activity against both test bacteria and fungus. Concentrations equal or less than 25 mg/mL did not show antimicrobial activity against A. niger and A. flavus except C. albicans with a zone of growth inhibition of 3.5 mm (). This may be attributed to the poor diffusion of the extracts into both solid media.
The activities of the ethanol extracts from the stem bark and leaves against selected bacteria and fungi were found be to almost the same and this may account for the application of extracts from leaves and bark for the treatment of similar infections (CitationSonibare et al., 2009). The antimicrobial activity exhibited by F. elastica extracts may be responsible for its use as agent for the treatment of wounds and veneral diseases such as syphilis and gonorrhea. In fact, conessine, a steroid alkaloid found in F. elastica, could possibly account for its strong antibacterial properties (CitationZirihi et al., 2005; CitationSonibare et al., 2009). The MICs of the FLE extract against the test organisms were found to be lower than that of FBE extract. For the agar diffusion method, the FBE extract exhibit relatively higher activity in terms of zones of growth inhibition compared to the FLE extract and this may be due to the inability of the bioactive components of FLE to diffuse into the agar (CitationCos et al., 2006).
Carrageenan induces acute inflammation mediated by cytokines [interleukin (IL)-1 and tumor necrosis factor (TNF)-α which cause vasodilatation, increased vascular permeability, and fluid exudation], and proinflammatory agents [PGE2, and PGI2 generated by the local tissues and blood vessels, PGD2 released mainly by mast cells, and leukotriene (LT) B4 a chemotaxin] in the foot of the chick, causing the foot volume to increase.
The level of FLE anti-inflammatory activity was not proportional to increases in the doses of the extract as the least dose of 10 mg/kg body weight was more effective at reducing the edema than high doses of 30 and 100 mg/kg body weight () which may be due to receptor desensitization or the presence of competing or antagonistic compounds in the extract.
The significant reduction in foot volume cause by FBE and FLE is a clear indication of anti-inflammatory effect comparable to dexamethasone and diclofenac. The mechanism of anti-inflammatory effect of FLE and FBE may possibly be similar to that for dexamethasone because the phytochemical screening showed that the extracts contained steroidal components. Dexamethasone is a steroidal anti-inflammatory agent which prevents phospholipase A2 (PLA2) from hydrolyzing arachidonic acid from phospholipids in cell membrane with a resultant reduction in the production of the prostaglandins (PGE2, PGI2 and PGD2) and thromboxanes. Diclofenac is a nonsteroidal anti-inflammatory agent (NSAID) that also reduces the production of prostaglandins and thromboxanes from arachidonic acid by inhibiting cyclooxygenase enzymes (CitationRang et al., 2007).
Extracts from several medicinal plants have been found to inhibit both acute and chronic phases of inflammation (CitationAbad et al., 1996; CitationMostafa et al., 2010). The anti-inflammatory property observed for F. elastica bark extract could be due to the steroids such as cyclofuntumienol and cycloeucalenol present in the leaves and stem bark (CitationZirihi et al., 2005). Phytosterols have iscos-modulating effects, normalizing an over-reactive antibody response as well as anti-inflammatory properties.
Conclusion
Both ethanol extracts of F. elastica (FBE and FLE) exhibited significant antimicrobial activity with MIC ranges of 125–1550 µg/mL and 125–1750 µg/mL, respectively, against the test organisms, and anti-inflammatory (30, 100, and 300 mg/kg) activities. This may justify its folkloric use for the treatment of wounds and various forms of pains. Further bioactivity-guided fractionation and isolation of the bioactive compounds responsible for the antimicrobial and anti-inflammatory activities would be performed.
Figure 2. The effects of 30, 100, and 300 mg/kg of ethanolic leaf extract (FLE) on carrageenan-induced inflammation in chicks. ***implies p < 0.001 which signifies a significant reduction in foot volume at all dose levels. Data are presented as mean ± SEM (n = 5), analyzed by one-way ANOVA followed by Newman-Keuls test for column graphs. Control is the untreated group.
