Abstract
Context. Ribes nigrum L. (Grossulariaceae) is among the most commonly used herbal medicines and it is popularized for its alleged tonic effect and curative and restorative properties. The current practice of identifying herbal extracts is by measuring the concentration of the main botanicals. Their concentrations are used to characterize the herbal preparations and fingerprinting is recommended by the main Pharmacopeias as a potential and reliable strategy for the quality control of complex mixtures.
Objective: The aim of this research was to perform an analytical study of R. nigrum bud-preparations, in order to identify and quantify the main bioactive compounds, obtaining a specific chemical fingerprint to evaluate the single class contribution to herbal preparation phytocomplex.
Materials and methods: The same analyses were performed using a high-performance liquid chromatograph-diode array detector both on University lab preparations and on commercial preparations from different Italian locations. Different chromatographic methods were used to analyse the macerated samples, two for polyphenols and one for terpenic compounds.
Results. Ribes nigrum was identified as a rich source of anti-inflammatory and antioxidant compounds. The observed analytical firgerprint demonstrated that these bud-preparations represent a rich source of terpenic and polyphenolic compounds, especially catechins and phenolic acids.
Discussion and conclusion: Analytical fingerprinting could be an important tool to study the assessment of chemical composition and bioactivities of plant-derived products, helping to find new sources of natural health-promoting compounds: this study allowed the development of an effective tool for quality control through botanical fingerprinting of bud preparations.
Introduction
Ribes nigrum L. (Grossulariaceae) is commonly used as herbal medicine and it is popularized for its alleged tonic effect and possible curative and restorative properties (Tabart et al., Citation2011, Citation2012). Ribes nigrum is a shrub spontaneously growing in the cold and temperate climate zones and today many orchards with different genetic materials are realized in order to produce fruit, leaves and buds. The most important industrial product of R. nigrum is fruit. However, due to their particular chemical composition and excellent flavor, leaves and buds are also used in some applications as a raw material for herbal and cosmetic industries. Many people use the buds as a medicinal preparation for anti-inflammatory activity and antidermal diseases (eczema and psoriasis) (Dvaranauskaite et al., Citation2008).
For this reason, bud-preparations, derived from embryonic fresh plant tissues, are important therapeutic remedies, prescribed in hepatic, respiratory, circulatory and inflammatory disorders, but data on their chemical composition are lacking as, until now, phytochemical studies have principally been performed on bark, roots and root exudates, leaves, fruit, and seeds (Donno et al., Citation2012a; Peev et al., Citation2007).
Polyphenols and terpenes are the dominant majority of biologically active plant compounds with antioxidative and anti-inflammatory properties: these secondary plant metabolites may be nutritionally important and play critical roles in human health in the prevention of chronic diseases such as pulmonary inflammation, cancer, cardiovascular and neurodegenerative conditions (Donno et al., Citation2012b; Komes et al., Citation2011; Mattila et al., Citation2011; Tabart et al., Citation2012; Zhang et al., Citation2009).
By nature, herbal preparations are complex matrices, comprising a multitude of compounds, which are prone to variation due to environmental factors and manufacturing conditions (Edwards et al., Citation2012; Donno et al., Citation2012a; Komes et al., Citation2011; Steinmann & Ganzera, Citation2011). The analysis of plant and herbal preparation secondary metabolites is a challenging task because of their chemical diversity: low variability is usually observed even within the same species and an herbal preparation detailed chemical profile is certainly necessary also to ensure the reliability and repeatability of clinical and pharmacological studies (Mok & Chau, Citation2006).
It is estimated that 100 000–200 000 metabolites occur in the plant kingdom (Oksman-Caldentey & Inze, Citation2004), and only highly selective and sensitive methods will be suitable for controlling their composition and quality because many traditional herbal preparations contain several medicinal plants (Steinmann & Ganzera, Citation2011). The most important chromatographic or electrophoretic techniques coupled to different detectors are employed for this purpose. High-performance liquid chromatography (HPLC) is still the preferred separation technique for the analysis of natural products (Gray et al., Citation2010).
