Abstract
Context: Pisonia aculeata leaves (Nyctagenaceae), a Folk medicinal plant used in the treatment of several inflammation, pain, and oxidative stress associated diseases.
Objective: To evaluate anti-inflammatory, analgesic, and antioxidant potential of crude methanol extract of P. aculeata leaves (MEPA).
Materials and methods: Analgesic and anti-inflammatory activities of MEPA (250 and 500 mg/kg) were evaluated using writhing, formalin, hot plate, tail flick, carrageenan-induced paw edema test, and membrane stabilizing activity. Free radical scavenging activity, total phenolic and flavonoid contents of MEPA were also determined using standard methods.
Results: Oral administration of MEPA showed significant (p < 0.001) inhibition of paw edema, pronounced at 4 h and 5 h after carrageenan injection, and at 200 µg/mL exerts 77.67 and 38.51% protective effect against hypotonic solution and heat induced hemolysis, respectively. MEPA (250 and 500 mg/kg) produced 35.21 and 79.14% inhibition of acetic acid-induced writhing. Furthermore, MEPA (500 mg/kg) inhibited 49.19% early and 73.14% late phase of formalin-induced hypernociception. In contrast, a lower dose of MEPA did not prevent hot plate induced nociception, while in the tail immersion method, pronounced analgesic activity was observed between 1 and 4 h postdosing. The extract possesses significant in vitro antioxidant activity and a lipid peroxidation inhibition effect. Total phenolic and total flavonoid content in MEPA were 87.99 ± 0.87 mg GAE/g and 58.98 ± 0.01 mg QE/g, respectively.
Discussion and conclusion: Our findings confirmed the analgesic, anti-inflammatory and antioxidant activities of Pisonia aculeata leaves. Contents of flavonoids and phenolic compounds in extract could be correlated with its observed biological activities.
Introduction
Pisonia aculeata L. (Nyctagenaceae) is a large, thorny climbing shrub used widely in India as a traditional folk medicine. Pisonia aculeata leaves and bark are used commonly to treat rheumatism, swelling, and pulmonary complaints, in addition to its other beneficial effects, such as diuretic, antiscabies, aphrodisiac; the roots are used to treat anemia (CitationCouncil of Scientific and Industrial Research, 1969; Chetty et al., Citation2008). Pisonia aculeata has been studied pharmacologically for its antitubercular (Wu et al., Citation2011b), antitumor (CitationSenthilkumar et al., 2008), hepatoprotective, and in vivo antioxidant activity (CitationAnbarasu et al., 2011; CitationPalanivel et al., 2008).
Inflammation, pain and oxidative stress play important roles in several ailments, such as rheumatoid arthritis, cancer, asthma, neurodegenerative, and other age related disorders, which show a high pervasiveness worldwide (CitationGupta et al., 2003; CitationZimmer et al., 2012). Recently, there has been a renewed interest in scientific research on traditional/folk medicinal plants and use of the knowledge of traditional medicinal systems to find new and effective drug molecules or therapy. In the present study, Pisonia aculeata leaf extract was assessed for anti-inflammatory, analgesic, and antioxidant activity. In addition, we measured total phenolic content and total flavonoid content in the extract in order to correlate them with the assayed activities.
Materials and methods
Plant material and preparation of the crude extract
Leaves of Pisonia aculeata were collected in August 2011 from Tirupathi, Andhra Pradesh, India. The leaves were identified and authenticated by Dr. Madhava Chetty, Department of Botany, SV University, Andhra Pradesh. A herbarium was prepared and deposited in our department for further reference (No. 331). Dried powered leaves (250 g) were extracted with methanol using a Soxhlet apparatus, and the solvent was evaporated to dryness to get solvent free extract. The yield of the dried extract was 18.5% w/w.
Chemicals
Sodium nitroprusside (SNP), 2-deoxyribose,trichloro-acetic acid (TCA), thiobarbituric acid (TBA), N-naphthyl ethylenediamine dihydrochloride, Folin-Ciocalteau reagent, aspirin and quercetin were procured from SD Fine Ltd. Mumbai. 2,2-Diphenyl-picrylhydrazyl (DPPH), ferrozine, ascorbic acid, and gallic acid were obtained from Sisco Research Laboratories Pvt. Ltd., Mumbai. All other drugs and chemicals used in the study were obtained commercially and were of analytical grade.
