Abstract
Context. Campomanesia xanthocarpa Berg. (Myrtaceae), popularly known in Brazil as guabiroba, is a plant used as antidiarrheic, anti-inflammatory and antirheumatic agents, and in stomach and hepatic disorders.
Objective: The antiproliferative and genotoxic effects of aqueous extracts and essential oil of C. xanthocarpa were evaluated.
Materials and methods: Cytotoxicity and genotoxicity of the aqueous extracts (6 and 30 mg/mL) and essential oil (0.25%, v/v) obtained from leaves of C. xanthocarpa were evaluated using the Allium cepa L. (Amaryllidaceae) assay. Mitotic index was calculated as the percentage of dividing cells of total cells observed; chromosome abnormalities were observed and counted during cell division. Additionally, the composition of the essential oil and the quantification of the main compounds of the extracts were determined by gas chromatography/mass spectrometry and high performance liquid chromatography coupled with diode array detector, respectively.
Results and discussion: Aqueous extracts (6 and 30 mg/mL) led to a reduction of 67.7% and 34.1% of the mitotic index, respectively, whereas the treatment with essential oil caused a 48.2% reduction in the mitotic index, when compared with negative control. Chromosomal mutations were observed and included anaphase bridges, delay chromosome, break chromosome, as well as metaphase with disorganized chromosomal and binuclear cells. The main compounds of the essential oil were β-caryophyllene (8.87%), viridiflorol (6.40%), spathulenol (5.16%), δ-cadinene (4.92%), linalool (4.46%) and α-cadinol (4.25%). Gallic acid (3.19%), chlorogenic acid (1.04%), quercetin (2.97%) and rutin (4.82%) were identified in an aqueous extract (30 mg/mL).
Conclusion: Our results demonstrated that genotoxic and antiproliferative activities are present in C. xanthocarpa infusions using the in vivo onion root-tip cell test.
Introduction
Medicinal plants are widely used by the human population, especially in developing countries, where the lack of financial resources hinders access to the treatment of diseases by conventional medicine. However, the majority of the species used in folk medicine have not been sufficiently studied, especially when it comes to studies regarding mutagenic compounds.
The Allium cepa L. (Amaryllidaceae) vegetal system has been reported as a bio-indicator of genotoxic and proliferative capacity of medicinal species (Bagatini et al., Citation2009). Biomarkers can be defined as indicator systems that typically included subsystems of a complete organism used for the identification of a specific target (Silva et al., Citation2003). Besides being a good biological indicator, the method of evaluation of chromosomal changes in roots of A. cepa is validated by the International Program on Chemical Safety (IPCS, WHO) and United Nations Environment Program (UNEP) as an effective test for analysis and in situ monitoring of genotoxicity of environmental substances (Cabrera & Rodriguez, Citation1999). Moreover, correlation studies on the sensitivity of the A. cepa test and other systems are important for the evaluation of the environmental risk and the data obtained can be extrapolated to other organisms, including humans (Leme & Marin-Morales, Citation2009).
The A. cepa test is important since it is an excellent model in vivo, where the roots grow in direct contact with the substance of interest (i.e., effluent or complex medicinal mix being tested) enabling possible damage to the DNA of eukaryotes to be predicted. Therefore, the data can be extrapolated for all animal and plant biodiversity. The analysis of chromosomal alterations can be equal to the test of mutagenicity mainly for the detection of structural alterations; however, it is possible to observe numerical chromosomal alterations, as well. The A. cepa test is one of the few direct methods for measuring damage in systems that are exposed to mutagens or potential carcinogens, and enables the evaluation of the effects of this damage through the observation of chromosomal alterations (Tedesco & Launghinghouse, Citation2012).
Campomanesia xanthocarpa Berg. (Myrtaceae), commonly known as guabiroba, guabirobeira, guabiroba-miúda, or guabirobeira-do-mato, is an herb used in popular medicine. In Brazil, it is present in several states of the central and southern regions including Minas Gerais, São Paulo, Mato Grosso do Sul and Rio Grande do Sul, among others (Corrêa, Citation1984). Traditionally, infusions obtained from its leaves are used as an antidiarrheic, and to treat dysentery, stomach, and hepatic disorders, as well as anti-inflammatory and anti-rheumatic agent (Corrêa, Citation1984; Dickel et al., Citation2007). Moreover, the plant is popularly used for weight loss and in the control of a number of conditions associated with obesity (Dickel et al., Citation2007). According to Klafke et al. (Citation2010), the treatment with encapsulated C. xanthocarpa reduced blood total cholesterol and LDL levels in hypercholesterolemic patients. Furthermore, the fruits of Campomanesia spp. have great economic potential, either as in natural food or in the preparation of sweets, ice cream and home-made liqueurs (Vallilo et al., Citation2008).
