Abstract
Context: Despite the many biological activities reported for essential oils, their anti-inflammatory ability is relatively underexplored considering the wide variation in plant sources and in their volatile composition. Oils from Syzygium cumini Skells (SC) and Psidium guajava L. (PG) (Myrtaceae) have been described as having diverse pharmacological activities.
Objective: The current study seeks to evaluate the anti-inflammatory activity of the essential oils from the leaves of SC and PG, as well as some of their terpene-enriched fractions (+V = more volatile and −V = less volatile) obtained by vacuum distillation. Both the pharmacological responses and chemical compositions were correlated.
Materials and methods: The relative contents of the oils and their fractions were evaluated by gas chromatography. Individual constituents in the oils were characterized by gas chromatography coupled to mass spectrometry. Anti-inflammatory activity was accessed in the lipopolysaccharide-induced pleurisy model, by measuring the inhibition of total leukocyte, neutrophil and eosinophil migration in the mice pleural lavage, after oil treatment with the oils at 100 mg/kg.
Results: Eosinophil migration was inhibited by SC (67%), SC (+V) (63%), PG (76%), PG (+V) (67%) and PG (−V) (74%). This efficacy was correlated with the presence of β-pinene and β-caryophyllene in the oils, a result that was reinforced by evaluating both these pure components (38 and 50% inhibition, respectively). Synergistic effects associated with the presence of α-pinene were speculated.
Discussion and conclusion: Essential oils from SC and PG may be useful to treat inflammatory diseases by mechanisms that include the inhibition of eosinophil migration.
Introduction
Essential oils extracted from many distinct plant species have shown pharmacological effects. For example, the oral or intraperitoneal administration of essential oils, at doses of 50–100 mg/kg, has shown dose-dependent inhibition of rat paw edema induced by carrageenan, PGE1 or bradykinin (Kavimani et al., Citation1997; Martin et al., Citation1993; Ocete et al., Citation1989; Veiga Jr. et al., Citation2001) of granulomas induced by croton oil (Kavimani et al., Citation1997; Ocete et al., Citation1989), and oxytocin-induced rat uterine contractions (Ocete et al., Citation1989). These oils have been shown to inhibit pleurisy in mice induced by zymosan or lipopolysaccharide (LPS), as shown by significant reductions in eosinophil and neutrophil migration (Ramos et al., Citation2006; Siani et al., Citation1999; Souza et al., Citation2003). In addition, essential oils from various plants have shown antinociceptive effects on writhing induced by acetic acid or formalin in mice (Golshani et al., Citation2004). Psidium guajava oil, at 100–400 mg/kg, has shown a dose-dependent analgesic effect (Santos et al., Citation1998), although lower doses had no activity (Siani et al., Citation1999). Essential oils have also been shown to be effective in treating wounds and ulcers (Woollard et al., Citation2007).
In general, the anti-inflammatory effects of essential oils are attributed to their terpenoid composition. These effects have been observed with several isolated mono- and sesquiterpenes, including the hydrocarbons Δ-3-carene (Ocete et al., Citation1989), myrcene and limonene (Souza et al., Citation2003), and α-bergamoptene, α-aromadendrene, and the pool of β-caryophyllene and α-humulene (Fernandes et al., Citation2007; Martin et al., Citation1993; Passos et al., Citation2007; Veiga Jr. et al., Citation2001) as well as the alcohol terpinen-4-ol (Hart et al., Citation2000). Essential oils may have synergistic effects (Perrucci et al., Citation1995), as suggested by the high structural similarity of the terpenoids present in these complex mixtures (Harris, Citation2002). For example, the treatment of Wistar rats with phenylpropanoid-rich essential oils from the species of Myrtaceae inhibited carrageenan-induced edema (Maridass, Citation2008), a finding previously observed with other oils of similar composition (Tung et al., Citation2008, Citation2010).
