Abstract
Context. Huperzia saururus (Lam.) Trevis. (Lycopodiaceae), an autochthonous plant species in Argentina, is used as a memory improver in traditional medicine. It was studied for this reason and the purified alkaloid extract did show significant activity on learning and memory. The species is mostly consumed in the form of infusions and decoctions.
Objectives: To evaluate the activity of the H. saururus infusion and decoction as inhibitors of acetylcholinesterase (AChE) and to determine the amino acid content in both extracts.
Material and methods: Infusion and decoction were purified by ionic exchange chromatography and analyzed by high-performance liquid chromatography HPLC-UV, and the AChE inhibition of these extracts was evaluated by using the Ellman method.
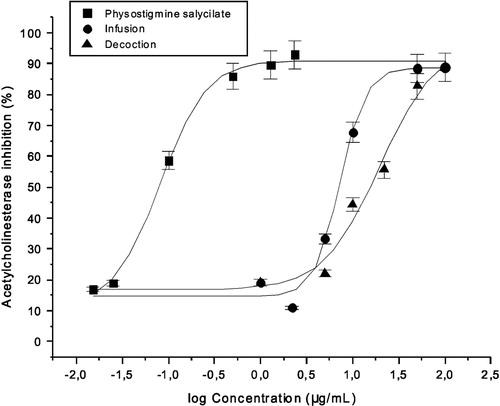
Results: Both infusion and decoction exerted strong AChE inhibitory activities (IC50 = 7.2 ± 0.4 and 22.7 ± 5.6 μg/mL, respectively). Among nine amino acids, arginine (Arg) was identified in a concentration greater than 9 mg/100 g of dried aerial parts in both extracts.
Discussion and conclusion: This high content of Arg could be considered a contributing factor to the activity on memory previously demonstrated for the H. saururus alkaloid extract, since Arg is implicated indirectly in mnemonic processes as a precursor in nitric oxide synthesis. Thus, the central effect of H. saururus could involve two different mechanisms, the cholinergic mechanism and the nitric oxide pathway.
Introduction
Huperzia saururus (Lam.) Trevis. (Lycopodiaceae) is an autochthonous species in Argentina where its common name is “cola de quirquincho”. Ethnomedicine attributes this species with aphrodisiac and memory-improving properties (Amorín, Citation1974; Martínez Crovetto, Citation1981). For this reason, H. saururus was chemically studied and 10 Lycopodium alkaloids were identified, sauroine being the major alkaloid (Ortega et al., Citation2004a, Citation2004b; Vallejo, Citation2009). At the same time, inhibition of acetylcholinesterase (AChE) was studied. The H. saururus alkaloid extract was shown to be active with an IC50 = 0.58 µg/mL (Ortega et al., Citation2004a).
In vitro as well as in vivo research work was done with the alkaloid extract and also with some purified alkaloids. These studies showed that they have an important facilitating effect on learning and memory processes. To this date, sauroine is the most active alkaloid isolated from this species (Ortega et al., Citation2006; Vallejo, Citation2009; Vallejo et al., Citation2007, Citation2009).
Ethnomedicine points out that infusions and decoctions are the common means of consuming H. saururus. Therefore, in this type of aqueous extracts, alkaloids as well as other metabolites can be extracted, among them amino acids that, as a consequence of their ionic nature, are highly soluble in water. It is important to point out that several studies indicate that Arg is related to processes of memory and learning since it is a precursor molecule in nitric oxide (NO) synthesis (Bredt & Snyder, Citation1989).
Given all these antecedents, the aim of this study was to determine the amino acid content in an infusion and in a decoction, in search for the presence of amino acids, especially Arg, as well as to determine the AChE inhibition of both infusion and decoction extracts, because this is the most popular use in ethnomedicine.
Material and methods
Plant material
Aerial parts of H. saururus were collected in Pampa de Achala, Department of San Alberto, Province of Córdoba, Argentina, in February 2008. They were identified by Dr. Gloria Barboza, Multidisciplinary Institute of Plant Biology (IMBIV), National University of Córdoba. A voucher specimen was deposited at the herbarium of the Museo Botánico de Córdoba (CORD) as CORD 684.
Amino acids extraction
Aerial parts of H. saururus were dried and ground before use. In accordance with the way this species is consumed in popular medicine, two methods were used for extraction, infusion and decoction. For infusion, 25 g of plant material were extracted by pouring 250 mL of boiling water and putting the lid on. After 5 min the infusion was filtered and concentrated under reduced pressure until acquiring a volume of approximately 100 mL.