Due to the importance of C. xanthocarpa and the extraction forms (infusion and essential oil) as well as the possibility of its use in a safe way, this work aimed, in a first approach, to evaluate the genotoxic effects and cellular proliferative activity of the aqueous extracts and essential oil obtained from the leaves of C. xanthocarpa. Additionally, the composition of the essential oil was determined by gas chromatography/mass spectrometry (GC/MS), whereas the polyphenolic profile and quantification of the main phenolic and flavonoid compounds was achieved by HPLC/DAD analyses.
Materials and methods
Material
Leaves of C. xanthocarpa were collected during the flowering stage (September 2009) from cultivated specimens in Santa Maria (RS), Brazil, and were identified by Dr Thais Scotti do Canto-Dorow. Voucher specimen numbers have been deposited in the SMDB Herbarium (number 12.422) of UFSM (Santa Maria, Brazil).
Glyphosate was purchased from Kelldrin Industrial Ltd (Goiânia, Brazil). Methanol, acetic acid, gallic acid and chlorogenic acid were purchased from Merck (Darmstadt, Germany). Quercetin and rutin were acquired from Sigma Chemical Co. (St. Louis, MO). All other chemicals and solvents presented pharmaceutical grade and were used as received. A. cepa bulbs were obtained from an organic farmer.
Preparation of extracts
Leaves of C. xanthocarpa were dried at room temperature for 3 weeks and used for the preparation of the aqueous extracts and the essential oil extraction. Infusion of the leaves were prepared in hot water for 10 min using two concentrations, 6 and 30 mg/mL, the 6 mg/mL being the traditional concentration used by the population in general to prepare the tea. The aqueous extracts were filtered and placed at room temperature for cooling. The essential oil was obtained by hydro-distillation with the Clevenger apparatus after 4 h. The oil concentration used in the test was 0.25% (w/v) in ethanol.
A. cepa assay
For the test, we used 30 A. cepa bulbs divided into six groups of five onion bulbs for each treatment, namely: negative control (water); aqueous extracts at 6 mg/mL; aqueous extract at 30 mg/mL; control-ethanol; essential oil at 0.25%; and positive control (glyphosate solution at 4.8% – known to cause chromosomal abnormalities in A. cepa cells). For each treatment, the bulbs were rooted in distilled water for 7 d and then placed in their respective group for 24 h. After, the radicles were collected and fixed in 3:1 (v/v) ethanol:acetic acid for 24 h. Subsequently, they were placed in 70% (v/v) aqueous ethanol under refrigeration (4 ± 2 °C) until analysis (Knoll et al., Citation2006). For each bulb, two slides were prepared using two root tips hydrolyzed in 1 N HCl for 5 min and washed in distilled water. The fragmented meristematic regions were stained with 2% (w/v) acetic orcein (Guerra & Souza, Citation2002). Each slide was assessed by bright-field optical microscopy at 40× magnifications and the number of interphase, prophase, metaphase, anaphase and telophase cells was recorded. At least 1000 cells for each bulb were analyzed, and a total of 5000 cells for each treatment were scored. Mean values for the different cell cycle phases and the mitotic index (MI), were calculated as the ratio between the number of mitotic cells and the total number of cells scored and expressed as percentage (MI %). The mitodepressive index was obtained through the mitotic index based on the study of Valencia-Quintana et al. (Citation1993).
