Abstract
Context: Bovine pancreatic trypsin inhibitor (BPTI) has been reported to relieve liver ischemia-reperfusion-induced injury in rats.
Objective: This study was designed to determine whether the recombinant BPTI (rBPTI) can prevent the chronic liver injury induced by CCl4 in rats.
Materials and methods: Fifty male Wistar rats were divided into five groups. Rats were treated with 40% CCl4 at a dose of 2 ml/kg body weight twice a week subcutaneously for 12 weeks. In the 8th week, they were administered intraperitoneally with rBPTI (80 MU/kg), BPTI (80 MU/kg) or hepatocyte growth-promoting factor (pHGF; 100 mg/kg) daily for the next 4 weeks.
Results: rBPTI significantly prevented the disruption of liver function of alanine aminotransferase (ALT; 172.7 ± 18.16 versus 141.2 ± 15.28, p = 0.003), aspartate aminotransferase (AST; 225.10 ± 36.54 versus 170.06 ± 27.14, p = 0.007) and hydroxyproline (Hyp; 1.14 ± 0.27 versus 0.62 ± 0.17, p = 0.001). rBPTI significantly decreased the level of thiobarbituric acid reactive substances (TBARS; 1.15 ± 0.16 versus 0.87 ± 0.15, p = 0.003) and increased the activities of superoxide dismutase (SOD; 6.07 ± 0.95 versus 7.75 ± 1.12, p = 0.007). rBPTI reduced the production of cytokines of IL-1β and TGF-β. The hepatocyte necrosis, fibrosis, fatty degeneration and inflammatory cell infiltration were ameliorated by rBPTI administration.
Conclusion: This study demonstrated that rBPTI exerted a hepatoprotective effect on chronic liver fibrosis induced by CCl4, which suggests that rBPTI may have the potential application for chronic liver injury induced by drugs metabolism and toxic substances.
Introduction
Bovine pancreatic trypsin inhibitor (BPTI), a 58 amino acid basic protein, is produced in several organs, such as lung, pancreas or parotid glands in bovine (Antuch et al., Citation1993; Pritchard & Dufton, Citation1999). Endogenous BPTI not only inhibits trypsin, but acts as an inhibitor for chymotrypsin, plasmin and kallikreine (Barbar et al., Citation2001; Hagihara et al., Citation2002). BPTI was applied as a potential treatment for acute pancreatitis and coagulation disorders, and hemorrhage in surgery (Bull et al., Citation2000; Molenaar et al., Citation2001). BPTI also demonstrated an inhibiting role of protease inhibitors in inflammation. Ulinastatin, a human trypsin inhibitor, was able to suppress endotoxin-induced thromboxane B2 production in human monocytes (Aibiki & Cook, Citation1997). Another protease inhibitor, urinary trypsin inhibitor (UTI) was shown to protect against bacterial endotoxin-induced systemic inflammatory response and decrease subsequent organ injury (Inoue et al., Citation2005a) and acute lung injury (Inoue et al., Citation2005b) through preventing expression of pro-inflammatory cytokines and chemokines. However, the anti-inflammatory effects of BPTI on patients with cardiopulmonary bypass surgery are controversial (Englberger et al., Citation2002; Mojcik & Levy, Citation2001; Schmartz et al., Citation2003; Tassani et al., Citation2000). Our previous study showed that recombinant BPTI (rBPTI) could be produced in the eukaryotic expression system-Pichia pastoris (Yang et al., Citation2008), and it has the same activity as the one that was prepared from the natural source such as bovine lung. We observed that rBPTI had a potential protective effect on acute liver injury in mouse (Yang et al., Citation2010). In the present study, we investigated whether there is a potential hepatoprotective effect of rBPTI on chronic liver injury induced by CCl4 in rats.
Materials and methods
Reagents
CCl4 was purchased from Beijing Chemistry Plant (Beijing, China). Hepatocyte growth-promoting factor (pHGF) was from Yangjiang Pharmaceutical Company (Guangdong, China). Alanine aminotransferase (ALT), aspartate aminotransferase (AST) and albumin (Alb) reagents kits are products of Beijing Chemistry Reagent Company (Beijing, China). The hydroxyproline (Hyp), thiobarbituric acid reactive substances (TBARS) and superoxide dismutase (SOD) detection kits were obtained from Jiancheng Institute of Biotechnology (Nanjing, China).