The current practice for herbal extract identification is by measuring the concentration of the main bioactive compounds, called “markers”. The concentrations of the main chemical components are used to characterize the herbal preparation (Mok & Chau, Citation2006) and referred to as the “fingerprint”. Indeed, some studies showed that synergistic or additive biological effects of different phytochemicals (phytocomplex) contribute to disease prevention better than a single compound or a group of compounds (Jia et al., Citation2012).
Chromatographic fingerprinting is recommended by the main national and international Pharmacopoeias as a potential and reliable strategy for the quality control of complex mixtures like herbal medicines. However, it should be noted that many traditional preparations are composed of multiple herbs, so that the analysis of selected constituents might not reflect their overall quality or efficacy (Qiao et al., Citation2010; Steinmann & Ganzera, Citation2011; Zhao et al., Citation2009; Zhou et al., Citation2008). Different kinds of features can be selected to characterize the herbal preparations, and referred to as the overall fingerprint: genetic, quality, sensory or morphological features could be used to create a fingerprint as showed in other studies (Canterino et al., Citation2012; Mellano et al., Citation2012). In this study, polyphenolic and terpenic composition was referred to as a chemical fingerprint.
The aim of this research was to perform an analytical study of R. nigrum bud-preparations, in order to identify and quantify the main bioactive polyphenolic and terpenic compounds, obtaining a specific profile of the main polyphenols and terpenes and the total bioactive compound content. The same analyses were performed using an HPLC-DAD both on University lab preparations and on commercial preparations in order to obtain a chemical fingerprint for the assessment of the single bioactive class contribution to total bud-preparation phytocomplex.
Materials and methods
Plant material
University lab preparations and commercial preparations were evaluated. In February 2012, samples of R. nigrum buds were picked up in a germplasm repository in San Secondo di Pinerolo, Turin Province (Italy). Two different varieties (Rozenthal and Daniels) were sampled, in order to test the genotype effect on the chemical composition of the final product. Fresh buds were used to prepare herbal preparations.
Commercial products from five different Italian herbal companies were also considered. The companies are located in San Gregorio di Catania (Catania Province), Predappio (Forlì-Cesena Province), Collepardo (Frosinone Province), Cambiasca (Verbania Province) and Binasco (Milano Province). University lab and commercial preparations were labeled with a code ().
Table 1. Source and identification code of the analysed samples.
Macerated sample preparation protocol
The protocol of bud-preparations is detailed in the monograph “Homeopathic preparations”, quoted in the French Pharmacopoeia, 8th edition, 1965 (Ordre national des pharmaciens, Citation1965). Bioactive compounds were extracted through a cold maceration process for 21 days, in a solution of ethanol (95%) and glycerol, followed by a first filtration (Whatman Filter Paper, Hardened Ashless Circles, 185 mm Ø), a manual pressing and, after two days of decanting, a second filtration (Whatman Filter Paper, Hardened Ashless Circles, 185 mm Ø). Macerated samples were then stored at normal atmosphere (N.A.), at 4 °C and 95% relative humidity (R.H.).
Solvents and chemicals
The maceration solvents, ethanol and glycerol, were purchased from Fluka Biochemika (Buchs, Switzerland) and Sigma Aldrich (St. Louis, MO), respectively. Analytic HPLC grade solvents, methanol and formic acid were purchased from Sigma Aldrich and Fluka Biochemika, respectively; potassium dihydrogen phosphate was also purchased from Sigma Aldrich. Milli-Q ultrapure water was produced by using Sartorius Stedium Biotech mod. Arium.
All calibration standards were purchased from Sigma Aldrich: caffeic acid, chlorogenic acid, coumaric acid, ferulic acid, hyperoside, isoquercitrin, quercetin, quercitrin, rutin, gallic acid, ellagic acid, catechin, epicatechin, limonene, phellandrene, sabinene, γ-terpinene and terpinolene.