GC and GC/MS analysis
The components of essential oil obtained from C. xanthocarpa leaves were determined by gas chromatography (GC; Laboratório de Óleos Essenciais e Extratos Vegetais, Instituto de Biotecnologia, Universidade de Caxias do Sul, Brazil). GC analyses were carried out on a chromatograph (Hewlett Packard 6890 Series) using an HP-Innowax column (30 m × 0.32 mm, 0.5 μm film thickness). Injector and detector temperature was set at 250 °C; and the oven temperature was programmed from 40 °C (8 min) to 180 °C at 3 °C/min, 180–230 °C at 20 °C/min, and then 230 °C (20 min). Helium was employed as carrier gas (34 Kpa). The oil was diluted in hexane (1:10) and 1 μL was injected. Percentage compositions were obtained from electronic integration measurements using flame ionization detection. GC/MS analyses were performed using a Hewlett Packard 6890/MSD5973, equipped with HP Chemstation software and Wiley 275 spectrolibrary, operating at 70 eV. A fused silica capillary column (30 m × 0.25 mm, 0.5 μm film thickness) was used, and the temperature program was performed as described above (interface at 280 °C). Helium was employed as carrier gas (56 Kpa, 1 mL/min). The sample was diluted in hexane (1:10) and 0.4 μL was injected.
Quantification of phenolic and flavonoid compounds by HPLC/DAD
The content of phenolic and flavonoid compounds in the aqueous extract at 30 mg/mL was evaluated by high-performance liquid chromatography coupled with a diode array detector (HPLC/DAD). The analysis was performed with the HPLC system (Shimadzu, Kyoto, Japan), Prominence Auto Sampler (SIL-20A), equipped with Shimadzu LC-20AT reciprocating pumps connected to the degasser DGU 20A5 with integrator CBM 20A, UV-VIS detector DAD (diode) SPD-M20A and Software LC solution 1.22 SP1. Reverse phase chromatographic analyses were carried out under gradient conditions using a C18 column (4.6 mm × 250 mm) packed with 5 μm diameter particles; water containing 2% acetic acid (A) and methanol (B) was used as mobile phase, and the composition gradient was 5% (B) for 2 min; 25% (B) until 10 min; 40, 50, 60, 70 and 80% (B) every 10 min. All the samples and mobile phase were filtered through 0.45 μm membrane filter (Millipore) and then degassed by ultrasonic bath prior to use. Stock solutions of standard references were prepared in the HPLC mobile phase at a concentration range of 0.031–0.250 mg/mL for quercetin and rutin, and 0.006–0.250 mg/mL for gallic and chlorogenic acids. Quantification was carried out by integration of the peaks using the external standard method, at 254 nm. The flow rate was 0.8 mL/min and the injection volume was 40 μL. The chromatography peaks were confirmed by comparing their retention time and Diode-Array-UV spectra with those of the reference standards. All chromatography operations were carried out at ambient temperature and in triplicate.
Statistical analysis
The statistical data analysis was performed using the Chi-squared (χ2) test at p ≤ 0.05, with the statistical program BioEstat 3.0 (Ayres et al., Citation2003). Calibration curves of the standards gallic and chlorogenic acids and quercetin and rutin were constructed with the aid of Excel program.
Results
Effects of aqueous extracts and essential oil on the A. cepa cellular cycle
Treatment with the extracts (aqueous and essential oil) of C. xanthocarpa resulted in a marked decrease in the mitotic activity of A. cepa cells. The number of interphase, prophase, metaphase, anaphase and telophase, as well as the MI for all tested groups are shown in . The lower concentration of the aqueous extract (6 mg/mL) led to a reduction of 67.7% of the MI, whereas the 30 mg/mL concentration led to a reduction of 34.1% of the MI, when compared with the negative control. Treatment with essential oil caused a 48.2% reduction in the MI. A significant difference (χ2 test, p ≤ 0.05) was obtained to the extracts and essential oil in relation to the control groups (water and ethanol; ). The mitodepressive index was also calculated and the values obtained were: 4.54, 1.82, 5.0, 2.52 and 3.56 for the positive control, control-ethanol, extract at 6 mg/mL, extract at 30 mg/mL and essential oil at 0.25%, respectively.
Table 1. Number of cells in different phases at different stages of cell cycle of onion root-tip cells treated for 24 h with water, glyphosate solution, ethanol, aqueous extracts and essential oil of Campomanesia xanthocarpa.
Cellular irregularities of the A. cepa cycle were observed for all treatments. The total and the percentage number of chromosome aberrations observed in each treatment are shown in . Aqueous extracts at 6 and 30 mg/mL showed 24.4 and 22.2% of cells with alterations, respectively, and the essential oil showed 26.7%. For the positive control (glyphosate solution at 4.8%), 27.5% of irregularities were found. In relation to cellular irregularities, chromosomal mutations were also observed in each treatment, including anaphase bridges, delay chromosome, break chromosome, as well as metaphase with disorganized chromosomal and binuclear cells (). The occurrence of these aberrations can be observed in .