Experimental animals
Albino mice (20–30 g) were used for the study. Animals were kept on a 12 h light/12 h dark cycle in a regulated environment (24 ± 2°C temperature, and 60% humidity). All animal procedures were approved by Institutional Ethical Committee (1305/ac/09/CPCSEA) in accordance with animal experimentation and care.
Preliminary phytochemical screening
A small amount of methanol extract of Pisonia aculeata leaves (MEPA) was used to determine the alkaloids, carbohydrates, proteins, amino acids, glycosides, tannins, flavonoids, steroid, and triterpinoids using standard methods (CitationKokate et al., 2007).
Estimation of total phenolic content and total flavonoid content
Total phenolic content in the extract was determined using the Folin-Ciocalteu reagent method and the result was expressed as mg gallic acid equivalents (GAE)/g dry extract (CitationAiyegoro & Okoh, 2010). Total flavonoid content extract was estimated using the aluminium nitrate reagent method as described by CitationAsokkumar et al. (2010), and the value was expressed as mg quercetin equivalent (QE)/g dry extract.
Anti-inflammatory activities
Carrageenan-induced paw edema in mice
Carrageenan (200 µg) was injected subcutaneously into the plantar surface of the left hind paw of mice 1 h after the oral administration of vehicle, MEPA (250 and 500 mg/kg), or indomethacin (10 mg/kg). The volume of the left hind paw of each animal was measured using a plethysmometer at hourly intervals up to 5 h. The degree of inflammation was determined by the paw volume increase (Vt − V0) where Vt and V0 are the volume of the left hind paw after and before the carrageenan injection, respectively (CitationNonato et al., 2011).
Membrane stabilizing activity
Membrane stabilizing activity of MEPA was performed using two separate methods, i.e., heat induced hemolysis assay and hypotonic solution induced hemolysis assay as described by CitationShinde et al. (1999). Blood was collected from mice in a heparinized tube and washed with 0.9% saline for three times. Isotonic buffer solution (pH 7.4) was used to prepare a 40% (v/v) erythrocyte suspension, which was used to determine the activities.
Analgesic activity
Hot plate test
The hot plate test was carried out using Eddy’s hot plate at a fixed temperature of 55 ± 0.5°C. Latency to exhibit analgesic responses (paw licking or jumping) was recorded. The reaction time was determined at 0, 30, 60, and 90 min after administration of vehicle, MEPA (250 and 500 mg/kg, p.o.), and morphine (10 mg/kg, s.c.) (CitationZhang et al., 2009).
Tail immersion method
The tail immersion test was performed as described by CitationAsongalem et al. (2004) with some modifications. The reaction time (withdrawing the tail) was recorded by immersing 2–3 cm of the mouse tail in a hot water (55 ± 0.5°C). The reaction time for each animal was recorded at 0, 0.5, 1, 4, 6 h following the administration of test substances.
Writhing test
The drug solutions were administered 60 min prior to treatment with 0.6% acetic acid (10 mL/kg, i.p.). Five minutes after the injection of acetic acid, we observed the number of writhes (abdominal wall contractions, pelvic rotation, followed by hind limb stretches) for a period of 25 min (CitationZhang et al., 2009).
Formalin-induced licking response in mice
Exactly 25 μL of 1% formalin in saline was injected subcutaneously in the right subplantar region of hind paw of each mouse 1 h after oral administration of test drug solutions. Mice were kept in the observation chamber, and the time spent in licking and biting the injected paw was recorded. The first period (early phase) was recorded at 0–5 min and the second period (late phase) was recorded at 10–35 min (Wu et al., Citation2011a).
In vitro antioxidant and radical scavenging activity
In vitro free radical scavenging activity of methanol extract was determined by several assays include DPPH radical (CitationHarlalka et al., 2007), hydroxyl radical (CitationOzyurek et al., 2008), and nitric oxide radical (CitationYen et al., 2001) scavenging activities using standard procedures. Hydrogen peroxide scavenging ability of the extract was estimated following the method used by CitationBozin et al. (2008). Reducing power ability of the extract (25–400 µg/mL) was determined using the method used by CitationPal et al. (2010). The chelating capacity of ferrous ions by the MEPA was determined by the method of CitationGulcin (2006). Several natural antioxidants, like ascorbic acid, gallic acid, quercetin, α-tocopherol, were used as standard.
