Abstract
Context. Cenostigma macrophyllum Tul. var. acuminata Teles Freire (Leguminosae- Caesalpinioideae) is popularly known as “caneleiro”. Previous studies showed antioxidant action and analgesic effects of the ethanol extract from the leaves of C. macrophyllum. The phytochemical evaluation of the stem bark revealed the presence of antinociceptive compounds.
Objective: To investigate the antinociceptive actions of the ethanol extract and ethyl acetate fraction from C. macrophyllum stem bark in streptozotocin (STZ)-induced diabetic rats and the involvement of opioid and nitrergic mechanisms.
Materials and methods: STZ-rats received the ethanol extract (E.EtOH 200 and 300 mg/kg, p.o.) during 5 weeks. In acute experiments, untreated diabetic rats were treated with the ethyl acetate fraction (F.EtOAc 250 and 500 mg/kg, p.o.), on the 28th day of diabetes induction when the opioid and nitrergic mechanisms were investigated. The mechanical nociceptive threshold (MNT) was determined by application of von Frey filaments.
Results: Data show that STZ-induced diabetic rats developed a significant tactile allodynia during 5 weeks. Diabetic rats that received E.EtOH (200 and 300 mg/kg) and F.EtOAc (250 and 500 mg/kg) had a pain threshold higher than those in the STZ-vehicle group. F.EtOAc effects were inhibited by pretreatment with naloxone and were not influenced by .-arginine.
Discussion and conclusion: The results suggest that the ethanol extract and ethyl acetate fraction of C. macrophyllum presented antinociceptive activity. Thus, F.EtOAc may be exerting its effect by affecting the opioid system, but nitrergic mechanisms are not detectable. The observed activity may be due to its gallic acid, lupeol and bergenin content.
Introduction
Diabetes mellitus is a complex of metabolic disorders associated with insufficiency of insulin secretion, insulin action, or both, and is manifested by hyperglycemia (American Diabetes Association (ADA), Citation2002) and elevation of glycated proteins, glucose auto-oxidation and oxidative stress (Lapolla et al., Citation2005). Chronic hyperglycemia is related to the long-term complications of diabetic patients, including retinopathy, peripheral vascular disease, renal failure, autonomic neuropathy, and cardiovascular disorders that cause both morbidity and premature mortality (The Expert Committee, Citation2002; UK Prospective Diabetes Study (UKPDS) Group, Citation1998).
The administration of streptozotocin is the most common animal model for the study of acute and chronic diabetic complications, including neuropathy (Rees & Alcolado, Citation2005). In this model, neuropathy is characterized by slowed nerve conduction velocity, thermal hypoalgesia, and axonal degeneration on morphometry (Apfel, Citation2006).
Several studies have examined the mechanisms underlying hyperglycemia-induced nerve damage in order to find a possible therapy. In this context, painful diabetic neuropathy (DN) is a recognized complication of diabetes with multifactorial pathogenesis. Thus, the distal peripheral neuropathy (DPN) involves many different mechanisms, including alterations in opioid and nitrergic systems (Akunne & Soliman, Citation1987; Levy & Zochodne, Citation2004). Nitric oxide (NO) has been implicated in the transmission and modulation of spinal afferent and pain processing (Budai, Citation2000). According to Levy and Zochodne (Citation2004) and Zochodne and Levy (Citation2005), NO plays a direct role in axonal injury in the periphery, and there are important relationships between NO, Wallerian degeneration, neuropathic pain, and axon regeneration. The associated actions of NO and AGEs induce irreversible nitrergic nerve degeneration in diabetes (Cellek et al., Citation2004). Considering the opioid system, previous studies showed that diabetes does not induce a reduction of the number of mu-opioid receptors in the spinal cord dorsal horn of diabetic rats (Chen et al., Citation2002; Chen & Pan, Citation2003). However, downregulation and/or desensitization of mu-opioid receptors in the dorsal horn of the spinal cord have been reported in DN (Chen et al., Citation2002; Chen & Pan, Citation2003). The mechanisms proposed for opioid resistance include increased production of protein kinase C and activation of N-methyl-.-aspartate (NMDA) receptors in postsynaptic cells (Mao et al., Citation1995; Rodríguez-Muñoz et al., Citation2012). The decreased inhibitory G protein function in DN is associated with protein kinase C (PKC) activation, as described by Shangguan et al. (Citation2003). These mechanisms are related to opioid resistance in diabetic-induced neuropathy (Nikura et al., Citation2010).