The current work describes our initial attempt to determine the synergistic activity of the constituents of essential oils by measuring the in vivo anti-inflammatory activity of essential oils from the leaves of two species of Myrtaceae, Syzygium cumini Skells and P. guajava L., both of which have been shown to have anti-inflammatory properties (Ramos et al., Citation2006). The oils from these species differ in their qualitative profiles: S. cumini has high amounts of monoterpenes (M) and low amounts of sesquiterpenes (S), whereas P. guajava has low amounts of M and high amounts of S. Oils from these leaves were assayed in LPS-induced pleurisy tests, as were their terpene-enriched fractions obtained by distillation. We also analyzed the correlation between the activity of these mixtures and their chemical composition, including the relative amounts of M and S and the relative amounts of hydrocarbons and oxygenated species (alcohols). Finally, four commercially available terpenes, all of which are present in these oils, were assayed under the same conditions to determine their ability to inhibit leukocyte migration.
Materials and methods
Plant material
Leaves of S. cumini (SC) and P. guajava (PG) were collected in January–February 2005 from sites on the campus of the Oswaldo Cruz Foundation, located in the municipality of Rio de Janeiro, Brazil (22°52′ S/41°35′ W). Vouchers under registration numbers RB-326235 (SC) and RB-326233 (PG) have been deposited in the Herbarium of the Botanical Garden of Rio de Janeiro.
Extraction and distillation of the volatile oils
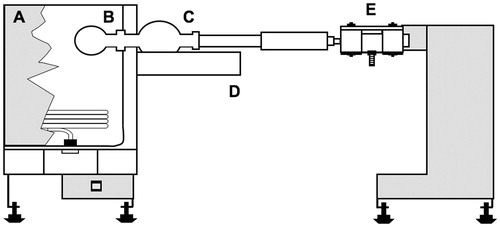
Essential oils were obtained from leaves by hydrodistillation for 6 h using a modified Clevenger apparatus (Wasicky, Citation1963). The samples were subsequently collected, centrifuged, carefully separated from water and stored at −20 °C in amber-sealed flasks. Refractive indices were determined using a VEE GEE Model C10 Abbe refractometer (Nova-Tech International, Kingwood, TX). The oils were distilled in vacuum, using a commercial ball-tube-type Kugelrohr distillation apparatus equipped with an oven (350 W), a vacuum outlet, a cooling bath (dry ice/acetone) for the collecting flask and a rotary system for the sample flask (Aldrich Chemical Co., Milwaukee, WI) coupled to a VacoBox B-177 equipped with a B-720 vacuum controller (Büchi Labortechnik AG, Flawil, Switzerland) (see the schematic diagram in ). Before use, we ensured that the oven temperature would respond linearly in the range of 120–180 °C, at atmospheric pressure, when the potency was varied from 20 to 40 units in the Variac equipment (Powermite mod. 3A). Parameters used for optimization included the respective yields and terpenoid compositions of fractions as functions of temperature and time. SC oil samples were distilled 12 times and PG oils five times to optimize parameters for separating M and S in each sample. Each distillation started with about 200 mg of oil. At the end of each process, the more volatile fraction was collected in flask C, and the less volatile fraction was carefully removed from the distillation flask B. Both were weighed in order to determine their respective yields. Commercial α-pinene, β-pinene, β-caryophyllene and β-caryophyllene oxide were purchased from Aldrich Chemical Co.
Chromatographic analysis
The chemical constituents of the oils and their fractions were characterized by gas chromatography coupled to a mass electron impact detector using a Hewlett Packard 6890 instrument (Palo Alto, CA) with an HP-5 MS (30 m × 0.32 mm i.d. × 0.25 μm film thickness) capillary column (Hewlett Packard, Palo Alto, CA) and helium as a carrier gas at a flow rate of 0.5 ml/min. One milliliter aliquots of 6 mg/100 μl solutions of oils in dichloromethane were injected at a 1/20 split ratio under the following conditions: injector temperature 250 °C, Ti 70 °C, ti 2 min, Tf 280 °C and heating rate 3 °C/min. Mass spectra were acquired from electron impact at 70 eV and an ion source at 250 °C. Individual components in the oils were identified by comparing their spectra with those stored in the Wiley library software HP 59943 B database and by considering their respective retention indices (Adams, Citation2007). The composition of the oils and fractions were estimated from the relative peak areas in the chromatograms obtained from identical chromatographs coupled to a flame ionization detector, operated at the same conditions as above, and assuming identical relative responses for all the oil components.