Lipid peroxidation inhibition assay
One milliliter of mice brain homogenate (5%) was mixed with 0.1 mL of sample solution and incubated for 2 h at 37°C. After incubation, 1.0 mL of TCA (15% w/v) and 1.0 mL (0.67% w/v) of TBA were added and warmed in a boiling water bath for 15 min, and the mixture final volume was made up to 5.0 mL with deionized water. The mixture was centrifuged at 2800 rpm for 10 min and the absorbance of the supernatant solution was recorded at 532 nm (CitationTai et al., 2011).
IC50 determination
The percentage of inhibition or radical scavenging activity (I%) of extract was calculated by using equation
where, Ac, absorbance control; As, absorbance extract/standard. The IC50 value was the concentration which causes 50% scavenging effect as determined graphically.
Statistical analysis
The data were subjected to analysis of variance (ANOVA) and expressed as mean ± SEM (n = 3 for in vitro and ex vivo model; n = 6 for in vivo tests). Statistical analysis was carried out by analysis of variance followed by Turkey tests, using SPSS (Statistical Package for Social Sciences) version 10.0 software. A level of p < 0.05 was used as the criterion for statistical significance.
Results
In an attempt to validate the folk use of Pisonia aculeata in pain, inflammation and oxidative stress induced diseases, this study was planned to assess the analgesic, anti-inflammatory and antioxidant effect of the plant extract, and to find its possible underlying mechanism(s) using several methods.
Preliminary phytochemical analysis
Qualitative phytochemical analysis results revealed that the methanol extract of P. aculeata leaves contained alkaloids, carbohydrates, tannins, flavonoids, steroids and triterpenoids. Saponin, glycosides, protein, and amino acid were absent in the extract.
Total phenolic content and total flavonoid content
Quantitative estimation of MEPA showed that the total phenolic content in the extract was 87.99 ± 0.87 mg GAE/g of dry material, while flavonoid content was 58.98 ± 0.01 mg QE/g of dry material.
Anti-inflammatory activity of MEPA
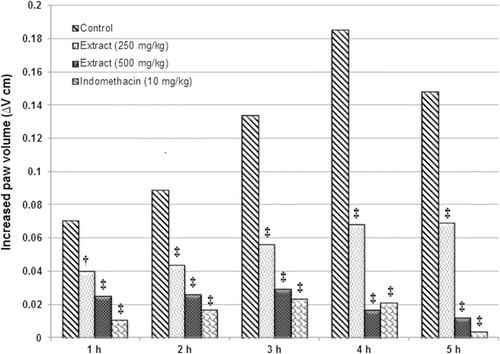
The methanol extract showed significant (p < 0.001) inhibition of carrageenan-induced paw edema in mice. In the control group, carrageenan caused a localized edema starting 1 h after injection and the swelling increased progressively to 0.329 ± 0.006 at 4 h, while oral administration of MEPA and indomethacin showed significant decrease in paw edema at 4 and 5 h, which was near to normal. A higher dose of MEPA registered a comparable activity with that of indomethacin ().
Figure 1. Effects of the leaf extracts of Pisonia aculeata on carrageenan-induced mouse paw edema. †p < 0.01, ‡p < 0.001 significantly different from control group (ANOVA followed by Tukey’s test).
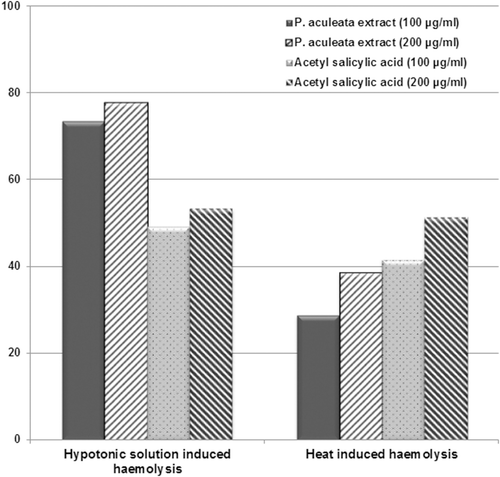
The extract was able to protect against erythrocyte hemolysis in a concentration-dependent manner and the inhibitory effect of MEPA was better than the standard drug acetyl salicylic acid in hypotonic solution induced hemolysis. The percentage inhibition of the methanol extract at 100 and 200 µg/mL concentration were 73.39, 77.67, 28.83, 38.51% against hypotonic solution and heat-induced hemolysis, respectively ().