The study of medicinal plants is interesting as a future perspective of the DN treatment. Cenostigma macrophyllum Tul. var. acuminata Teles Freire, popularly named caneleiro, is a plant from the family Leguminosae, included in the subfamily Caesalpinioideae and tribe Caesalpiniaceae (Freire, Citation1994). The stem bark, flowers, and fruits are popularly used in the cases of gastric or intestinal diseases.
According to Silva et al. (Citation2007), the phytochemical investigation of the ethanol extract (E.EtOH) from the stem bark of C. macrophyllum var. acuminata resulted in the isolation and identification of several compounds, such as valoneic acid dilactone, ellagic acid, lupeol, alkyl ferulate, cholesterol, campesterol, stigmasterol, sitosterol and mixtures of 3-β-hydroxysterols and fatty acids. Previous studies showed antioxidant action (Sousa et al., Citation2007) and antinociceptive effects of the ethanol extract from the leaves of C. macrophyllum (Carvalho, Citation2009). According to Alves et al. (Citation2012), the phytochemical study of the F.EtOAc obtained by partition of the methanol extract from the stem bark of C. macrophyllum revealed the presence of antinociceptive compounds, such as gallic acid and bergenin.
In the present research, we investigated the antinociceptive actions of ethanol fraction (E.EtOH) and ethyl acetate fraction (F.EtOAc) from the stem bark of C. macrophyllum, as well as the involvement of opioid and nitrergic mechanisms in streptozotocin-induced diabetic rats.
Materials and methods
Chemicals
Streptozotocin (STZ), N-nitro-.-arginine, .-arginine and naloxone (Sigma, Northbrook, IL), sodium citrate (Vetec, Rio de Janeiro, Brazil), citric acid (Dinamica, Fazenda, Brazil), insulin Novolin (Novo Nordisk, Montes Claros, Brazil), sodium thiopental (Cristalia, Itapira, Brazil), glucose oxidase kit (Labtest, Lagoa Santa, Brazil) were used in this work. All solutions were prepared with substances immediately before each experiment, using as a vehicle saline (NaCl 0.9%) solution or distilled water. The extract and fraction concentrations were adjusted for treatment to yield a volume of 10 mL/kg with vehicle solution containing distilled water and dimethyl sulfoxide (DMSO 1%, v/v).
Preparation of extracts
Stem bark of C. macrophyllum var. acuminata was collected in the surroundings of the Federal University of Piaui, Teresina, Brazil, in July 2010. The species is found as an ornamental tree in urban landscaping and is considered the “symbol tree” of the city of Teresina in Brazil (Lorenzi, Citation1998). The species is also found as a native species of the cerrado vegetation in northeast of Brazil (Warwick & Lewis, Citation2009). A voucher specimen (Freire, Citation1994) was deposited in the Graziela Barroso Herbarium of the Federal University of Piaui, Brazil (TEPB 10.374).