Animals
Male Swiss mice (15–20 g) were obtained from the Oswaldo Cruz Foundation Breeding Unit (Fiocruz, Rio de Janeiro, Brazil) and caged, with free access to food and fresh water, in a room at the Farmanguinhos Experimental Animal Facility maintained at a temperature of 22–24 °C under a 12 h light/dark cycle. All experimental procedures were performed according to guidelines provided by the Committee on Ethical Use of Laboratory Animals of the Oswaldo Cruz Foundation.
Treatments
One hour prior to stimulation, mice fasted for 12 h were orally (p.o.) administered essential oils, volatile fractions or commercial samples (100 mg/kg), diluted to 1 µl/mg in sterile saline in a final volume of 200 µl. Negative control mice were administered the same volume of vehicle p.o., whereas positive control mice were administered dexamethasone (2 mg/kg) diluted in sterile saline to a final volume of 200 µl, 1 h before stimulation.
Induction of pleurisy
Pleurisy was induced in mice as described by Spector (Citation1956) and modified by Henriques (Citation1990). Briefly, an adapted needle was inserted into the right side of the thoracic cavity to enable intrathoracic (i.t.) administration of E. coli endotoxin (LPS; 250 ng/cavity). Control animals received an equal volume (50 µl/cavity) of pyrogen-free sterile saline (NaCl 0.9%). Twenty-four hours later, the animals were killed by CO2 inhalation, their thoracic cavities were washed with 1 ml of PBS containing heparin (20 IU/ml) and the lavage fluid was collected for the assessment of leukocyte accumulation. Total leukocyte counts were performed in an automated particle counter (Z1; Coulter, Brea, CA). Cytospin smears (Shandon, San Luis Obispo County, CA) were performed and stained by the May–Grünwald Giemsa method for differential leukocyte counts under light microscopy (100×). Results are presented as mean ± SEM and were statistically evaluated by one-way ANOVA followed by the Student–Newman–Keuls test. The significance level was set at p ≤ 0.05.
Results and discussion
Analysis of essential oils and their more and less volatile fractions
The yields of oils from SC and PG leaves and their refractive indexes were 0.05% ( = 1.4970) and 0.14% (
= 1.4828), respectively. Oil from SC leaves contained a high content of monoterpenes (69%) and oil from PG contained a high content of sesquiterpenes (85%), with these proportions calculated relative to the total pool of terpenoid types, as determined by GC-FID, in the original crude oils, and by considering their relative abundance in the chromatograms ().
Table 1. Constitution of the leaf essential oils and the more (+V) and less (−V) volatile fractions from S. cumini and P. guajava after the Kugelrhor distillation.
To achieve maximum separation between mono- and sesquiterpene pools as well as between hydrocarbons and oxidized species, we determined the optimum conditions for the Kugelrohr distillation of each sample, by varying the pressure, temperature and time of each process and measuring the yields and terpenoid composition of the more (+V) and less (−V) volatile fractions. Better separation was generally obtained by varying the pressure. In SC, where monoterpenes predominate, distillation at atmospheric pressure almost completely eliminated sesquiterpenes and enriched +V for monoterpenes. At lower pressures, there was significant loss of the original samples, reducing the final recovery of the +V and −V fractions. In contrast, lower pressure during the distillation of PG leaves optimized the separation of the two groups of terpenoids. Optimal separation conditions for the Kugelrohr distillation of SC leaves were a pressure of 750 mbar, a temperature of 90 °C and a time of 1 h, whereas optimal conditions for PG samples were a pressure of 50 mbar, a temperature of 100 °C and a time of 2 h. Under these conditions, the yields of +V and −V fractions yields were 31% and 42%, respectively, for SC, and 52% and 22%, respectively, for PG. The relative contents of terpenoid species are displayed in and detailed in .