Standard preparation
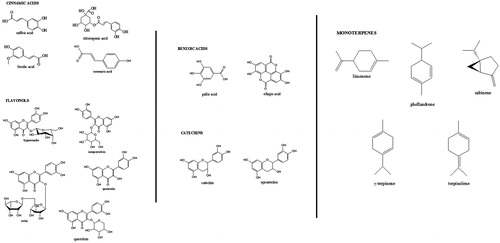
Chemical structures of all the compounds are shown in . Stock solutions of cinnamic acids and flavonols with a concentration of 1.0 mg/mL were prepared in methanol: from these solutions, four calibration standards were prepared by dilution with methanol. Stock solutions of benzoic acids and catechins with a concentration of 1.0 mg/mL were prepared in 95% methanol and 5% water. From these solutions, four calibration standards were prepared by dilution with 50% methanol--water.
Stock solutions of monoterpenes with a concentration of 1.0 mg/mL were prepared in methanol: from these solutions, four calibration standards were prepared by dilution with methanol.
HPLC sample preparation and storage
Macerated University lab and commercial preparations were filtered with circular pre-injection filters (0.45 µm, polytetrafluoroethylene membrane, PTFE) and then stored for a few days at N.A., 4 °C and 95% R.H.
Apparatus and chromatographic conditions
An Agilent 1200 high-performance liquid chromatograph, equipped with a G1311A quaternary pump, a manual injection valve and a 20 μL sample loop, coupled to an Agilent GI315D UV-Vis diode array detector, was used for the analysis.
Three different chromatographic methods were used to analyse the macerated samples, two for polyphenols and one for terpenic compounds. The first method (A) was used for the analysis of cinnamic acids and flavonols; bioactive compound separation was achieved on a ZORBAX Eclipse XDB – C18 column (4.6 × 150 mm, 5 μm), while the mobile phase consisted of methanol and a solution of 40 mM potassium dihydrogen phosphate in water. The flow rate was 1.0 mL min−1 (gradient analysis, 60 min) and the detector wavelength was 330 nm (Donno et al., Citation2012a; Peev et al., Citation2007). The second method (B) was used for the analysis of benzoic acids and catechins. Bioactive molecules were separated on a ZORBAX Eclipse XDB – C18 column (4.6 × 150 mm, 5 μm), while the mobile phase consisted of a solution of methanol:water:formic acid (5:95:0,1 v/v/v) and a mix of methanol:formic acid (100:0,1 v/v). The flow rate was 1.0 mL min−1 (gradient analysis, 35 min) and the detector wavelengths were 250, 280 and 320 nm (Donno et al., Citation2012a; Moller et al., Citation2009).
The third method (C) was used for the analysis of monoterpenes; chromatographic separation was performed using a ZORBAX Eclipse XDB – C18 column (4.6 × 150 mm, 5 μm). The liquid flow rate was 1.0 mL min−1 using water and methanol as mobile phase with a linear gradient of 75 min. UV spectra were recorded at 220 and 235 nm (Zhang et al., Citation2009).
Identification and quantification of bioactive compounds
All single compounds were identified in samples by comparison of their retention times and UV spectra with those of standards in the same chromatographic conditions. Quantitative determinations were performed using an external standard method. Calibration curves in the 125–1000 mg L−1 range with good linearity for a four point plot were used to determine the concentration of polyphenolic and terpenic compounds in bud-preparation samples. The linearity for each compound was established by plotting the peak area (y) versus the concentration (x) of each analyte. The limit of detection (LOD) and the limit of quantification (LOQ) of the three chromatographic methods were defined as the lowest amount of analyte that gives a reproducible peak with a signal-to-noise ratio (S/N) of 3 and 10, respectively. Calibration curve equations, linearity (R2), LOD and LOQ for all of the compounds are summarized in .
Table 2. Calibration curve equations, R2, LOD and LOQ of the used chromatographic methods for each calibration standard.