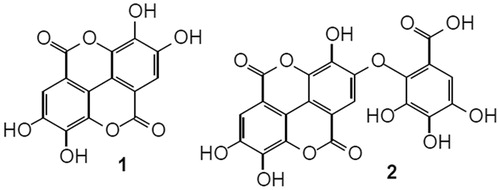
The dried and powdered bark (1.5 kg) was extracted with ethanol 99.5% at room temperature (Silva et al., Citation2007). The solvent was removed by evaporation under reduced pressure using a Hedolph Rotary Evaporator to yield the ethanol extract (E.EtOH, 202.6 g, 13.5%). The ethanol extract was suspended in methanol/water (1:2) and successively extracted with diethyl ether and ethyl acetate, yielding the aqueous fraction (F.H2O, 78.8 g, 38.9%), ether fraction (F.Et2O, 29.3, 14.5%), and ethyl acetate fraction (F.EtOAc, 64.4 g, 31.8%). The chemical study of the ethanol extract was already performed by Silva et al. (Citation2007). Analysis by thin layer chromatography of F.EtOAc using authentic standard indicated the presence of ellagic acid (1) and valoneic acid dilactone (2) as shown in . These substances had been found in the ether fraction by Silva et al. (Citation2007). Based on the phytochemical profile and preliminary experiments, the E.EtOH and F.EtOAc were chosen for this study.
Animals and diabetes induction
Male Wistar rats (260–290 g) from Animal House of the Federal University of Piaui (Teresina, Brazil) were housed at 22 ± 2° under a 12 h light:12 h dark cycle with access to a standard commercial diet and water ad libitum. The experiments were performed after the approval of the protocols by the Institutional Ethics Committee and were carried out in accordance with the current guidelines for the care of laboratory animals and the ethical guidelines for investigations of experimental pain in conscious animals (Zimmermann, Citation1983). The animals were fasted for at least 12 h and received streptozotocin (STZ, 40 mg/kg, i.v.) dissolved in 0.01 mol/L citrate buffer (pH 4.5) at a concentration of 20 mg/mL (Pepato et al., Citation2001). Glucose levels were determined spectrophotometrically using an assay kit (Labtest) based on the method of glucose oxidase. After 48 h of streptozotocin injection, a fasting serum glucose concentration above or equal to 250 mg/dL was considered to indicate diabetes mellitus.
Chronic treatment
After 48 h of diabetes induction, rats were divided into 5 groups of 8–10 animals each for daily treatment through a gastric catheter (gavage) until the 35th as follows: non-diabetic group (control) and diabetic group (STZ-vehicle) received vehicle (DMSO 1% in distilled water, p.o.), treated diabetic groups received ethanol extract (STZ-E.EtOH 200 and STZ-E.EtOH 300 mg/kg, p.o.) or insulin (STZ-insulin, 2.5 U s.c. per rat twice at 8:00 am and 6:00 pm).
E.EtOH doses were chosen based on lethal dose values and preliminary experiments in diabetic rats. All groups were used to assess tactile allodynia by measuring the mechanical nociceptive threshold (MNT) by means of von Frey filaments.
The MNT was tested before and 1, 2, 3, 4 and 5 weeks after STZ injection based on the method described previously (Chaplan et al., Citation1994). Rats were placed individually in clear boxes on elevated wire mesh platforms, to access to the ventral surface of the hindpaws. The withdrawal response was measured following applications of 0.1, 0.16, 0.4, 0.6, 1.0, 2.0, 4.0, 6.0, 8.0 and 10.0 g von Frey filaments (North Coast Medical, Gilroy, CA), perpendicularly to the lateral plantar surface. The force of application was increased until the animal withdrew its foot, and the force applied at the moment of foot withdrawal was taken as the threshold. The antinociceptive response was evaluated by the increase of the MNT. Thirty-five days after STZ injection, the animals were anesthetized with sodium thiopental 40 mg/kg intraperitoneally (Cherksey & Altszuler, Citation1974). Blood was collected from the superior vena cava for measuring the fasting serum glucose.
Acute treatment
Twenty-eight days after diabetes induction, non-diabetic rats (control group) and diabetic rats were submitted to von Frey filaments and animals with mechanical nociceptive threshold lower or equal to 2 g were included in the acute experiments.