For the decoction, another 25 g of plant material were extracted, this time by adding 250 mL of water and bringing to boil during 10 min. After that, the decoction was filtered and concentrated to approximately 100 mL. Both methods were developed according to Farmacopea Nacional Argentina (1978).
Materials and reagents
The materials and reagents used in this study were as follows: the amino acids used were Mann Research Laboratories (New York, NY), resin Amberlite IR-120 (BDH, Philadelphia, PA), TLC plates (Macherey-Nagel, Düren, Germany), acetonitrile (Sintorgan S.A., Buenos Aires, Argentina). Physostigmine salicylate was supplied by Merck (Darmstadt, Germany), and phenyl isothiocyanate, 5,5′-dithiobis-2-nitrobenzoic acid and acethylthiocholine iodide were purchased from Sigma-Aldrich Co (St. Louis, MO); triethylamine and nynhidrine from Anedra (San Fernando, Argentina). Other reagents used were of analytical grade.
Amino acids purification
Since the species contains a high concentration of polysaccharides, these substances were eliminated by adding ethanol in an equal volume to the aqueous extracts, then centrifugating at 2500 g, and finally eliminating the ethanol under reduced pressure.
Infusion and decoction free of polysaccharides were subjected to ion-exchange chromatography using Amberlite IR-120, standard grade (25 g) and a 50 × 1.5 cm2 column. Before purification, the resin was stabilized with a 0.01 M hydrochloric acid solution. The infusion (1.4 g) and 1.3 g of decoction were dissolved in 2.5 mL of 0.01 M hydrochloric acid and then subjected to column chromatography packed with the previously mentioned resin, adjusting the flow to 60 drops/min. For elution, 50 mL of 0.01 M hydrochloric acid were used. After the first fraction was collected, 30 mL of Mili-Q water were used to obtain a second fraction of each. Finally, 120 mL of 2 M ammonium hydroxide solution were used as eluent, and a third fraction of infusion and decoction was obtained, respectively. All the fractions were monitored by TLC (silica gel 60 F254) with phenol/water (75:25) as mobile phase, sprayed with 0.3% nynhidrine in ethanol and heated at 100 °C, during 5 min for visualization. Fraction 3 was positive to the detection reagent for amino acids in both infusion and decoction.
Standards preparation
Standards and amino acid fractions were derivatized with phenyl isothiocyanate (Gheshlaghi et al., Citation2008). Twelve aqueous solutions were prepared with the following amino acids: alanine (Ala), arginine (Arg), asparagine (Asn), aspartic acid (Asp), cysteine (Cys), glutamic acid (Glu), glutamine (Gln), glycine (Gly), proline (Pro), serine (Ser), threonine (Thr) and tryptophan (Trp). All of them were of different concentration in such a way that an aliquot of 10 µL of each solution corresponded to 3 µmol of each amino acid. After concentration to dryness, 30 µL of ethanol:water:triethylamine (2:2:1) were added to each aliquot and then concentrated at reduced pressure to dryness. By using a vortex, 60 µL of ethanol:water:triethylamine:phenyl isothiocyanate (7:1:1:1) were added in order to derivatize the amino acids. The reaction took place at room temperature during 20 min and it was finished at 50 °C by evaporation at reduced pressure. Then, 200 µL of acetonitrile were added to the dried product, concentrated to dryness to eliminate the remaining phenyl isothiocyanate from the reaction media. The procedure was repeated twice.
Samples preparation
The same procedure was employed with infusion and decoction samples but using 4.5 and 3.6 mg of fraction 3, respectively. The aliquot of ethanol:water:triethylamine (2:2:1) was 175 µL and, for derivatization, 200 µL of ethanol:water:triethylamine:phenyl isothiocyanate (7:1:1:1) were used. The time and volume of the acetonitrile added were the same as in the preparation of standards mentioned above.
HPLC analysis
HPLC analysis was carried out on a Varian model instrument (Varian, Walnut Creek, CA), with manual injector, gradient bomb system and multiple wavelength detector. Work conditions were λ = 254 nm, and T = 35 °C. A Hypersil ODS (5 µm, 4.6 mm × 250 mm2) C18 column from Phenomenex (Torrance, CA) was used. Standard amino acids and phenyl isothiocyanate were purchased from Sigma-Aldrich Co. (St. Louis, MO). HPLC-grade solvents were from Sintorgan S.A. (Buenos Aires, Argentina) and all the other solvents were of analytical grade. Two different solvent systems were used for the amino acids identification (). The pH of Phase A was adjusted with glacial acetic acid.