Figure 1. Allium cepa cells treated with aqueous extracts, essential oil and control-ethanol of Campomanesia xanthocarpa. (1) Cell in metaphase disorganization (6 mg/mL); (2) anaphase with several anaphasic bridges (6 mg/mL); (3) cell binuclear (6 mg/mL); (4) metaphase disorganization and with chromosomal breaks (30 mg/mL); (5) anaphase with chromosomal bridges (30 mg/mL); (6) irregular metaphase with chromosome lost; (7) irregular anaphase with chromosomal breaks (ethanol); (8) irregular anaphase with bridges (essential oil). Scale bar = 10 μm.
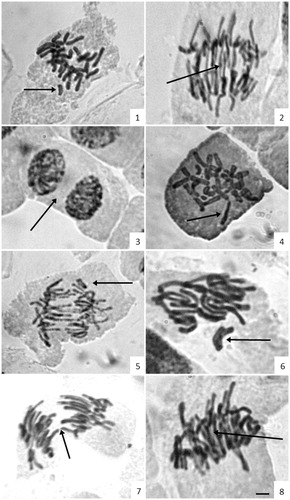
Table 2. Number and percentage (%) of chromosome aberration observed on A. cepa root-tip cells treated with aqueous extracts essential oil and controls of C. xanthocarpa.
GC/MS and HPLC/DAD analysis
The yield of essential oil of the dried leaves was 0.2% (v/w). The chemical composition of leaf oil from C. xanthocarpa was analyzed by GC/MS and the main compounds obtained were β-caryophyllene (8.87%), viridiflorol (6.40%), spathulenol (5.16%), δ-cadinene (4.92%), linalool (4.46%) and α-cadinol (4.25%).
The HPLC/DAD polyphenolic profile of the aqueous extract at 30 mg/mL from the leaves of C. xanthocarpa is shown in . The quantification of quercetin, rutin, gallic and chlorogenic acids was determined based on the references of the standard calibration curves (). Calibration curves obtained were gallic acid: y = 53 985x − 1033.9 (r = 0.9892); chlorogenic acid: y = 52 542x − 1082.3 (r = 0.9951); rutin: y = 49 761x − 1235.7 (r = 0.9829), and quercetin: y = 50 833x − 1741.5 (r = 0.9936). The extract contains gallic acid (retention time – tR = 12.44 min, peak 1), chlorogenic acid (tR = 22.31 min, peak 2), rutin (tR = 38.17 min, peak 3) and quercetin (tR = 47.62 min, peak 4) besides other minor compounds. The quantification of the compounds in aqueous extract at 6 mg/mL was not evaluated due to the lower concentration of leaves used in this preparation.
Figure 2. High-performance liquid chromatography phenolics and flavonoids profile of aqueous extract at 30 mg/mL from the leaves of C. xanthocarpa. Gallic acid (peak 1), chlorogenic acid (peak 2), rutin (peak 3) and quercetin (peak 4).
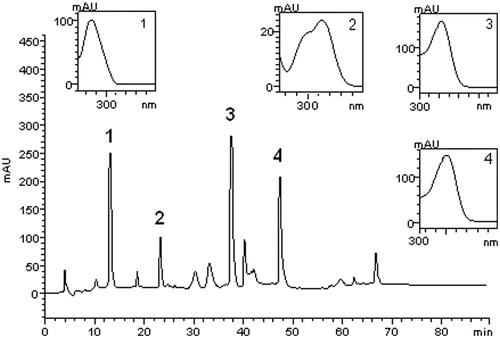
Table 3. Phenolics and flavonoids composition of Campomanesia xanthocarpa leaves.