In order to elucidate the involvement of opioid mechanism in the ethyl acetate fraction (F.EtOAc) action, non-diabetic and diabetic rats were separated into eight groups of 8–17 rats and treated as follows: control and STZ-vehicle (DMSO 1% in distilled water, p.o.), STZ- F.EtOAc 250 and STZ-F.EtOAc 500 received F.EtOAc 250 and 500 mg/kg, p.o., respectively, STZ-mor (morphine 5 mg/kg, i.p.), STZ-nalox (naloxone 5 mg/kg, i.p., an opioid receptor antagonist), (Bian et al., Citation1995), STZ-F.EtOAc-nalox (F.EtOAc 500 mg/k/g, p.o., and naloxone 5 mg/kg, i.p.), and STZ-mor-nalox (morphine 5 mg/kg and naloxone 5 mg/kg, i.p.). The assessment of MNT by means of von Frey filaments was determined in the times of 0, 60, 120 and 180 min after treatment.
In other experiments, the involvement of a nitrergic mechanism was investigated. The rats were divided into seven groups of 8–17 rats for treatment as follows: control and STZ-vehicle (DMSO 1% in distilled water, p.o.), STZ-F.EtOAc 500 (F.EtOAc 500 mg/kg, p.o.), STZ-.-arg (.-arginine 600 mg/kg, i.p.), (Pietrovski et al., Citation2006), STZ-.-NOARG (N-nitro-.-arginine 60 mg/kg, i.p., a NO synthase inhibitor), STZ-F.EtOAc-.-arg; F.EtOAc 500 mg/k/g p.o. and .-arginine 600 mg/kg, i.p.) and STZ-.-arg-.-NOARG (.-arginine 600 mg/kg and N-nitro-.-arginine 60 mg/kg, i.p.). The MNT was assessed in the times of 0, 60, 120 and 180 min after treatment. The antinociceptive response was evaluated by the increase of the MNT.
Statistical analyses
Statistical analysis was performed using the GraphPad Prism® 5.0 software (Graphpad Software Inc., San Diego, CA). Statistical significance of the results was determined using one way analysis of variance (ANOVA) followed by Tukey as the post hoc test and Student’s t-test for paired observations. Data were considered significant at p < 0.05.
Results
The data in show that serum glucose level and body weight did not differ between STZ-vehicle and E.EtOH-treated diabetic rats (STZ-E.EtOH 200 STZ-E.EtOH 300 mg/kg). Body weights in all diabetic rats were significantly lower than in the control at the end of experiment (p < 0.05). On the other hand, the daily administration of the insulin (5 U/day per rat) produced a significantly decrease in glycemia and an increase in body weight (p < 0.05).
Table 1. Serum glucose levels and body weight in control and streptozotocin-induced diabetic rats.
The DN symptoms that were observed by measuring the pain threshold induced by mechanical stimulation throughout the treatment period of the animals began in the first week after injection of STZ (p < 0.05), as shown in . From the second week, the reduction of the pain threshold in untreated diabetic rats was more drastic, and stabilized over 35 days.
Figure 2. Mechanical nociceptive threshold in control and diabetic rats during chronic treatment with E.EtOH of C. macrophyllum. Values represent mean and SEM. ap < 0.05 versus control, bp < 0.05 versus STZ-vehicle, and cp < 0.05 versus STZ-insulin.
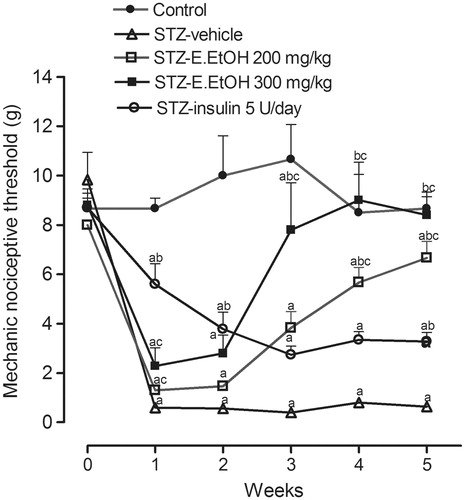
Diabetic rats that received chronic treatment with the E.EtOH 300 mg/kg had a pain threshold higher than those in the STZ-vehicle group during the third, fourth, and fifth weeks (p < 0.05). From the fourth week, STZ-E.EtOH 200 mg/kg presented an increase in the pain threshold when compared with STZ-vehicle, but lower than that of the control group. In this study, rats treated with insulin (STZ-insulin) showed MNT higher than those in the STZ-vehicle group during the first, second, and fifth weeks (p < 0.05), but the values were lower as compared with control rats during 5 weeks.