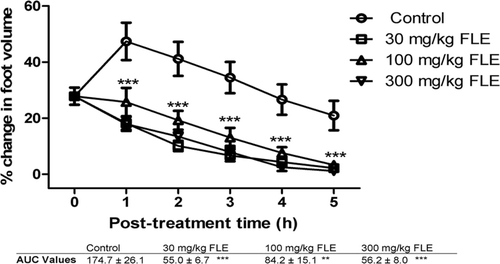
Figure 3. The effects of 0.25, 0.5, and 1.0 mg/kg of dexamethasone on carrageenan-induced inflammation in chicks. ***implies p < 0.001, which signifies a significant reduction in foot volume at all dose levels. Data are presented as mean ± SEM (n = 5), analyzed by one-way ANOVA followed by Newman-Keuls test for column graphs. Control is the untreated group.
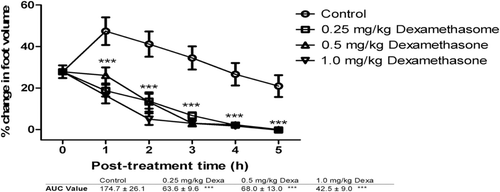
Figure 4. The effects of 10, 30, and 100 mg/kg of diclofenac on carrageenan-induced inflammation in chicks. ***implies p < 0.001, which signifies a significant reduction in foot volume at all dose levels. Data are presented as mean ± SEM (n = 5), analyzed by one-way ANOVA followed by Newman-Keuls test for column graphs.Control is the untreated group.
Acknowledgements
The authors wish to express their gratitude to Mr. Thomas Ansah of the Department of Pharmacology, KNUST, Kumasi, Ghana for his technical assistance and Mr. Kwame Opoku Agyemang for working on the project with us in the laboratory.
Declaration of interest
The authors report no conflicts of interest.
References
- Abad MJ, Bermejo P, Carretero E, Martínez-Acitores C, Noguera B, Villar A. (1996). Antiinflammatory activity of some medicinal plant extracts form Venezuela. J Ethnopharmacol, 55, 63–68.
- Adekunle AA, Ikumapayi AM. (2006). Antifungal property and phytochemical screening of the crude extracts of Funtumia elastica and Mallotus oppositifolius. West Indian Med J, 55, 219–223.
- Alderman DJ, Hastings TS. (2003). Antibiotic use in aquaculture: Development of antibiotic resistance – potential for consumer health risks. Int J Food Sci Technol, 33, 139–155.
- Annadurai S, Basu S, Ray S, Dastidar SG, Chakrabarty AN. (1998). Antibacterial activity of the antiinflammatory agent diclofenac sodium. Indian J Exp Biol 36, 86–90.
- Baser MJ. (1993). Helicobacter pylori: Microbiology of a ‘slow’ bacterial infection. Trend Microbiol, 1, 255–260.
- Berridge MV, Herst PM, Tan AS. (2005). Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol Annu Rev, 11, 127–152.
- Burkill HM. (1995). The Useful Plants of West Tropical Africa, 2nd edition, Vol 3:11. London: Royal Kew Botanical Gardens, 522–527.
- Centers for Disease Control (CDC). (2007). MRSA: Methicillin-resistant Staphylococcus aureus in health care settings. www.cdc.gov/mrsa/index.html (Accessed on April 2011).
- Chattopadhyay D, Arunachalam G, Mandal AB, Sur TK, Mandal SC, Bhattacharya SK. (2002). Antimicrobial and anti-inflammatory activity of folklore: Mallotus peltatus leaf extract. J Ethnopharmacol, 82, 229–237.
- Cos P, Vlietinck AJ, Berghe DV, Maes L. (2006). Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol, 106, 290–302.
- Daels-Rakotoarison DA, Kouakou G, Gressier B, Dine T, Brunet C, Luyckx M, Bailleul F, Trotin F. (2003). Effects of a caffeine-free Cola nitida nuts extract on elastase/α-1-proteinase inhibitor balance. J Ethnopharmacol, 89, 143–150.
- Dutta NK, Dastida SG, Kumar A, Mazumdar K, Ray R, Chakrabarty AN. (2004). Antimycobacterial activity of the anti-inflammatory agent diclofenac sodium and its synergism with streptomycin. Brazilian J Microbiol, 35, 316–323.