Table 1. Characteristics of solvent systems.
HPLC analysis of derivatized fractions and derivatized forms of the standards was carried out according to Gheshlaghi et al. (Citation2008). Standards and amino acids fractions were solubilized in 500 µL of 70% mobile phase A and 30% of mobile phase B and filtered through a 0.22 μm Millipore membrane (Indústria e Comercio Ltda, Sao Paulo, Brasil). Injection volume was 20 µL.
Method validation, arginine quantitation and statistical analysis
Following the International Conference on Harmonization Guidelines (International Conference on Harmonization, Citation2005a,Citationb) the subsequent parameters were evaluated. Calibration curve was created with seven solutions of Arg, each one in triplicate and in a concentration range of 0.1–1.00 µM. Linearity was determined by calculating the regression plots by the linear regression method and expressed as determination coefficient (R2). Precision was determined by the evaluation of intra and inter-day precision. Three concentrations, each one in triplicate, were selected from the calibration curve and analyzed during two different days to evaluate repeatability. This parameter gives the smallest value of precision because the results were obtained by the same operator, with the same equipment and within short intervals of time. Precision was expressed as relative standard deviation. Accuracy: three triplicate concentrations (0.2, 0.3 and 0.4 µM) were selected from the calibration curve and the value was expressed as average of recovery. Limits of detection and quantitation were determined from the y-intercept standard deviation (Sb) and the slope (a) of the calibration curve (de Sousa et al., Citation2008).
Chromatograms were analyzed by Star Chromatography Workstation 6.0, validation results by Origin 6.0. To compare both of the concentration results of Arg quantitation in infusion and decoction, a t-test was calculated. A p value under 0.05 was considered statistically significant.
AChE inhibition assay
Erythrocyte membranes were used as an enzyme source and were obtained as previously described (Ortega et al., Citation2004a). The AChE assay was performed using the colorimetric method of Ellman et al. (Citation1961) with some modifications incorporated by our group (Ortega et al., Citation2004a). Briefly, the following procedure was employed to calculate the activity: 750 μL of phosphate buffer (50 mM, pH 7.2) containing 0.25 mM of 5,5′-dithiobis-2-nitrobenzoic acid (buffer + DTNB), 5 µL enzyme preparation, 25 µL of acetylthiocholine iodide 5 mM and 100 µL of distilled water were mixed and incubated for 30 min. Then, each 30 s during 3 min and at 25 °C, the absorbance was measured at 405 nm. All experiments were repeated three times. Different concentrations of infusion and decoction (range of 1–100 µg/mL) were required for 50% enzyme inhibition (IC50). The IC50 values were estimated from a non-linear fitting of the concentration--response data, using the Origin 5.0 software (Northampton, MA) on a PC compatible computer. Physostigmine salicylate was employed as reference inhibitor for the comparison of the anticholinesterase activity (Ortega et al., Citation2004a).
Results
Amino acids identification
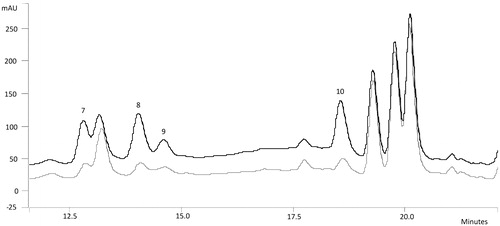
By means of solvent system 1, a good resolution for the majority of amino acids was obtained () with the exception of Asn, Ser, Gly and Gln. Therefore, solvent system 2 was used in order to obtain a better resolution for these two pairs of amino acids. An enlargement of the interest zone can be seen in . To achieve an unequivocal identification, the derivatized form of standard amino acids were added to derivatized fraction 3 of infusion and decoction, respectively. Chromatograms of the derivatized fraction 3 of infusion was overlapped with one of the same derivatized fraction plus the derivatized form of the standard amino acids, in order to determine which signal areas were increased ( and ). The same procedure was applied to derivatized fraction 3 of decoction.
Figure 1. Derivatized fraction 3 of infusion and standard amino acids + fraction 3 overlapped chromatograms by using SS1. 1. Asp (RT = 7.13), 2. Glu (RT = 7.85), 3. Thr (RT = 14.20), 4. Ala (RT = 15.26), 5. Pro (RT = 15.79), 6. Arg (RT = 16.77), A = Ser + Asn, B = Gly + Gln.