All samples were analysed in triplicate (three repetitions for three plants for each University lab sample and three repetitions for three products for each commercial sample), and all data are given in order to assess the repeatability of the used methods (standard deviation). Accuracy was checked by spiking samples with a solution containing each bioactive compound in a concentration of 10 mg mL−1.
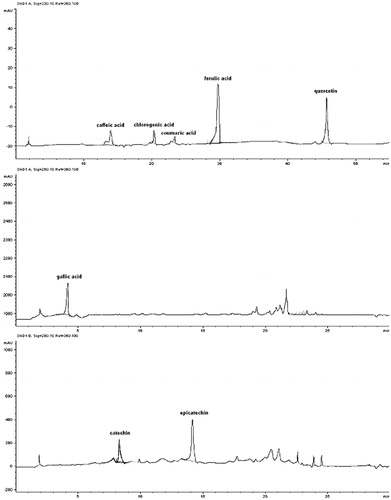
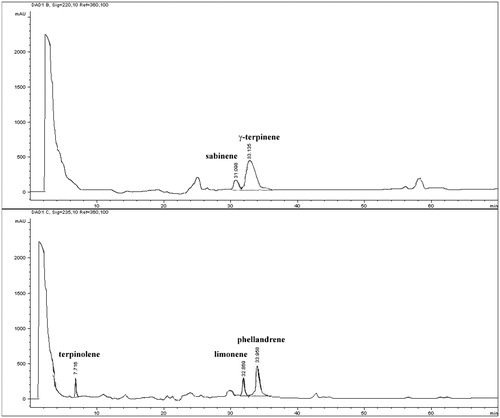
Examples of R. nigrum bud-preparation chromatographic profiles are reported in and . Total bioactive compound content (TBCC) were determined as the sum of the most important classes of polyphenols and terpenic compounds present in the samples. Four polyphenolic classes were considered: benzoic acids (gallic and ellagic acids), catechins (catechin and epicatechin), cinnamic acids (caffeic, chlorogenic, coumaric, and ferulic acids) and flavonols (hyperoside, isoquercitrin, quercetin, quercitrin and rutin); one terpenic class was considered: monoterpenes (limonene, phellandrene, sabinene, γ-terpinene, terpinolene). All results were expressed as mg per 100 g of buds fresh weight (FW).
Statistical analysis
Results were subjected to ANOVA and Student’s t-test for mean comparison (SPSS 18.0 Software, Chicago, IL) and HSD Tukey multiple range test (p < 0.05). Principal component analysis (PCA) was performed on the single botanical concentration data.
Results
Total bioactive compound content
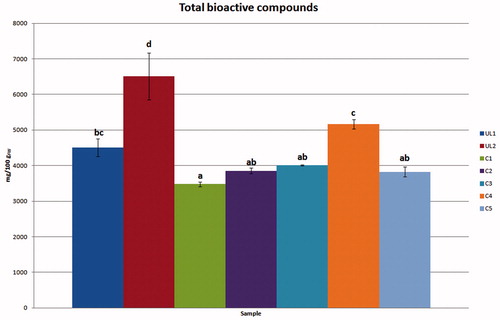
The content of total bioactive compounds in the evaluated bud-preparations is reported in . Statistically significant differences were observed among the analysed samples, with a lower total bioactive compound content (TBCC) value of 3478.95 mg/100 gFW (sample C1) and a higher value of 6507.29 mg/100 gFW (sample UL2).
Figure 4. TBCC in University lab and commercial bud-preparations. Different letters for each sample indicate the significant differences at p < 0.05.