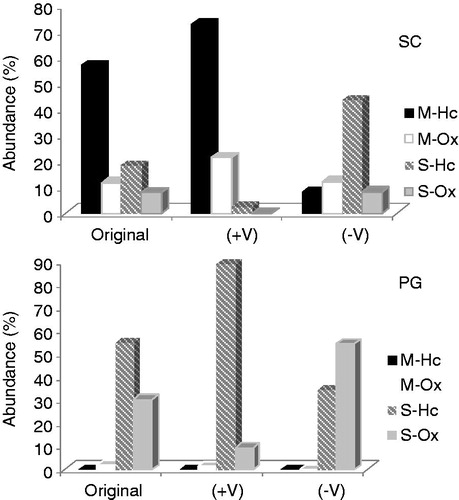
Figure 2. Relative contents of monoterpenes and sesquiterpenes in the original samples and the more (+V) and less (−V) volatile fractions from distillation of S. cumini (SC) and P. guajava (PG) essential oils. Identified compounds: M-Hc = monoterpenes hydrocarbons; M-Ox = oxydized monoterpenes; S-Hc = sesquiterpenes hydrocarbons; S-Ox = oxydized sesquiterpenes. Identified compounds: M-Hc = monoterpenes hydrocarbons; M-Ox = oxydized monoterpenes; S-Hc = sesquiterpenes hydrocarbons; S-Ox = oxydized sesquiterpenes.
The individual constituents of +V and −V, obtained from the optimized distillations, were characterized by their retention indices and their fragmentation patterns in mass spectra (Adams, Citation2007). In general, the ability to characterize sesquiterpenes from their retention indices on chromatograms was reduced for −V from SC (51.6%), in agreement with the low relative content of sesquiterpenes in the original samples. The complexity of the sesquiterpene profile in these chromatograms was exacerbated by the emergence of trace compounds after enrichment by distillation. This enhancement of the sesquiterpene relative peak areas, however, was not quantitatively relevant.
The amount of sesquiterpene alcohols in S. cumini was low, whereas the sesquiterpene hydrocarbons (plus bornyl acetate, the least volatile monoterpene present) were concentrated in the −V fraction (43.4% of the characterized signals). In contrast, +V contained a highly significant amount of monoterpenes (94% of the characterized signals). The selected distillation conditions led to a loss of β-caryophyllene (18% in the original oil), as verified by its drastic reduction in both +V and −V (). The original oil in PG leaves consisted solely of sesquiterpenes, restricting any possible comparison to the contents in each fraction of hydrocarbons and oxygenated species. Hydrocarbons constituted 88.6% of oils in the +V fraction (54.6% in the crude oil), whereas oxygenated species constituted 54.4% of the oils in the −V fraction (30.2% in the crude oil) ().
Anti-inflammatory activity
Generally, SC and PG oils effectively inhibited the migration of eosinophils and, to a lesser extent, neutrophils stimulated by LPS ( and ). The ability of SC (67%) to inhibit eosinophil migration was matched by its more volatile fraction, +V (63%). SC and its +V fraction consisted of 22.2% and 55.0% α-pinene, respectively, an effective monoterpene in pinenes-rich oil from Bupleurum fruticosum (Umbelliferae) (Lorente et al., Citation1989). Furthermore, the limonene and ocimene-isomers present in SC (23%) and in +V (16%) have been shown to have anti-inflammatory activity in the LPS-induced pleurisy model (Souza et al., Citation2003). Although not statistically significant, the inhibitory activity of the −V fraction of SC may be related to the presence of caryophyllene-type sesquiterpenes (β-caryophyllene plus its α-isomer humulene) (Fernandes et al., Citation2007; Passos et al., Citation2007; Veiga Jr. et al., Citation2001) and putatively to δ-cadinene. This isolated compound has not been assessed directly, although active essential oils have been shown to contain cadinane-type compounds (Lenfeld et al., Citation1986; Tung et al., Citation2008). The concentrations of monoterpenes and monoterpene-rich oils have been correlated with the inhibition of eosinophil and neutrophil migration (Martin et al., Citation1993; Siani et al., Citation1999; Souza et al., Citation2003). In each case, the amount of hydrocarbons in the mixture was higher than that of oxidized compounds (Ramos et al., Citation2006), similar to our findings with SC oil.