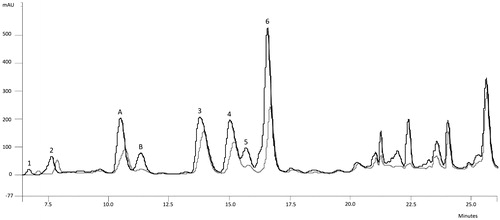
Figure 2. Derivatized fraction 3 of infusion and standard amino acids + fraction 3 overlapped chromatograms by using SS2 enlargement. 7. Ser (RT = 12.78), 8. Gln (RT = 14.00), 9. Gly (RT = 14.55), 10. Asn (RT = 18.55).
As a consequence, 10 amino acids were separated and identified in both infusion and decoction, alanine (Ala), arginine (Arg), asparagine (Asn), aspartic acid (Asp), glutamic acid (Glu), glutamine (Gln), glycine (Gly), proline (Pro), serine (Ser) and threonine (Thr).
Validation of arginine quantitation
A comprehensive validation of the present method was conducted. The results indicated that a good linearity with a correlation coefficient of R2 = 0.999 (n = 7) was achieved for Arg, with linearity range = 0.1–1.0 µM.
Precision was evaluated and relative standard deviation values were within 1.07–3.09% and 1.41–2.63%, for intra-day and inter-day precision, respectively. Relative standard deviation was lower than 5%, so the reproducibility was acceptable according to the working conditions [low concentrations used (Quattrocchi et al., Citation1992) ].
Table 2. Accuracy and precision data of quantitation.
The average recovery rate was 99.38% (t-test: tobs = 1.29 < ttab = 2.30, no significant differences between average recovery and 100% were observed, ). According to the results obtained, the method was accurate. The detection and quantitation limits of Arg were 0.04 µM and 0.09 µM, respectively, the latter near the calibration curve inferior point, but below the minimal concentration used within linearity range. This study demonstrated the suitability of this method for arginine detection with precision and accuracy as well as for its quantitation.
Arginine quantitation in infusion and decoction
After validation, Arg concentration was determined. It was present in a concentration of 9.71 ± 0.20 mg for infusion and 9.26 ± 0.21 mg/100 g of plant material for decoction. Statistical evaluation was performed using Student’s t-test, to compare the results obtained in infusion and decoction; at 95% confidence level (p = 0.0947; p > 0.05) no significant difference between the Arg content in infusion and decoction was observed.
Discussion
The Lycopodiaceae family has been extensively studied in relation to the alkaloid content. Some secondary metabolites other than alkaloids have also been studied (Ya-Bing et al., Citation2008). However, little is known in relation to primary metabolites (Sakai et al., Citation1992).
Related to H. saururus, the methodology used allowed the identification of 10 amino acids for the first time in the species, and it is important to point out that there are no previous reports in relation to amino acids neither in the genus, nor in the family.
The results obtained showed a high concentration of Arg, this primary metabolite is a precursor molecule in NO synthesis. This, in turn, has different physiological functions, such as its role as retrograde messenger in the synapses of some neuronal cells. Unlike most other neurotransmitters that only transmit information from presynaptic to postsynaptic neurons, the small and fat soluble NO molecule can diffuse widely and readily enter cells. NO is involved in learning and memory through the maintenance step of the hippocampal long-term potentiation (LTP; Mysliveček et al., Citation1996; Taqatqeh et al., Citation2009). The LTP phenomenon is considered the paradigm that underlies certain types of memory and learning processes (Bliss & Collingridge, Citation1993).
Comprehensive knowledge about the chemistry of a medicinal plant species contributes to a better understanding of its pharmacological effects. In some cases, the effect of a species extract that is consumed in popular medicine can be attributed to Known active principles, but the extract is a complex matrix where the actions of the different components can be due to the sum of each individual activities, or to have a synergic effect, or to be antagonistic (Phillipson, Citation2001).