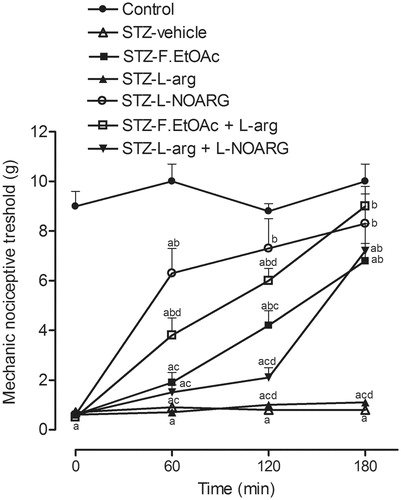
Moreover, in order to clarify the effects of E.EtOH of C. macrophyllum stem bark, acute experiments were carried out by treating diabetic rats with ethyl acetate fraction (F.EtOAc) obtained from E.EtOH partition at the 28th day after the STZ injection when the MNT decreased in diabetic rats. According to , diabetic rats treated with F.EtOAc 250 and F.EtOAc 500 mg/kg showed increased pain threshold when compared with STZ-vehicle. At 180 min, the group STZ-F.EtOAc 500 mg/kg presented values of MNT that were similar to STZ-mor group.
Table 2. Mechanical nociceptive threshold (g) of control and diabetic rats in acute treatment at the 28th day after diabetes induction.
The antinociceptive effect of F.EtOAc in diabetic rats was inhibited by pretreatment with naloxone () and was not influenced by .-arginine, which failed to reverse the analgesic effect caused by F.EtOAc (). On the other hand, in diabetic rats that received l-NOARG (STZ-.-NOARG), the pain threshold increased and was similar to controls in the times of 120 and 180 min after administration.
Discussion
The destruction of pancreatic β cells by STZ mimicks the insulin-dependent diabetic status (Pepato et al., Citation2001) which was characterized by hyperglycemia and a significant decrease in body weight. On the other hand, the daily administration of insulin decreased serum glycemia and reduced the loss of body weight.
DN has been considered the most common and earliest complication of long-term hyperglycemia (Apfel, Citation2006). DPN is related to many different mechanisms (Dobretsov et al., Citation2007; Ueda, Citation2006). This study evaluated the effects of the E.EtOH obtained from C. macrophyllum stem bark on DN characterized by greater sensitivity to pain.
The detection of tactile allodynia in the first week after injection of STZ is consistent with described data in the literature, which reports the onset of mechanical hyperalgesia in animals with a 20–40% of pain threshold reduction (Dobretsov et al., Citation2003). The mechanical nociceptive threshold is one of the most consistent signs of peripheral neuropathy in diabetic animals (Ahlgren & Levine, Citation1993; Dobretsov et al., Citation2003). Some authors have reported that hypersensitivity to mechanical stimulation is detectable 1 week after induction of DM and fully develops between 2 and 8 weeks of DM (Chen & Pan, Citation2002; Fox et al., Citation1999), being influenced by direct and indirect effects of hyperglycemia (Courteix et al., Citation1993; Sasaki et al., Citation1998; Zhang et al., Citation1999). The elevation in the pain threshold during treatment with E.EtOH 200 and 300 mg/kg in the 4th week is suggestive of antinociceptive effects of the E.EtOH in this model of DN. Besides, evidence is accruing that insulin deficiency rather than hyperglycemia contributes to the development of DN. In fact, rats treated with insulin had an increase in the MNT. In this context, several reports have showed trophic properties of insulin in the prevention of DN. Thus, for short-term diabetes, the effects of insulin therapy on regulation of axon-glia relationships, vascular permeability, and function of nociceptive primary afferent neurons may protect against early DN (Dobretsov et al., Citation2007; Sugimoto et al., Citation2000, Citation2002). However, for long-term diabetes, there is evidence for insulin-induced resistance to its trophic actions (Singh et al., Citation2012).