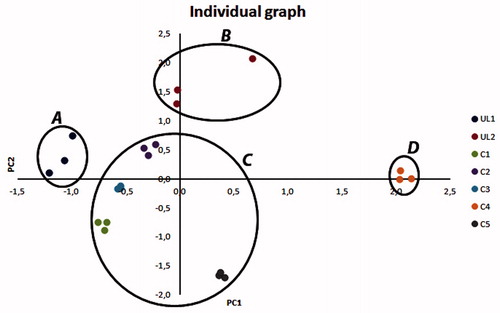
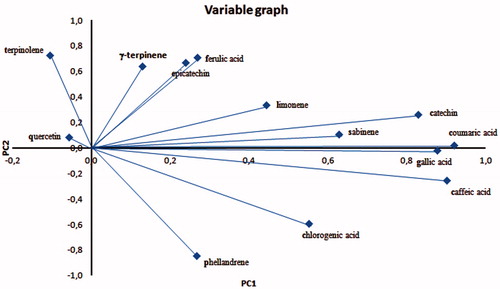
PCA was performed on all samples and it reduced the initial variables (single bioactive compound concentration) into four principal components (83.15% of total variance) and the initial seven groups into four groups, confirming the statistically significant differences in TBCC (ANOVA Test). The new groups were called A (UL1), B (UL2), C (C1, C2, C3, C5) and D (C4) (). PCA variable graph () showed a correlation between the most of polyphenols and PC1 (32.62% of total variance) and a correlation between monoterpenes, except limonene and sabinene, and PC2 (24.77% of total variance).
Single bioactive compound profile
All data are reported in (method A), 4 (method B) and 5 (method C). Ribes nigrum bud-preparations showed the following botanical composition: four cinnamic acids (caffeic acid, chlorogenic acid, coumaric acid, ferulic acid), one flavonol (quercetin), one benzoic acid (gallic acid), two catechins (catechin, epicatechin) and five monoterpenes (limonene, phellandrene, sabinene, γ-terpinene, terpinolene). Hyperoside, isoquercitrin, quercitrin, rutin and gallic acid were not detected. Single bioactive compound concentration ranged from 0.84 mg/100 gFW (chlorogenic acid, C1 sample) to 1309.19 mg/100 gFW (γ-terpinene, UL2 sample).
Table 3. Single polyphenolic profile of University bud–preparations and commercial bud–preparations (method A).
Table 4. Single polyphenolic profile of University bud-preparations and commercial bud–preparations (method B).
Table 5. Single terpenic profile of University bud–preparations and commercial bud–preparations (method C).
Statistically significant differences were observed both in the University lab bud-preparations and in commercial bud preparations. The most important differences were observed in the concentrations of catechin, limonene and terpinolene.
Fingerprinting
The chemical fingerprint of R. nigrum bud-preparations was determined. In total, 13 botanicals were identified by HPLC/DAD. By a single bioactive compound profile, botanicals were grouped into polyphenolic and terpenic classes to evaluate the contribution of each class to total phytocomplex composition.
The chemical fingerprint showed the prevalence of monoterpenes and catechins (mean values were considered). The most important class was monoterpenes (82.94%), followed by catechins (9.46%), cinnamic acids (3.64%), flavonols (2.67%) and benzoic acids (1.29%) ().
Table 6. Contribution (%) of botanical classes to the phytocomplex in analysed R. nigrum bud-preparations.
Therefore, monoterpenes and catechins were the two main groups of bioactive compounds in the evaluated bud-preparations. Monoterpene contribution ranged from 77.75% in the C4 sample to 87.01% in the UL2 sample, while catechins contributed to total phytocomplex in a different range, from 6.67% (UL2) to 13.52% (C2).
Discussion
The HPLC analysis of botanicals is nowadays a widespread and well developed characterization tool and some analytical reports were found in the literature. These compounds are very interesting because of their wide structural variability (5000 derivatives are known up to now), which explains their broad spectrum of pharmacological effects and medicinal uses (Ganzera, Citation2008). In most reports, comparable analytical conditions were described, which are based on reverse-phase (RP) stationary phases and acid mobile phases (Guo et al., Citation2008; Matsui et al., Citation2007).