Analgesic effect of MEPA
In the hot plate method, the higher dose of MEPA showed significant analgesic activity, but the lower dose failed to produce any significant activity. Analgesic activity of MEPA was higher at 60 min, which was significantly decreased at 90 min. MEPA (250 and 500 mg/kg) cause a significant reduction in painful sensation in the tail immersion method. Inhibitory effects of MEPA became pronounced between 1 and 4 h postdosing but activity decreased after 4 h ().
Table 1. Analgesic effect of the methanol extract of Pisonia aculeata leaves against several test models.
MEPA showed significant analgesic activity against acetic acid-induced pain. A higher dose of MEPA showed similar activity with the standard. Indomethacin and extract (250 and 500 mg/kg) inhibit pain (writhing response) by 82.28, 35.21, and 79.14% as compared to the control, respectively ().
We found that Pisonia aculeata produced analgesic activity both in the early and late phases of the formalin test, but the activity of the extract was better in second phase. In the first phase of the formalin-induced pain model, indomethacin, MEPA (250 and 500 mg/kg) produced 56.68, 25.93, and 49.19% inhibition of pain response, while during second phase, the inhibition was 75.53, 42.96, and 73.58%, respectively ().
In vitro and ex vivo antioxidant activity of MEPA
In vitro antioxidant activity of Pisonia aculeata leaf extract and their possible mode of free radical scavenging activities were investigated by several assay models. Concentration-dependent scavenging activity of extract was observed against all tested methods. IC50 values of MEPA in different assay model are tabulated in . In the DPPH radical scavenging assay, at 48 µg/mL, methanol extract produced 76.86% inhibition, while ascorbic acid (16 µg/mL) possesses 91.30% scavenging activity. MEPA possess better hydroxyl radical scavenging activity (IC50 = 18.9 µg/mL) than the standard quercetin (IC50 = 20.8 µg/mL). MEPA showed excellent nitric oxide scavenging activity. At a concentration of 120 µg/mL, nitric oxide scavenging activity of the methanol extract and ascorbic acid were 86.53 and 76.76%, respectively. The IC50 values of ascorbic acid and methanol extract were 23.9 and 12.5 µg/mL.
Table 2. Antioxidant activities of the leaf extract Pisonia aculeata by using different in vitro and ex vivo models.
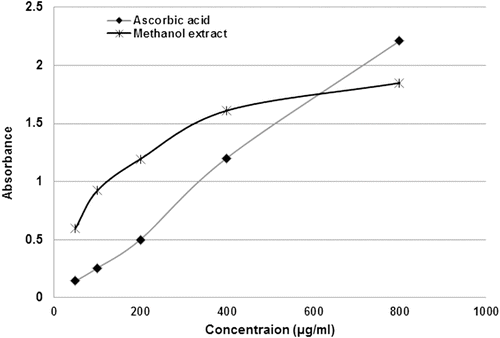
Moderate hydrogen peroxide scavenging effects were observed for methanol extract (IC50 113.0 µg/mL). MEPA and α-tocopherol interfered with the formation of ferrous and ferrozine complex, suggesting that extracts possess significant ferrous ion chelating activity. The IC50 value of iron chelating activity for the methanol extract and α-tocopherol were 119.1 and 107.1 µg/mL. shows the concentration-response curves for the reducing powers of the MEPA. The reducing power of the methanol extract increased from 0.601 ± 0.007 at 50 µg/mL to 1.850 ± 0.008 at 800 µg/mL.
Figure 3. Reducing power ability of the methanol extract of Pisonia aculeata leaves at different concentrations. Results are of triplicate measurements.
Malondialdehyde (MDA), a cytotoxic product generated during lipid peroxidation, which was taken to indicate the oxidation. The IC50 values methanol extract and ascorbic acid were 76.9 and 68.9 µg/mL, respectively (). At a concentration of 160 µg/mL, lipid peroxidation inhibition of methanol extract and ascorbic acid was 77.57 and 82.30%, respectively.