Figure 3. Effect of S. cumini (SC), more (+V) and less (−V) volatile fractions on LPS-induced total leukocyte, mononuclear cells, neutrophils and eosinophils recruitment. Oil (100 mg/kg; hatched columns) or vehicle (open and closed columns) was administered p.o. 1 h prior to LPS (250 ng/cavity) and pleural fluid was collected 24 h later. + and * indicate p < 0.05 when compared to control non-stimulated saline injected (open columns) or LPS-stimulated saline-treated group (closed columns), respectively.
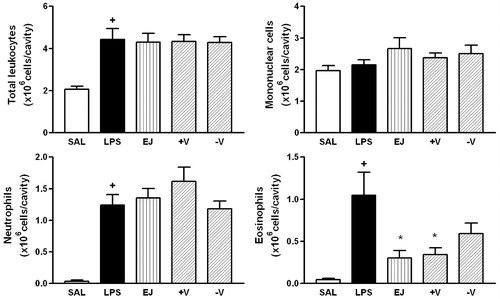
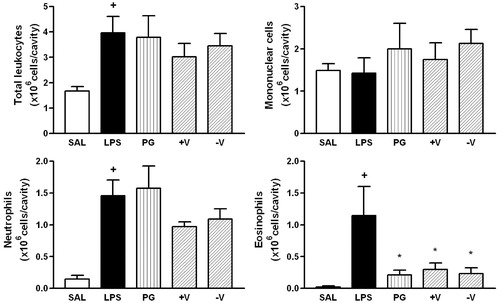
Figure 4. Effect of P. guajava (PG), more (+V) and less (−V) volatile fractions on LPS-induced total leukocyte, mononuclear cells, neutrophils and eosinophils recruitment. Oil (100 mg/kg; hatched columns) or vehicle (open and closed columns) was administered p.o. 1 h prior to LPS (250 ng/cavity) and pleural fluid was collected 24 h later. + and * indicate p < 0.05 when compared to control non-stimulated saline injected (open columns) or LPS-stimulated saline-treated group (closed columns), respectively. Oil (100 mg/kg; hatched columns) or vehicle (open and closed columns) was administered p.o. 1 h prior to LPS (250 ng/cavity) and pleural fluid was collected 24 h later. + and * indicate p < 0.05 when compared to control non-stimulated saline injected (open columns) or LPS-stimulated saline-treated group (closed columns), respectively.
The extent of the anti-inflammatory effect of Bupleurum fruticescens oil was also achieved by an artificial mixture of α-pinene and β-caryophyllene in which the relative proportion of each was similar to that found in the original oil (Martin et al., Citation1993). This study also pointed to a probable synergism between the two terpenes, since the addition of α-pinene clearly potentiated the moderate effect of β-caryophyllene administered at 150 mg/kg, particularly after the periods of 3 and 5 h. However, pure α-pinene only showed a notable anti-inflammatory effect at 300 mg/kg although a moderate effect could be seen at half of this dosage after 5 h. When the same experimental protocol was applied in adrenolectomized female Wistar rats, β-caryophyllene (300 and 600 mg/kg) demonstrated a dose-dependent anti-inflammatory capacity, whilst α-pinene did not exert any effect or could be even pro-inflammatory. On the other hand, at the same doses, both α-pinene and β-caryophyllene showed good acute anti-inflammatory responses to the rat hindpaw edema induced by PGE1 after 1 h. These results indicate that α-pinene and β-caryophyllene act through distinct anti-inflammatory mechanisms.