Reports on the analysis of phenolic acids (e.g., caffeic acid and its derivatives) by HPLC coupled to diode array or mass detectors have been published. They describe phenolic acid determination in medicinal plants and preparations, as R. nigrum bud-preparations (Castro et al., Citation2010; Urpi-Sarda et al., Citation2009), according to the single bioactive compound concentrations shown in this research. Among other identified classes, flavonols and catechins were also selected for quantitative studies (Huang et al., Citation2008; Surveswaran et al., Citation2010; Yi et al., Citation2009). Based on the obtained results, most of the research pointed out that the identified polyphenolic compounds significantly contribute to the total phytocomplex of these herbal preparations. The obtained fingerprints were useful for authentication and quality control purposes (Amaral et al., Citation2009; Dugo et al., Citation2009). The present study confirmed these results, adding as well as the terpenic compounds also significantly contributed to the R. nigrum bud-preparation phytocomplex, such as anti-inflammatory constituents in herbal preparations (Zhang et al., Citation2009). Few studies emphasized the identification of single terpenoids in plant material by HPLC analysis (Steinmann & Ganzera, Citation2011).
It is well-known that the chemical composition of secondary plant metabolites highly depends on some factors such as climate conditions, harvesting time and plant genotype (Donno et al., Citation2012a; Dvaranauskaite et al., Citation2008), and the results of this research confirmed this hypothesis. ANOVA and PCA results showed that the R. nigrum bud-preparation composition (different locations) was similar in all the samples but the single compound concentrations were different. Moreover, observing the chemical composition, results showed that few compounds were not detected in herbal medicines. Chromatographic fingerprinting could be applied in the differentiation of R. nigrum bud-preparations by other species (Zhao et al., Citation2009; Donno et al., Citation2012a).
In this study, effective HPLC–DAD methods were developed for fingerprint analysis and component identification of R. nigrum bud-preparations from different locations. Comparing with other analytical studies (Dugo et al., Citation2009; Tsao & Yang, Citation2003), the chromatographic conditions were optimized to obtain an effective fingerprint containing enough information of constituents with good resolution and reasonable analysis time. For optimizing the elute conditions, linear gradients of different slopes were used for the compound separation, because some constituents were similar in the structure with each other. In the macerated samples, most constituents were also weakly acid, so adding formic acid was necessary for enhancing the resolution and eliminating peak tailing (Zhao et al., Citation2009). The choice of detection wavelength was a crucial step for developing a reliable fingerprint (Zhao et al., Citation2009; Zhou et al., Citation2008). A full-scan on the chromatogram from 190 to 400 nm was performed and only selected wavelengths were suitable to achieve more specific peaks as well as a smooth baseline.
The methods showed a good resolution for most peaks and could be routinely used to evaluate bud-preparation overall quality. The results indicate that the developed methods are feasible for comprehensive authentication and quality control of R. nigrum bud-preparations. Knowledge of molecular structure, composition and quantity is necessary to understand the botanical role in determining potential health effects, because many traditional preparations contain multiple herbs. Moreover, pretending to have a natural origin, these preparations sometimes contain a mixture of synthetic adulterants (e.g., sildenafil, diazepam, captopril and amoxicillin), which explains their (unexpected) power but is also responsible for side effects of “unknown” reason (Kesting et al., Citation2010; Liang et al., Citation2006; Uchiyama et al., Citation2009) so that only highly selective, sensitive and versatile analytical techniques will be suitable for quality control purposes (Hager et al., Citation2008).
This study is only a preliminary research about R. nigrum bud-preparation chemical composition. By hyphenating high-performance liquid chromatography and mass spectrometry, the high quality demand of the consumer is fulfilled, providing the lab technicians with a multitude of technical options and applications (Gray et al., Citation2010; Steinmann & Ganzera, Citation2011).
Conclusions
Regarding the bud-preparations evaluated in this study, R. nigrum was identified as a rich source of anti-inflammatory and antioxidant compounds. The observed analytical fingerprint demonstrated that these bud-preparations represent a rich source of terpenes and polyphenolic compounds, especially catechins. This research suggested that identified botanicals might contribute to the total phytocomplex of these herbal preparations.