Discussion
The results of present study demonstrate that the methanol extract of Pisonia aculeata possesses anti-inflammatory, analgesic and antioxidant activity. Carrageenan-induced edema is an experimental model of acute inflammation and represents a biphasic response. The first phase reaction mediated by release of histamine and serotonin, while neutrophil infiltrations, eicosanoid release, production of free radicals are responsible for second phase (CitationArawwawala et al., 2010). MEPA was effective against carrageenan-induced paw edema supporting its use in traditional medicine. A protective effect on heat and hypotonic solution induced erythrocyte lysis is often seen as an index of stabilization of lysosomal membranes, and considered as a biochemical parameter of anti-inflammatory activity. Plants with membrane stabilizing properties can interfere with the early phase of inflammatory mediator release, specifically in the release of phospholipase A2 (CitationArawwawala et al., 2010; CitationShinde et al., 1999). Therefore, the inhibition of release of chemical mediators and the membrane stabilizing effect of extract may contribute to its anti-inflammatory activity.
The tail immersion method and hot plate method were selected to investigate central analgesic activity. The validity of the hot plate method has been shown even in the presence of considerable impairment of motor performance (CitationGupta et al., 2003). Drugs acting against the tail immersion test induced pain mediated their pharmacological actions by mu (µ) opioid receptors rather than kappa (κ) and delta (δ) receptors (CitationAsongalem et al., 2004). The extract at higher dose exhibited significant activity against these two models, but their potency and duration of action were significantly lower than the standard. Acetic acid-induced writhing response was selected to evaluate peripheral analgesic mechanism of MEPA. This model is considered as a model of chemonociception and it provokes pain sensation by increasing fluids of PGE2, PGE2α, serotonin, and histamine (CitationLoganayaki et al., 2012). Results showed that MEPA produced potent activity against acetic acid-induced pain. Hence, the mechanism of action of extract may be associated with the peripheral inhibition of algogen substances such as bradykinins, prostanoids, and other mediators. Pain induced by formalin test represents two distinct phase, early phase of formalin test represents centrally mediated pain, while the late phase pain indicates pain mediated by a peripheral effect via release of transmitters such as histamine, serotonin, prostaglandins, kinins, etc. (CitationNonato et al., 2009). Results indicated that the extract produced better activity in second phase, therefore analgesic effects of the extract might be mediated through a peripheral mechanism rather than a central mechanism.
In the DPPH scavenging assay, antioxidant compounds cause reduction of alcoholic DPPH solution due to their hydrogen-donating capacity (CitationGulcin, 2006). Therefore, DPPH radical scavenging activity of extract might be attributed to a direct role in trapping free radicals by donating hydrogen atoms. Hydroxyl radical is a potent cytotoxic agent and considered as the most reactive radical produced in living system and responsible for enormous biological damage (CitationGuzman et al., 2001). NO• is an imperative pleiotropic mediator of several physiological processes. However, excess production of NO• is associated with cancer, atherosclerosis, arthritis, and inflammation (CitationHazra et al., 2008). Thus, the finding justifies the application of P. aculeata in folk medicine augmenting its therapeutic value.
Hydrogen peroxide involved in the inactivation of different enzymes by oxidation of essential thiol groups, and can initiate the generation of hydroxyl radical (CitationBozin et al., 2008; CitationHazra et al., 2008). The methanol extract was able to neutralize H2O2 in a concentration-dependent manner, which could be seen by its graded increase in percentage inhibition. Iron is a highly reactive transition metal, and known as a most significant prooxidant for lipid oxidation (CitationHazra et al., 2008; CitationNagulendran et al., 2007). Therefore, ferrous ion chelating activity of extract may be related to its iron binding capacity. Reducing power capacity may provide a key indication about the antioxidant capacity of a compound (CitationNagulendran et al., 2007). The presence of reductone is related with the reducing power capacity of extract and considered as one of the key mechanisms for action of MEPA.
Ex vivo antioxidant activity was evaluated by inhibition of lipid peroxidation. Lipid peroxidation is considered as an oxidative deterioration process of polyunsaturated fatty acids, and may be responsible for the damage of almost every molecule of the body. Inhibition of lipid peroxidation is a vital property of antioxidant compounds by which they can inhibit the initiation and/or propagation of free radical reaction (CitationNiki, 2010; CitationTai et al., 2011). Results suggested that the extract effectively inhibited lipid peroxidation. These findings rationalize the traditional usage of this plant in the treatment of several inflammation, pain, and oxidative stress associated diseases.