Animals models of diabetes are thought to be useful for understanding the effects of medicinal plants and their mechanisms. Thus, this study was designed to experimentally certify the antinociceptive effects of E.EtOH and F.EtOAc obtained from the stem barks of C. macrophyllum, as well as the involvement of opioid and nitrergic mechanisms. Animal studies have suggested an altered response to opiate agonists and antagonists, as well as an increased pain threshold in diabetic animals (Desmeules et al., Citation1993; Dewan et al., Citation2000). Although, diabetes and hyperglycemia affect the sensitivity of laboratory animals to various pharmacological agents (Kamei et al., Citation1995), in acute experiments, the MNT values increased in STZ-rats treated with F.EtOAc 250 and 500 mg/kg or morphine. These effects were reversed by pretreatment with naloxone. In fact, there is evidence of opioid receptor involvement in the painful DN by naloxone administration in diabetic rats (Lynch et al., Citation1999). Thus, the results suggest that opioid receptors may contribute to the action mechanism of F.EtOAc.
Moreover, it has been reported that .-arginine NO pathways are involved in several models of nociception (Haley et al., Citation1992; Snyder, Citation1992; Yaksh & Rudy, Citation1977). The antinociceptive effect of F.EtOAc in diabetic rats was not influenced by .-arginine which failed to reverse the analgesic effect caused by F.EtOAc. On the other hand, the pain threshold increased in diabetic rats that received .-NOARG. Although nitrergic mechanisms are involved in this model of pain neuropathic (Meller & Gebhart, Citation1993), the oxide nitric pathway does not seem to participate in F.EtOAc action mechanism.
The effects of E.EtOH and F.EtOAc can be related to the analgesic compounds already isolated from the stem bark of C. macrophyllum, such as, lupeol (Silva et al., 2007), gallic acid, and bergenin (Alves et al., Citation2012).
In conclusion, the results of the present study exhibited analgesic effects of the ethanol extract (E.EtOH) and ethyl acetate fraction (F.EtOAc) in STZ-diabetic rats. Data showed that naloxone was effective in blocking the anti-allodynic effects of the morphine and F.EtOAc in this model, whereas, nitrergic mechanisms were not detectable in F.EtOAc activity. Further investigation is necessary to evaluate the toxicological profile of E.EtOH and F.EtOAc, as well as the involvement of others mechanisms. Based on these findings, C. macrophyllum appears to have therapeutic potential in the treatment of painful DN.
Declaration of interest
This work was supported by grants from CAPES, CNPq, FAPEPI/FINEP/MCT.
References
- Ahlgren SC, Levine JD. (1993). Mechanical hyperalgesia in streptozotocin-induced diabetic rats. Neuroscience 52:1049–55
- Akunne HC, Soliman KFA. (1987). The role of opioid receptors in diabetes and hyperglycemia-induced changes in pain threshold in the rat. Psychopharmacology 93:167–72
- Alves CQ, David JM, David JP, et al. (2012). Flavonoids and other bioactive phenolics isolated from Cenostigma macrophyllum (Leguminosae). Quim Nova 35:1137–40
- American Diabetes Association. (2002). Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 25:S5–20
- Apfel SC. (2006). Diabetic neuropathy models: Are they relevant? Drug Discov Today: Dis Models 3:397–402.