With gaining popularity of herbal remedies worldwide, the need of assuring safety and efficacy of these products increases as well. Analytical fingerprinting could be an important tool to assess the chemical composition and bioactivities of the plant-derived products, helping to find new sources of natural health-promoting compounds. Only in this way it will be possible to develop a new generation of standardized products that fulfill today’s standards for quality, safety and efficiency of herbal preparations.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Amaral S, Mira L, Nogueira JMF, et al. (2009). Plant extracts with anti-inflammatory properties – A new approach for characterization of their bioactive compounds and establishment of structure--antioxidant activity relationships. Bioorg Med Chem 17:1876–83
- Canterino S, Donno D, Mellano MG, et al. (2012). Nutritional and sensory survey of Citrus sinensis (L.) cultivars grown at the most northern limit of the Mediterranean latitude. J Food Quality 35:108–18
- Castro J, Krishna MVB, Choiniere JR, Marcus RK. (2010). Analysis of caffeic acid derivatives in echinacea extracts by liquid chromatography particle beam mass spectrometry (LC-PB/MS) employing electron impact and glow discharge ionization sources. Anal Bioanal Chem 397:1259–71
- Donno D, Beccaro GL, Mellano GM, et al. (2012a). Effect of agronomic and environmental conditions on chemical composition of tree-species buds used for herbal preparations. Int J Plant Res (VEGETOS) 25:21–9
- Donno D, Beccaro GL, Mellano MG, et al. (2012b). Application of sensory, nutraceutical and genetic techniques to create a quality profile of ancient apple cultivars. J Food Quality 35:169–81
- Dugo P, Cacciola F, Donato P, et al. (2009). High efficiency liquid chromatography techniques coupled to mass spectrometry for the characterization of mate extracts. J Chromatogr A 1216:7213–21
- Dvaranauskaite A, Venskutonis PR, Raynaud C, et al. (2008). Characterization of steam volatiles in the essential oil of black currant buds and the antioxidant properties of different bud extracts. J Agric Food Chem 56:3279–86
- Edwards JE, Brown PN, Talent N, et al. (2012). A review of the chemistry of the genus Crataegus. Phytochemistry 79:5–26
- Ganzera M. (2008). Quality control of herbal medicines by capillary electrophoresis: Potential, requirements and applications. Electrophoresis 29:3489–503
- Gray MJ, Chang D, Zhang Y, et al. (2010). Development of liquid chromatography/mass spectrometry methods for the quantitative analysis of herbal medicine in biological fluids: A review. Biomedl Chromatogr 24:91–103
- Guo XR, Chen XH, Li L, et al. (2008). LC-MS determination and pharmacokinetic study of six phenolic components in rat plasma after taking traditional Chinese medicinal-preparation: Guanxinning lyophilized powder for injection. J Chromatogr B – Anal Technol Biomed Life Sci 873:51–8
- Hager TJ, Howard LR, Liyanage R, et al. (2008). Ellagitannin composition of blackberry as determined by HPLC-ESI-MS and MALDI-TOF-MS. J Agricul Food Chem 56:661–9
- Huang WY, Cai YZ, Xing J, et al. (2008). Comparative analysis of bioactivities of four Polygonum species. Planta Med 74:43–9
- Jia N, Xiong YL, Kong B, et al. (2012). Radical scavenging activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on gastric cancer cell proliferation via induction of apoptosis. J Functional Foods 4:382–90
- Kesting JR, Huang J, Sorensen D. (2010). Identification of adulterants in a Chinese herbal medicine by LC-HRMS and LC-MS-SPE/NMR and comparative in vivo study with standards in a hypertensive rat model. J Pharmaceut Biomed Anal 51:705–11
- Komes D, Belščak-Cvitanović A, Horžić D, et al. (2011). Phenolic composition and antioxidant properties of some traditionally used medicinal plants affected by the extraction time and hydrolysis. Phytochem Anal 22:172–80