- Eloff JN. (1998). A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med, 64, 711–713.
- Getie M, Gebre-Mariam T, Rietz R, Höhne C, Huschka C, Schmidtke M, Abate A, Neubert RH. (2003). Evaluation of the anti-microbial and anti-inflammatory activities of the medicinal plants Dodonaea viscosa, Rumex nervosus and Rumex abyssinicus. Fitoterapia, 74, 139–143.
- Glasl H. (1983). Zur Photometrie in der Drogenstandiserung. Deutsche Apotheker Zeitung, 123, 1979–1987.
- Harborne JB. (1998). Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, 3rd edition. London: Chapman and Hall, 302.
- Isolauri E, Kirjavainen PV, Saminen S. (2002). Probiotics: A role in the treatment of intestinal infection and inflammation. Gut 50 (Suppl. III), S54–S59.
- Ke-Jia M, Zhan-Zhou Z, Cheng-Hao Y, Hong Z, Juan L, Lu-Ping Q. (2011). Analgesic, anti-inflammatory and antipyretic activities of the ethanol extract from Desmodium caudatum. Pharm Biol, 49, 403–407.
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK; Active Bacterial Core surveillance (ABCs) MRSA Investigators. (2007). Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA, 298, 1763–1771.
- Lambert PA. (2002). Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J R Soc Med, 95 Suppl 41, 22–26.
- Micet ST. (2007). Alternatives to vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis, 45, S184–S190.
- Mostafa M, Appidi JR, Yakubu MT, Afolayan AJ. (2010). Anti-inflammatory, antinociceptive and antipyretic properties of the aqueous extract of Clematis brachiata leaf in male rats. Pharm Biol, 48, 682–689.
- National Committee for Clinical Laboratory Standards. (1998). Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi: Proposed standard. NCCLS document M38-P. Pennsylvania: National Committee for Clinical Laboratory Standards.
- Odugbemi T. (2006). Outlines and pictures of medicinal plants from Nigeria. Unilag: University of Lagos Press, 158.
- Olaniyi AA. (1989). Essential Medicinal Chemistry, 4th edition. Ibadan, Nigeria: Shaneson C I Ltd, 474–475.
- Rang HP, Dale MM, Ritter JM, Flower RJ. (2007). Rang and Dale’s Pharmacology, 6th edition. London: Churchill Livingstone, 207.
- Roach JT, Sufka KJ. (2003). Characterization of the chick carrageenan response. Brain Res, 994, 216–225.
- Sethi S. (2000). Bacterial infection and the pathogenesis of COPD. Chest, 117, 286S–291S.
- Sherwood L. (2006). Fundamentals of physiology: A human perspective, 3rd edition. Kendallville: Courier/Kendallville Inc, 329.
- Sonibare MA, Soladoye MO, Esan OO, Sonibare OO. (2009). Phytochemical and antimicrobial studies of four species of Cola Schott & Endl. (Sterculiaceae). Afr J Tradit Complement Altern Med, 6, 518–525.
- Srinivasan D, Nathan S, Suresh T, Lakshmana Perumalsamy P. (2001). Antimicrobial activity of certain Indian medicinal plants used in folkloric medicine. J Ethnopharmacol, 74, 217–220.
- Steinegger E, Hansel R. (1992). Pharmacokognosie, 5th edition. Berlin-Heidelberg: Springer Verlag, 142–158.
- Tenover FC. (2006). Mechanism of antimicrobial resistance in bacteria. Am J Med, 119, S3–S10.
- Wagner H, Bladt S. (1996). Plant Drug Analysis: A Thin Layer Chromatography, 2nd edition. New York: Springer Verlag.
- Zetola N, Francis JS, Nuermberger EL, Bishai WR. (2005). Community-acquired meticillin-resistant Staphylococcus aureus: An emerging threat. Lancet Infect Dis, 5, 275–286.
- Zirihi GN, Grellier P, Guédé-Guina F, Bodo B, Mambu L. (2005). Isolation, characterization and antiplasmodial activity of steroidal alkaloids from Funtumia elastica (Preuss) Stapf. Bioorg Med Chem Lett, 15, 2637–2640.