Discussion
Plant-based bioassays have gained popularity among the toxicological assessment procedures, and some reasons for their wide use are comparative simplicity, sensitivity and cost effectiveness as well as a good correlation with other toxicity tests. Cytotoxicity tests using an in vivo vegetal system, such as A. cepa, have been applied by several researches, and one of the advantages of A. cepa test is the possibility to expose the test organism directly to complex mixtures without previous treatment of the test sample (Çelik & Aslantürk, Citation2010; Teixeira et al., Citation2003). Cytotoxicity and genotoxicity of C. xanthocarpa extracts and oil were estimated by observing cytological parameters such as the mitotic index and number of chromosome abnormalities, including breakage, lost chromosome, retardation chromosome, bridges, bridge and delayed, micronucleous, binuclear cells and disorganized division. It should be noted that the genotoxicity refers to the ability of certain agents produce chromosomal alterations in the genetic material, consequently, altering the structure and function of the DNA. Furthermore, cytotoxicity is a broader term and used to refer to the ability of certain agents produce some kind of morphological change in the cell.
The analysis of A. cepa meristematic cells at different stages of cellular cycle treated with the extracts (aqueous and oily) showed an inhibition in the number of divisions from the root tips when compared with negative control (). This result indicates that the extracts and the oil, and the ethanol and glyphosate applied in A. cepa bulbs have antimitotic activity, which was also observed by others researches, when studying the effects of Pterocaulon polysthachyum DC. (Asteraceae; Knoll et al., Citation2006), Achyrocline satureioides Lam. D.C. (Asteraceae; Fachinetto et al., Citation2007), and Solidago microglossa DC. (Asteraceae; Bagatini et al., Citation2009) extracts. Moreover, in the negative control, the occurrence of a higher number of cells in the prophase, metaphase and anaphase phases was indicative of the regular sequence of cell division. Analysis of the MI after treatment with the extracts and the oil revealed differences in their effect on mitotic activity. In the analysis of MI after 24 h, the values obtained showed a decrease of cells in division according to the treatment employed when compared to negative control (). Interestingly, when the A. cepa root tips were treated with the aqueous extract at 6 mg/mL, the highest inhibition of cell division was observed, indicating that the increase of extract concentration did not interfere in this effect. Essential oil (0.25%) also reduced the number of dividing cells significantly. The reduction in MI showed that substances in the extracts may have cytotoxic effects, but a dose-dependent effect was not observed. This result is similar to those described by Akinboro and Bakare (Citation2007) working with decoctions of Azadirachta indica A. Juss. (Meliaceae) and Cymbopogon citratus (D.C.) Stapf. (Poaceae), where the MI obtained at the 20% concentration was higher than that of 10% concentration. In effect, MI measures the proportion of cells in the M phase of the cell cycle and its inhibition could be interpreted as cellular death or a delay in the cell proliferation kinetics. On the other hand, dose-dependent antiproliferative effects of Psychotria myriantha Mull. Arg. (Rubiaceae) and P. leiocarpa Cham. et Schlecht infusions on the A. cepa cell cycle were reported by Lubini et al. (Citation2008), by Souza et al. (Citation2010), working with Artemisia verlotorum Lamotte (Compositae), and by Çelik & Aslantürk (Citation2010) testing Inula viscosa L. Ainton (Tribe Inulae, Asteraceae) leaf extracts by the same assay. In the same line, observing the morphology and count of cells in division, it was possible to demonstrate the genotoxic activity of the species studied, since chromosome aberrations were observed in significant proportions (above 20%) for the C. xanthocarpa oil and for both extracts concentrations, and these values, in percentage, were similar to glyphosate, a broad spectrum herbicide (). Chromosome aberration in onion root tips is an efficient method to the analysis and in situ monitoring of genotoxicity of environmental substances (Cabrera & Rodriguez, Citation1999; Leme & Marin-Morales, Citation2009; Silva et al., Citation2004).
Studies using aqueous extracts are appropriate because traditional medicinal herbs are generally used as crude extracts, but extracts of natural origin usually contain a range of chemically diverse constituents occurring in different concentrations that makes it a complex mixture of biologically active compounds. Some of these compounds can be cytotoxic and/or genotoxic; others can be cytoprotective and/or antigenotoxic. Therefore, it is important to use chromatographic methods to analyze these inherently complex mixtures in order to locate the major constituents of each extract tested and also, to look for possible correlations between the constitution and biological activities observed. The phytochemical analysis of the aqueous extract at 30 mg/mL showed the presence of phenolics, such as gallic (3.19%) and chlorogenic acids (1.04%), besides the flavonols, quercetin (2.97%) and rutin (4.82%). Though many studies indicated an antiproliferative activity of phenolics, mechanisms of action have not been clearly determined yet. The antiproliferative capacity showed for the extracts studied may be related to the presence of substances with antioxidant activity found in the samples analyzed. Gallic acid, a naturally occurring plant polyphenol with antioxidative activity, has been found to induce cell death in promyelocytic leukemia HL-60RG cells and human colon adenocarcinoma cells (Inoue et al., Citation1994; Salucci et al., Citation2002). Several studies have shown that flavonoids, such as quercetin and its glycosilated form rutin, are potentially harmful chemicals, since some are mutagenic in bacterial and mammalian systems; this may be due to their activity as pro-oxidants in generating free radicals that damage DNA or their inhibition of DNA associated enzymes such as topoisomerase (Skibola & Smith, Citation2000). However, the level of flavonoids required to induce mutations and cytotoxicity in humans may not be physiologically achievable through dietary sources, there remains uncertainty regarding the conditions and the levels of flavonoids intake necessary to pose a potential health hazard.