- Liang QL, Qu J, Luo GA, Wang YM. (2006). Rapid and reliable determination of illegal adulterant in herbal medicines and dietary supplements by LC/MS/MS. J Pharmaceut Biomed Anal 40:305–11
- Matsui Y, Nakamura S, Kondou N, et al. (2007). Liquid chromatography-electrospray ionization-tandem mass spectrometry for simultaneous analysis of chlorogenic acids and their metabolites in human plasma. J Chromatogr B Anal Technol Biomed Life Sci 858:96–105
- Mattila PH, Hellström J, Mcdougall G, et al. (2011). Polyphenol and vitamin C contents in European commercial blackcurrant juice products. Food Chem 127:1216–23
- Mellano MG, Beccaro GL, Donno D, et al. (2012). Castanea spp. biodiversity conservation: Collection and characterization of the genetic diversity of an endangered species. Genet Resour Crop Evol 59:1727–41
- Mok DKW, Chau FT. (2006). Chemical information of Chinese medicines: A challenge to chemist. Chemometr Intel Lab Sys 82:210–17
- Moller C, Hansen SH, Cornett C. (2009). Characterisation of tannin-containing herbal drugs by HPLC. Phytochem Anal 20:231–9
- Oksman-Caldentey KM, Inze D. (2004). Plant cell factories in the post-genomic era: New ways to produce designer secondary metabolites. Trends Plant Sci 9:433–40
- Ordre national des pharmaciens. (1965). Monographie: préparations homéopathiques. In: Codex medicamentarius gallicus, codex français: pharmacopée française/rédigé par ordre du gouvernement Ministére de la santé publique et de la population. 8th ed. Paris: Ordre national des pharmaciens; 1965
- Peev CI, Vlase L, Antal DS, et al. (2007). Determination of some polyphenolic compounds in buds of Alnus and Corylus species by HPLC. Chem Nat Comp 43:259–62
- Qiao X, Han J, He WN, et al. (2010). Chemical fingerprint of commercial Radix Echinopsis and quantitative analysis of alpha-terthienyl. J Separation Sci 33:530–8
- Steinmann D, Ganzera M. (2011). Recent advances on HPLC/MS in medicinal plant analysis. J Pharmaceut Biomed Ana 55:744–57
- Surveswaran S, Cai YZ, Xing J, et al. (2010). Antioxidant properties and principal phenolic phytochemicals of Indian medicinal plants from Asclepiadoideae and Periplocoideae. Nat Prod Res 24:206–21
- Tabart J, Franck T, Kevers C, et al. (2012). Antioxidant and anti-inflammatory activities of Ribes nigrum extracts. Food Chem 131:1116–22
- Tabart J, Kevers C, Evers D, Dommes J. (2011). Ascorbic acid, phenolic acid, flavonoid, and carotenoid profiles of selected extracts from Ribes nigrum. J Agric Food Chem 59:4763–70
- Tsao R, Yang R. (2003). Optimization of a new mobile phase to know the complex and real polyphenolic composition: Towards a total phenolic index using high-performance liquid chromatography. J Chromatogr A 1018:29–40
- Uchiyama N, Kikura-Hanajiri R, Kawahara N, Goda Y. (2009). Identification of a cannabimimetic indole as a designer drug in a herbal product. Forensic Toxicol 27:61–6
- Urpi-Sarda M, Monagas M, Khan N, et al. (2009). Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1216:7258–67
- Yi T, Chen HB, Zhao ZZ, et al. (2009). Identification and determination of the major constituents in the traditional Uighur medicinal plant Saussurea involucrata by LC-DAD-MS. Chromatographia 69:537–42
- Zhang L, Shu X, Ding A, et al. (2009). LC-DAD-ESI-MS-MS separation and chemical characterization of the inflammatory fraction of the roots of Euphorbia kansui. Chromatographia 70:805–10
- Zhao ZY, Dong LL, Lin F. (2009). Fingerprint analysis of Euonymus alatus (Thuhb) siebold by LC-DAD and LC-ESI-MS. Chromatographia 69:429–36
- Zhou Y, Jiang SY, Ding LS, et al. (2008). Chemical fingerprinting of medicinal plants “Gui-jiu” by LC-ESI multiple-stage MS. Chromatographia 68:781–9