A similar trend was observed for Bupleurum gibraltaricum, in which the most active oil, containing Δ-3-carene and β-pinene, was always derived from the less-active α-pinene chemotype. Moreover, mixtures of equal amounts of Δ-3-carene and α-pinene inhibited carrageenan-induced plantar edema in rats after 1–24 h (Gil et al., Citation1989). These findings indicate that, at certain concentrations, pure α-pinene would be effective. However, its activity in oil is dependent on the composition of each oil, in that it may potentiate the overall inflammatory activity either positively or negatively. Its contribution may represent a dose-regulated effect, possibly arising from the triggering of different mechanisms and suggesting that the molecular mechanism underlying the anti-inflammatory activity of α-pinene remains to be fully understood. In LPS-stimulated THP-1 cells, α-pinene has shown dose-related upregulation of IkBα expression through the dose-related inhibition of nuclear translocation of NG-κB (Zhou et al., Citation2004).
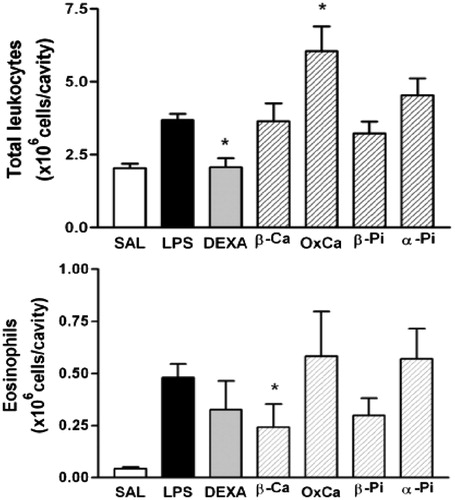
The sesquiterpenes in PG showed a 1.8:1 ratio of hydrocarbons to alcohol species. The original oil from PG leaves and both its +V (89% hydrocarbons) and −V (55% alcohols) fractions were similarly effective in inhibiting eosinophil migration by 76, 67 and 74%, respectively (). Moreover, these fractions tended to inhibit neutrophil migration, although these effects were not statistically significant. Caryophyllene isomers constitute 41% of the +V fraction, whereas hydrocarbons and oxidized species derived from caryophyllene/humulene constitute 25% of the −V fraction, thus accounting for the efficacy of both fractions. We also assayed commercial pure β-pinene, α-pinene, β-caryophyllene and β-caryophyllene oxide, at doses of 100 mg/kg, for their ability to inhibit LPS-induced pleurisy in mice. We found that β-caryophyllene and β-pinene inhibited eosinophil migration 50 and 38%, respectively, whereas pure β-caryophyllene oxide had pro-inflammatory activity ().
Figure 5. Effect of β-caryophyllene (β-Ca), β-caryophyllene oxide (OxCa), β-pinene (β-Pi) and α-pinene (α-Pi) on LPS-induced total leukocyte and eosinophils recruitment. Sample (100 mg/kg; hatched columns) or vehicle (open and closed columns) was administered p.o. 1 h prior to LPS (250 ng/cavity) and pleural fluid was collected 24 h later. * Indicates p < 0.05 when compared to control non-stimulated saline injected (open columns) or LPS-stimulated saline-treated group (closed columns), respectively. Dexametasona (DEXA, 2 mg/kg) was the positive control.
Conclusion
The essential oils from S. cumini and P. guajava were confirmed as useful to inhibit inflammatory diseases, by mechanisms that include the inhibition of eosinophil and, to a lesser extent, neutrophil and mononuclear cell migration. The presence of limonene, ocimenes, α-pinene and caryophyllene-type sesquiterpenes contributed decisively to the anti-inflammatory activity of SC oil. Both essential oils may be potentially useful for the treatment of inflammation associated with microorganisms, especially bacterial infections. Moreover, α-pinene may synergize with essential oils containing mono- and sesquiterpenes.
Declaration of interest
Authors declare no conflict of interest.