- Bian D, Nichols ML, Ossipov MH, et al. (1995). Characterization of the antiallodynic efficacy of morphine in a model of neuropathic pain in rats. Neuroreport 6:1981–4
- Budai D. (2000). Neurotransmitters and receptors in the dorsal horn of the spinal cord. Acta Biol Szeged 44:21–38
- Carvalho FCB. (2009). Avaliação dos efeitos de Cenostigma macrophyllum na neuropatia diabetica [unpublished master’s thesis]. Available from: http://www.dominiopublico.gov.br/pesquisa/DetalheObraForm.do?select_action=&co_obra=159871 [last accessed 14 May 2012]
- Cellek S, Qu W, Schmidt AM, Moncada S. (2004). Synergistic action of advanced glycation end products and endogenous nitric oxide leads to neuronal apoptosis in vitro: A new insight into selective nitrergic neuropathy in diabetes. Diabetologia 47:331–9
- Chaplan SR, Bach FH, Pogrel JW, et al. (1994). Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Method 53:55–63
- Chen SR, Sweigart KL, Lakoski JM, Pan HL. (2002). Functional mu opioid receptors are reduced in the spinal cord dorsal horn of diabetic rats. Anesthesiology 97:1602–8
- Chen SR, Pan HL. (2002). Hypersensitivity of spinothalamic tract neurons associated with diabetic neuropathic pain in rats. J Neurophysiol 87:2726–33
- Chen SR, Pan HL. (2003). Antinociceptive effect of morphine, but not mu opioid receptor number, is attenuated in the spinal cord of diabetic rats. Anesthesiology 99:1409–14
- Cherksey BD, Altszuler N. (1974). On the mechanism of potentiation by morphine of thiopental sleeping time. Pharmacology 12:362–71
- Courteix C, Eschalier A, Lavarenne J. (1993). Streptozotocin-induced diabetic rats: Behavioural evidence for a model of chronic pain. Pain 53:81–8
- Desmeules JA, Kayser V, Guilbaud G. (1993). Selective opioid receptor agonists modulate mechanical allodynia in an animal model of neuropathic pain. Pain 53:277–85
- Dewan S, Sangraula H, Kumar VL. (2000). Preliminary studies on the analgesic activity of latex of Calotropris procera. J Ethnopharmacol 73:307–11
- Dobretsov M, Hastings SL, Romanovsky D, et al. (2003). Mechanical hyperalgesia in rats models of systemic and local hyperglycemia. Brain Res 960:174–83
- Dobretsov M, Romanovsky D, Stimers JR. (2007). Early diabetic neuropathy: Triggers and mechanisms. World J Gastroenterol 13:175–91
- Fox A, Eastwood C, Gentry C, et al. (1999). Critical evaluation of the streptozotocin model of painful diabetic neuropathy in the rat. Pain 81:307–16
- Freire FMT. (1994). Revisão taxonômica do gênero Cenostigma TUL (Leguminosae – Caesalpinioideae) para o Brasil [Unpublished Master’s thesis]. Available from: http://www.scielo.br/pdf/abb/v10n2/v10n2a15.pdf [last accessed 14 May 2012]
- Haley JE, Dickenson AH, Schachter M. (1992). Electrophysiological evidence for a role of nitric oxide in prolonged chemical nociception in rat. Neuropharmacology 31:251–8
- Kamei J, Suzuki T, Misawa M, et al. (1995). Antinociceptive effect of dihydroethorphine in diabetic mice. Eur J Pharmacol 275:109–13
- Lapolla A, Traldi P, Fedele D. (2005). Importance of measuring products of non-enzymatic glycation of proteins. Clin Biochem 38:103–15
- Levy D, Zochodne DW. (2004). No pain: Potential roles of nitric oxide in neuropathic pain. Pain Pract 4:11–18
- Lorenzi H. (1998). Leguminosae-Caesalpinioideae, Arvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil 2:143–44. Nova Od essa, Brazil: Editora Plantarum