In the chemical composition of C. xanthocarpa essential oil, the results showed that the sample analyzed was rich in sesquiterpene compounds (48.6%), and β-caryophyllene was the main component, followed by viridiflorol, spathulenol, δ-cadinene, linalool and α-cadinol. Different quantities of β-caryophyllene, spathulenol and linalool were detected in the leaf essential oil of C. xanthocarpa collected in Rio Grande do Sul State (Limberger et al., Citation2001). The anti-oxidant activity of oil obtained from the fruits has been reported in the literature, and several known constituents of the myrtaceous leaf oils and extracts were also found in the essential oils from fruit, suggesting that the fruit may display functional properties similar to those of the leaf (Marin et al., Citation2008).
The mutagenic activity of the extracts of C. xanthocarpa was evaluated by Fernandes and Vargas (Citation2003) for Salmonella/microssome test. The authors reported that C. xanthocarpa showed frameshift (TA97 strain) signs of mutagenic activity without exogenous metabolism (S9 fraction), and these results were attributed to different active substances acting on the nDNA. The compounds found were listed as flavonoids, saponins and tannins.
Clinical studies and scientific reports have indicated the use of C. xanthocarpa in the treatment of hypercholesterolemia (Biavatti et al., Citation2004). In our study, infusions obtained from the C. xanthocarpa leaves in usual concentrations were evaluated and their antiproliferative capacity as well as their genotoxic potential were evidenced. This raises concern about the potential mutagenic or genotoxic hazards resulting from the long-term use of such plant.
Conversely, it became of interest to set up the efficacy of plant bioassays in establishing good correlation with other bioassays. The extrapolation of the results obtained with plant cells to human cells or generally from one species to another is probably the most important problem discussed in the context of biological testing. For these reasons, the therapeutic use of this plant in eukaryote organisms should be assessed, since significant chromosomal aberrations were found.
Conclusion
Our results demonstrated that leaf infusions presented antiproliferative and genotoxic activities using the in vivo onion (Allium cepa) root-tip cell test and an increase in the frequency of chromosome aberrations. According to the results obtained, the extracts of this plant should be studied in animal models to verify its safe use.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Akinboro A, Bakare AA. (2007). Cytotoxic and genotoxic effects of aqueous extracts of five medicinal plants on Allium cepa Linn. J Ethnopharmacol 112:470–5
- Ayres M, Ayres Jr M, Ayres DL, Santos AS. (2003). BioEstat. 3.0: Aplicações estatísticas nas áreas das Ciências Biológicas e Médicas. Brasília: CNPP, 290
- Bagatini MD, Fachinetto JM, Silva ACF, Tedesco SB. (2009). Efeitos citotóxicos das infusões (chá) de Solidago microglossa DC. (Asteraceae) no ciclo celular de Allium. Rev Bras Farmacogn 19:632–6
- Biavatti MW, Farias C, Curtius F, et al. (2004). Preliminary studies on Campomanesia xanthocarpa (Berg.) and Cuphea carthagenensis (Jacq.) J. F. Macbr. aqueous extract: Weight control and biochemical parameters. J Ethnopharmacol 93:385–9
- Cabrera GL, Rodriguez DMG. (1999). Genotoxicity of soil from farmland irrigated with wastewater using three plant biossays. Mutat Res 426:211–14