Taking into consideration that extracts are complex mixtures of compounds, the high concentration of Arg found in the infusion as well as in the decoction, the important inhibitory activity of both aqueous extracts demonstrated in this work show that in the way that the species is consumed in popular medicine there is a correlation between the present results and those stated by ethnomedicine in relation to a memory improvement (Martínez Crovetto, Citation1981). Besides, these results are consistent with those previously reported (Ortega et al., Citation2004a) in relation to the inhibitory activity shown by the purified alkaloid extract of H. saururus. Thus, Arg could be considered as a contributing factor to the memory-enhancing effect previously demonstrated for H. saururus (Ortega et al., Citation2006; Vallejo et al., Citation2007, Citation2009). At the same time, the present work contributes to a more comprehensive chemical and biological study of the species.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors are grateful to Dr. G. Barboza for the species identification, Dr. A. Kaplan and Dr. A. A. Kamensky (Moscow University) for arginine information, Dr. William Darbyshire for the revision of the article.
The authors are grateful to SeCyT-UNC 05/C375, and FONCyT (ANPCyT) BID–PICT 2398, and CONICET D32/10 for the grants.
References
- Amorín JL. (1974). “Cola de Quirquincho” Urostachis saururus (Lam.) Herter (Lycopodiaceas): Una peligrosa planta usada en la medicina popular Argentina. Farmacobotánica 116:3–6
- Bliss T, Collingridge G. (1993). A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361:3–9
- Bredt DS, Snyder SH. (1989). Nitric oxide: A physiologic messenger molecule. Proc Natl Acad Sci USA 86:9030–3
- de Sousa JP, da Silva Filho AA, Bueno PC, et al. (2008). A validated reverse-phase HPLC analytical method for the quantitation of phenolic compounds in Baccharis dracunculifolia. Phytochem Anal 20:24–32
- Ellman GL, Courtney KD, Andres JrV, Featherstone RM. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
- Farmacopea Nacional Argentina VI Edición (1978). Ministerio de Salud. Buenos Aires: Imprenta del Congreso de la Nación
- Gheshlaghi R, Scharer JM, Moo-Young M, Douglas PL. (2008). Application of statistical design for the optimization of amino acid separation by reverse-phase HPLC. Anal Biochem 383:93–102
- International Conference on Harmonization. (2005a). ICH Q2A: Text on validation of analytical procedures. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073381.pdf [last accessed October 2011]
- International Conference on Harmonization. (2005b). ICH Q2B: Validation of analytical procedures: methodology. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073384.pdf [last accessed October 2011]
- Martínez Crovetto R. (1981). Las plantas utilizadas en medicina popular. Miscelánea 69:15
- Mysliveček J, Hassmannová J, Barcal J, et al. (1996). Inhibitory learning and memory in newborn rats influenced by nitric oxide. Neuroscience 71:299–312
- Ortega MG, Agnese AM, Cabrera JL. (2004a). Anticholinesterase activity in an alkaloid extract of Huperzia saururus. Phytomedicine 11:539–43
- Ortega MG, Agnese AM, Cabrera JL. (2004b). Sauroine-a novel Lycopodium alkaloid from Huperzia saururus. Tetrahedron Lett 45:7003–5
- Ortega MG, Vallejo MG, Cabrera JL, et al. (2006). Huperzia saururus, activity on synaptic transmission in the hippocampus. J Ethnopharmacol 104:374–8
- Phillipson JD. (2001). Phytochemistry and medicinal plants. Phytochemistry 56:237–43
- Quattrocchi OA, Abelaira de Andrizzi SI, Laba RF. (1992). Introducción a la HPLC. Buenos Aires, Argentina: Artes Gráficas Farro SA
- Sakai H, Kamide K, Sue S, et al. (1992). Amino acid sequence study of ferredoxin from a club moss. Lycopodium clavatum L. J Plant Res 105:71–82
- Taqatqeh F, Mergia E, Neitz A, et al. (2009). More than a retrograde messenger: Nitric oxide needs two cGMP pathways to induce hippocampal long-term potentiation. J Neurosci 29:9344–50
- Vallejo MG. (2009). Alcaloides en Huperzia saururs: Actividad sobre memoria y aprendizaje [Ph. D. thesis]. National University of Córdoba
- Vallejo MG, Ortega MG, Cabrera JL, et al. (2007). Huperzia saururus increases memory retention in rats. J Ethnopharmacol 111:685–7
- Vallejo MG, Ortega MG, Cabrera JL, et al. (2009). Sauroine, an alkaloid from Huperzia saururus with activity in Wistar rats in electrophysiological and behavioral assays related to memory retention. J Nat Prod 72:156–8
- Ya-Bing Y, Xue-Qiong Y, Yan-Qun X, et al. (2008). A new flavone glycoside from Huperzia serrata. Chin J Nat Med 6:408–10