Different scientific reports proved that chemical constituents like alkaloids, volatile and essential oils, phenolic compounds, triterpenoids, saponins, phytosterols, tannins, flavanoids possess analgesic, anti-inflammatory, and antioxidant activity (Sen et al., Citation2010a, Citation2010b). Our report also suggested the presence of alkaloids, carbohydrates, tannins, flavonoids, steroids and triterpenoids in the extract. The extract also contained a significant amount of phenolic and flavonoid. The free radical scavenging activity of phenolic compounds is due to the presence of hydroxyl groups in their structure. Flavonoids are also useful to prevent and treat cardiovascular, neurodegenerative, gastrointestinal, endocrine diseases, and cancer. Their planar structure, number and position of their hydroxyl groups as well as the presence of the C2-C3 double bond are essential for metal chelation, antioxidant and free radical scavenging activities (CitationAtmani et al., 2009). Phenolic components, flavonoids and other phytochemicals present in the extract may be responsible for its antioxidant, analgesic, anti-inflammatory activities.
Conclusion
The present work revealed that leaves of Pisonia aculeata exhibited anti-inflammatory, antiociceptive and antioxidant activity. High levels of phenolics and flavonoids in extract may be responsible for its observed biological activities. The possible antioxidant mechanism of the extract can be due to hydrogen or electron donating and direct free radical scavenging activities of extract. Membrane stabilizing activity and inhibition of release of inflammatory mediators may be responsible for its anti-inflammatory activity. The extract possesses better peripheral analgesic activity rather than central analgesic activity. We suggests that the leaves of plant can be viewed as a potential source of natural antioxidants and can afford a precious functional component which may be used for the prevention of diseases related to oxidative stress, pain, and inflammation.
Acknowledgments
The authors wish to thank the Management of Creative Education Society’s College of Pharmacy for providing facilities and support.
Declaration of interest
The authors declare that they have no conflicts of interest to disclose. This research received no specific grant from any funding agency in the public, commercial, or not for profit sectors.
References
- Aiyegoro OA, Okoh AI. (2010). Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement Altern Med, 10, 21.
- Anbarasu C, Rajkapoor B, Kalpana J. (2011). Protective effect of Pisonia aculeata on rifampicin and isoniazid induced hepatotoxicity in rats. Int J Phytomed, 3, 75–83.
- Arawwawala M, Thabrew I, Arambewela L, Handunnetti S. (2010). Antiinflammatory activity of Trichosanthes cucumerina Linn. in rats. J Ethnopharmacol, 131, 538–543.
- Asokkumar K, Umamaheswari M, Baharudeen A, Sivashanmugam AT, Subhdhradevi V, Ravi TK. (2010). Antioxidant and hepatoprotective activities of the fractions of Ficus microcarpa using in vitro and ex vivo models. Funct Plant Sci Biotechnol, 4, 17–27.
- Asongalem EA, Foyet HS, Ngogang J, Folefo GN, Dimo T, Kamtchouing P. (2004). Analgesic and antiinflammatory activities of Erigeron floribundus. J Ethnopharmacol, 91, 301–308.
- Atmani D, Chaher N, Atmani D, Berboucha M, Debbache N, Boudaoud H. (2009). Flavonoids in human health: From structure to biological activity. Curr Nutr Food Sci, 5, 225–237.
- Bozin B, Mimica-Duki N, Samojlik I, Goran A, Igic R. (2008). Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem, 111, 925–929.
- Chetty MK, Shivaji K, Rao TK. 2008. The Flowering Plants of Chittor District. Tirupathi: Students Offset Printers.
- Council of Scientific and Industrial Research (1969). The Wealth of India, Vol 8. New Delhi: Council of Scientific and Industrial Research.
- Gulcin I. (2006). Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicol, 217, 213–220.
- Gupta M, Mazumder UK, Kumar RS, Kumar TS. (2003). Studies on anti-inflammatory, analgesic and antipyretic properties of methanol extract of Caesalpinia bonducella leaves in experimental animal models. Iran J Pharmacol Ther, 2, 30–34.
- Guzman S, Gato A, Calleja JM. (2001). Antiinflammatory, analgesic and free radical scavenging activities of the marine microalgae Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother Res, 15, 224–230.
- Harlalka GV, Patil CR, Patil MR. (2007). Protective effect of Kalanchoe pinnata Pers. (Cressulaceae) on gentamycin-induced nephrotoxicity in rats. Indian J Pharmacol, 39, 201–205.
- Hazra B, Biswas S, Mandal N. (2008). Antioxidant and free radical scavenging activity Spondias pinnata. BMC Complement Altern Med, 8, 1–10.