References
- Adams, R. (2007). Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Illinois: Allured Publ Co
- Fernandes ES, Passos GF, Medeiros R, et al. (2007). Anti-inflammatory effects of compounds α-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur J Pharmacol 569:228–36
- Gil ML, Jimenez J, Ocete MA, et al. (1989). Comparative study of different essential oils of Bupleurum gibraltaricum Lamarck. Pharmazie 44:284–7
- Golshani S, Karamkhani F, Monsef-Esfehani HR, Abdollabi M. (2004). Antinociceptive effects of the essential oil of Dracocephalum kotschyi in the mouse writhing test. J Pharm Sci 7:76–9
- Harris R. (2002). Synergism in the essential oil world. Int J Aromather 12:179–86
- Hart PH, Brand C, Carson CF, et al. (2000). Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm Res 49:619–26
- Henriques MGMO, Weg VB, Martins MA, et al. (1990). Differential inhibition by two hetrazepne PAF antagonists of acute inflammation in the mouse. Br J Pharmacol 99:164–8
- Kavimani S, Karpagam RI, Jaykar B. (1997). Anti-inflammatory activity of volatile oil of Psidium guajava. Indian J Pharm Sci 59:142–4
- Lenfeld J, Motl O, Trka A. (1986). Anti-inflammatory activity of extracts from Conyza canadensis. Pharmazie 41:268–89
- Lorente I, Ocete MA, Zarzuelo A, et al. (1989). Bioactivity of the essential oil of Bupleurum fruticosum. J Nat Prod 52:267–72
- Maridass M. (2008). Composition and anti-inflammatory activity of the essential oil of Eugenia discifera (Myrtaceae). Int J Essent Oil Ther 2:163–6
- Martin S, Ocete MA, Galvez J, et al. (1993). Anti-inflammatory activity of the essential oil of Bupleurum fruticescens. Planta Med 59:533–6
- Ocete MA, Risco S, Zarzuelo A, Jimenez J. (1989). Pharmacological activity of the essential oil of Bupleurum gibraltaricum: Anti-inflammatory activity and effects on isolated uteri. J Ethnopharmacol 25:305–3
- Passos GF, Fernandes ES, Cunha FM, et al. (2007). Anti-inflammatory and anti-allergic properties of the essential oil and active compounds from Cordia verbenacea. J Ethnopharmacol 110:323–33
- Perrucci S, Macchioni G, Cioni PL, et al. (1995). Structure/activity relationship of some natural monoterpenes as acaricides against Psoroptes cuniculi. J Nat Prod 58:1261–4
- Ramos MFS, Siani AC, Souza MC, et al. (2006). Avaliação da atividade antiinflamatória dos óleos essenciais de cinco espécies de Myrtaceae. Revista Fitos 2:58–66
- Santos FA, Rao VSN, Silveira ER. (1998). Investigation on the antinociceptive effect of Psidium guajava leaf essential oil and its major constituents. Phytother Res 12:24–7
- Siani AC, Ramos MFS, Menezes-De-Lima Jr O, et al. (1999). Evaluation of anti-inflammatory-related activity of essential oils from the leaves and resin of species of Protium. J Ethnopharmacol 66:57–69
- Souza MC, Siani AC, Ramos MFS, et al. (2003). Evaluation of anti-inflammatory activity of essential oils from two Asteraceae species. Pharmazie 58:582–6
- Spector WG. (1956). The mediation of altered capillary permeability in acute inflammation. J Pathol Bacteriol 72:367–80
- Tung Y-T, Chua M-T, Wang S-Y, Chang S-T. (2008). Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour Technol 99:3908–13
- Tung YT, Yen PL, Lin CY, Chang ST. (2010). Anti-inflammatory activities of essential oils and their constituents from different provenances of indigenous cinnamon (Cinnamomum osmophloeum) leaves. Pharm Biol 48:1130–6
- Veiga Jr VF, Zunino L, Calixto JB, et al. (2001). Phytochemical and antioedematogenic studies of commercial copaíba oils available in Brazil. Phytother Res 15:476–80
- Wasicky R. (1963). Uma modificação do aparelho de Clevenger para extração de óleo essencial. Rev. Farm Bioquím Univ S. Paulo 1:77–81
- Woollard AC, Tatham KC, Barker S. (2007). The influence of essential oils on the process of wound healing: A review of the current evidence. J Wound Care 16:255–7
- Zhou J-Y, Tang F-D, Mao G-G, Bian R-L. (2004). Effect of α-pinene on nuclear translocation of NF-κB in THP-1 cells. Acta Pharmacol Sin 25:480–4