- Çelik TA, Aslantürk OS. (2010). Evaluation of cytotoxicity and genotoxicity of Inula viscosa leaf extracts with Allium test. J Biomed Biotechnol 2010:1–8
- Corrêa MP. (1984). Dicionário das plantas úteis do Brasil e das exóticas cultivadas. Rio de Janeiro: Imprensa Nacional, 509–10
- Dickel ML, Rates SMK, Ritter MR. (2007). Plants popularly used for loosing weight purposes in Porto Alegre, South Brazil. J Ethnopharmacol 109:60–71
- Fachinetto JM, Bagatini MD, Durigo J, et al. (2007). Efeito anti-proliferativo das infusões de Achyrocline satureioides DC (Asteraceae) sobre o ciclo celular de Allium cepa. Rev Bras Farmacogn 17:49–54
- Fernandes, JBF, Vargas, VMF. (2003). Mutagenic and antimutagenic potential of the medicinal plants M. laevigata and C. xanthocarpa. Phytother Res 17:269–73
- Guerra M, Souza MJ. (2002). Como Observar Cromossomos: Um Guia de Técnica em Citogenética Vegetal, Animal e Humana. São Paulo: Funpec
- Inoue M, Suzuki R, Koide T, et al. (1994). Antioxidant, gallic acid, induces apoptosis in HL-60RG cells. Biochem Biophs Res Commun 204:898–904
- Klafke JZ, Silva MA, Panigas TF, et al. (2010). Effects of Campomanesia xanthocarpa on biochemical, hematological and oxidative stress parameters in hypercholesterolemic patients. J Ethnopharmacol 127:299–305
- Knoll MF, Tedesco SB, Silva ACF, Canto-Dorow TS. (2006). Effects of Pterocaulon polystachium DC. (Asteraceae) on Allium cepa root-tip cells. Genet Mol Biol 29:539–42
- Leme DM, Marin-Morales MA. (2009). Allium cepa test in environmental monitoring: A review on its application. Mutat Res 682:71–81
- Limberger RP, Apel MA, Sobral M, et al. (2001). Chemical composition of essential oils from some Campomanesia species (Myrtaceae). J Essent Oil Res 13:113–15
- Lubini G, Fachinetto JM, Laughinghouse HD, et al. (2008). Extracts affecting mitotic division in root-tip meristematic cells. Biologia (Bratislava) 63:647–51
- Marin R, Apel MA, Limberger RP, et al. (2008). Volatile components and antioxidant activity from some Myrtaceous fruits cultivated in southern Brazil. Lat Am J Pharm 27:172–7
- Salucci M, Stivala LA, Maiani G, et al. (2002). Flavonoids uptake and their effect on cell cycle of human colon adenocarcinoma cells (Caco2). Brit J Cancer 86:1645–51
- Silva CR, Monteiro MR, Caldeira-de-Araújo A, Bezerra RJAC. (2004). Absence of mutagenic and citotoxic potentiality of senna (Cassia angustifolia Vahl.) evaluated by microbiological tests. Rev Bras Farmacogn 14:1–3
- Silva J, Erdtmann B, Henriques JAP. (2003). Genética toxicológica. Porto Alegre: Alcance
- Skibola CF, Smith MT. (2000). Potential health impacts of excessive flavonoid intake. Free Radical Bio Med 29:375–83
- Souza LFB, Laughinghouse IV HD, Pastori T, et al. (2010). Genotoxic potential of aqueous extracts of Artemisia verlotorum on the cell cycle of Allium cepa. Int J Environ Stud 67:871–7
- Tedesco SB, Launghinghouse IV HD. (2012). Bioindicator of genotoxicity: The Allium cepa test. In: Tedesco SB & Languinghouse IV HD, eds. Org. Environmental Contamination. Rijeka: Intech, 137–56
- Teixeira RO, Camparoto ML, Mantovani MS, Vicentini VEP. (2003). Assessment of two medicinal plants, Psidium guajava L. and Achillea millefolium L. in in vivo assays. Genet Mol Biol 26:551–5
- Valencia-Quintana R, Gómez-Arroyo S, Villalobos-Pietrini R. (1993). Cytological effects of some carbamate insecticides. I. Methomyl and oxamyl in Vicia faba. Rev Int Contam Ambient 9:65–9
- Vallilo MI, Moreno PRH, Oliveira E, et al. (2008). Composição química dos frutos de Campomanesia xanthocarpa Berg-Myrtaceae. Ciência e Tecnologia de Alimentos 28:231–7