- Lynch JJ, Michael FJ, Kowaluk EA. (1999). An adenosine kinase inhibitor attenuates tactile allodynia in a rat model of diabetic neuropathic pain. Eur J Pharmacol 364:141–6
- Mao J, Price DD, Mayer DJ. (1995). Experimental mononeuropathy reduces the antinociceptive effects of morphine: Implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain 61:353–64
- Meller ST, Gebhart GF. (1993). Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain 52:127–36
- Nikura K, Narita M, Butelman ER, et al. (2010). Neuropathic and chronic pain stimuli downregulate central mu-opioid and dopaminergic transmission. Trends Pharmacol Sci 31:299–305
- Pepato MT, Folgado VB, Kettelhut IC, Brunetti IL. (2001). Lack of antidiabetic effect of a Eugenia jambolana leaf decoction on rat streptozotocin diabetes. Braz J Med Bio Res 34:389–95
- Pietrovski EF, Rosa KA, Facundo VA, et al. (2006). Antinociceptive properties of the ethanolic extract and of the triterpene 3β,6β,16β-trihidroxilup-20(29)-ene obtained from the flowers of Combretum leprosum in mice. Pharmacol Biochem Behav 83:90–9
- Rees DA, Alcolado JC. (2005). Animal models of diabetes mellitus. Diabet Med 22:359–70
- Rodríguez-Muñoz M, Sánchez-Blazquez P, Vicente-Sánchez A, et al. (2012). The mu-opioid receptor and the NMDA receptor associate in PAG neurons: Implications in pain control. Neuropsychopharmacology 37:338–49
- Sasaki T, Yasuda H, Maeda K, Kikkawa R. (1998). Hyperalgesia and decreased neuronal nitric oxide synthase in diabetic rats. Neuroreport 9:243–7
- Shangguan Y, Hall KE, Neubig RR, Wiley JW. (2003). Diabetic neuropathy: Inhibitory G protein dysfunction involves PKC-dependent phosphorylation of Goα. J Neurochem 86:1006–14
- Silva HR, Silva CCM, Caland Neto LB, et al. (2007). Chemical constituents from bark of Cenostigma macrophyllum: Cholesterol occurrence. Quim Nova 30:1877–81
- Singh B, Xu Y, McLaughlin T, et al. (2012). Resistance to trophic neurite outgrowth of sensory neurons exposed to insulin. J Neurochem 121:263–76
- Snyder SH. (1992). Nitric oxide: First in a new class of neurotransmitters. Science 257:494–6
- Sousa CMM, Silva HR, Vieira-Jr GM, et al. (2007). Total phenolics and antioxidant activity of five medicinal plants. Quim Nova 30:351–5
- Sugimoto K, Murakawa Y, Sima AA. (2000). Diabetic neuropathy – A continuing enigma. Diabetes Metab Res Rev 16:408–33
- Sugimoto K, Murakawa Y, Sima AA. (2002). Expression and localization of insulin receptor in rat dorsal root ganglion and spinal cord. J Peripher Nerv Syst 7:44–53
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. (2002). Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 25:S5–20
- Ueda H. (2006). Molecular mechanisms of neuropathic pain–phenotypic switch and initiation mechanisms. Pharmacol Ther 109:57–77
- UK Prospective Diabetes Study (UKPDS) Group. (1998). Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–53
- Warwick C, Lewis GP. (2009). A revision of Cenostigma (Leguminosae-Caesalpinioideae-Caesalpinieae), a genus endemic to Brazil. Kew Bull 64:135–46
- Yaksh TL, Rudy TA. (1977). Studies on the direct spinal action of the narcotics in the production of analgesia in the rat. J Pharmacol Exp Ther 202:411–28
- Zhang JM, Song XJ, Lamotte RH. (1999). Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol 82:3359–66
- Zimmermann M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–10
- Zochodne DW, Levy D. (2005). Nitric oxide in damage, disease and repair of the peripheral nervous system. Cell Mol Biol 5:255–67