- Kokate CK, Purohit AP, Gokhale SB. (2007). Pharmacognosy. Pune: Nirali Prakashan.
- Loganayaki N, Siddhuraju P, Manian S. (2012). Antioxidant, anti-inflammatory and anti-nociceptive effects of Ammannia baccifera L. (Lythracceae), a folklore medicinal plant. J Ethnopharmacol, 140, 230–233.
- Nagulendran KR, Velavan S, Mahesh R, Begumm VH. (2007). In vitro antioxidant activity and total polyphenolic content of Cyperus rotundus rhizomes. E-J Chem, 4, 440–449.
- Niki E. (2010). Assessment of antioxidant capacity in vitro and in vivo. Free Radic Biol Med, 49, 503–515.
- Nonato FR, Barros TAA, Lucchese AM, Oliveira CEC, Santos RR, Soares MBP, Villarreal CF. (2009). Antiinflammatory and antinociceptive activities of Blechnum occidentale L. extract. J Ethnopharmacol, 125, 102–107.
- Nonato FR, Nogueira TM, Barro TA, Lucchese AM, Oliveira CE, Santos RR, Soares MB, Villarreal CF. (2011). Antinociceptive and antiinflammatory activities of Adiantum latifolium Lam.: Evidence for a role of IL-1β inhibition. J Ethnopharmacol, 136, 518–524.
- Ozyurek M, Bektasoglu B, Guclu K, Apak R. (2008). Hydroxyl radical scavenging assay of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method using catalase for hydrogen peroxide degradation. Anal Chim Acta, 616, 196–206.
- Pal J, Ganguli S, Tahsin KS, Acharya K. (2010). In vitro free radical scavenging activity of wild edible mushroom Pleurotus squarrosulus (Mont.) Singer. Indian J Exp Biol, 47, 1210–1218.
- Palanivel M, Rjakapoor B, Kumar R, Einstein J, Kumar E, Kumar M, Kavitha K, Kumar M, Jayakar B. (2008). Hepatoprotective and antioxidant effect of Pisonia aculeate L. against CCl4-induced hepatic damage in rats. Sci Pharm, 76, 203–215.
- Sen S, Chakraborty R, De B, Ganesh T, Raghavendra HG. (2010a). Analgesic and anti-inflammatory drugs: a potential source of herbal medicine. Int J Pharma Sci Res, 1, 32–44.
- Sen S, Chakraborty R, Sridhar C, Reddy YSR, De B. (2010b). Free radicals, antioxidants, diseases and phytomedicines: Current status and future prospect. Int J Pharma Sci Rev Res, 3, 91–100.
- Senthilkumar R, Manivannan R, Balasubramaniam A, Sivakumar T, Rajkapoor B. (2008). Effects of ethanol extract of Pisonia aculeata Linn. on Enrich ascites carcinoma tumour bearing mice. Int J Green Pharm, 2, 50–53.
- Shinde UA, Phadke AS, Nair AM, Mungantiwar AA, Dikshit VJ, Saraf MN. (1999). Membrane stabilizing activity – a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia, 70, 251–257.
- Tai Z, Cai L, Dai L, Dong L, Wang M, Yang Y, Cao Q, Ding Z. (2011). Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem, 126, 1648–1654.
- Wu C, Hseu Y, Lien J, Lin L, Lin Y, Ching H. (2011a). Triterpenoid contents and anti inflammatory properties of the methanol extracts of Ligustrum species leaves. Molecules, 16, 1–15.
- Wu M, Peng C, Cheng I, Tsai L. (2011b). Antitubercular chromones and flavonoids from Pisonia aculeata. J Nat Prod, 74, 976–982.
- Yen G, Lai H, Chou H. (2001). Nitric oxide-scavenging and antioxidant effects of Uraria crinita root. Food Chem, 74, 471–478.
- Zhang L, Hu J, Lin J, Fang W, Du G. (2009). Anti-inflammatory and analgesic effects of ethanol and aqueous extracts of Pterocephalus hookeri (C.B. Clarke) Höeck. J Ethnopharmacol, 123, 510–514.
- Zimmer AR, Leonardi B, Miron D, Schapoval E, de Oliveira JR, Gosmann G. (2012). Antioxidant and anti-inflammatory properties of Capsicum baccatum: From traditional use to scientific approach. J Ethnopharmacol, 139, 228–233.