Preparation of the recombinant BPTI
The production of rBPTI is described in our previous publications (Yang et al., Citation2008). Briefly, in a eukaryotic expression system-Pichia pastoris, with the human serum albumin as a signal peptide, rBPTI was expressed, which has the same N-termnal as the natural BPTI does. The rBPTI was produced by the large-scale fermentation. After the fermentation, the rBPTI was purified using cation exchange chromatography and reverse-phase chromatography. The purity of rBPTI was higher than 95% and the purified coefficient was more than 60%. The purified rBPTI was evaporated using a rotary evaporator under reduced pressure at 50 ± 1 °C and the water extract was freeze-dried into powder.
Animals and treatments
Fifty 7-week old male Wistar rats (Weight: 215 ± 15 g) were purchased from Changchun High-tech Animal Medical Center (Changchun, China). The animals were housed in a controlled environment at 25 ± 1 °C and 50 ± 5% relative humidity under a 12-h light cycle. All experiments were carried out in accordance with the guidelines of the Animal Care and Use Committee of Tianjin Medical University. The rats were divided into five groups: Saline (group 1); Saline/CCl4 (group 2); pHGF/CCl4 (group 3); BPTI/CCl4 (group 4) and rBPTI/CCl4 (group 5). The rats were treated subcutaneously with 40% (v/v) CCl4 dissolved in corn oil at a dose of 2 ml/kg body weight twice a week for 12 weeks. The rats in saline group received the corn oil only. In the 8th week, the rats were administered intraperitoneally with rBPTI (80 MU/kg), BPTI (80 MU/kg) and pHGF (100 mg/kg) every day for the next 4 weeks. In saline and saline/CCl4 groups, the rats received the saline (5 ml/kg) only. During the administration of rBPTI, BPTI, pHGF and saline, the rats still kept the same administration of 40% (v/v) CCl4 for the next 4 weeks.
Determination of liver enzyme and components
After 12 weeks, the animals were weighed and sacrificed under ether anesthesia. Blood was collected from the abdominal aorta, the plasma samples were separated and frozen at −80 °C. The livers were perfused through the portal vein with ice-cold 0.15 M KCl for 5 min to remove blood as much as possible, isolated and weighed (Ohta & Sahashi, Citation2002) and followed by snap frozen in liquid nitrogen and stored at −80 °C. Plasma levels of ALT, AST and albumin were detected according to the protocol of kit and measured in the autoanalyser (AU2700; Olympus, Tokyo, Japan). For detecting the levels of Hyp, TBARS and SOD, the liver was homogenized in 0.15 M KCl and centrifuged at 3000 rpm/min for 20 min at 4 °C and collected the supernatant for analysis (Sarban et al., Citation2005).
Real-time RT-PCR
Total RNA was extracted from livers using the RNeasy Mini Kit (Qiagen, Chatsworth, CA). Reverse transcription was performed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Branchburg, NJ) in accordance with the manufacturer’s suggestions. Total RNA (1 μg) was reverse transcribed (RT) in a final volume of 20 μl containing 2 μl of 10 × RT buffer; 0.8 μl of 25 × dNTP Mix, 10 × RT Random primers; 1 μl of RNase inhibitor and 1 μl of reverse transcriptase. Quantitative real-time polymerase chain reaction (Q-PCR) was performed in a final volume of 20 μl containing 1 μl primer and probe (Applied Biosystems), 10 μl of Taqman mixture and 2 μl of cDNA. The mRNA levels of IL-1β (NM-008361), transforming growth factor (TGF)-β (NM-011577) and tumor necrosis factors (TNF-α; NM-013693) were assayed using a 7900HT fast real time PCR system. All samples were tested in triplicate in the same plate. We used GAPDH as the internal standard. Thermal cycle conditions were as follows: hold at 95 °C for 10 min, then repeat 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The expression level for each sample was calculated with the ΔΔCt method.
Pathological examination
Liver specimens were fixed in 10% buffered formalin and embedded in paraffin for histological analysis. Liver sections were stained with hematoxylin and eosin (H&E) and evaluated with light microscopy. The incidence and the degree of liver fibrosis, necrosis, fatty degeneration and inflammatory cell infiltrate were scored as normal (0), slight (1), moderate (2) or severe (3) (Frei et al., Citation1984).
Statistical analysis
To analyze post hoc multiple comparisons, one-way analysis of variance and Student--Newman–Keuls test were used. Statistical analysis was performed using the SPSS software (SPSS Inc, Chicago, IL). Liver pathological examination data were analyzed by the Kruskal–Wallis non-parametric test, followed by the Mann--Whitney test. All data obtained are presented as mean ± SD. Values of p < 0.05 were considered significant.
Results
rBPTI ameliorates the disruption of liver function
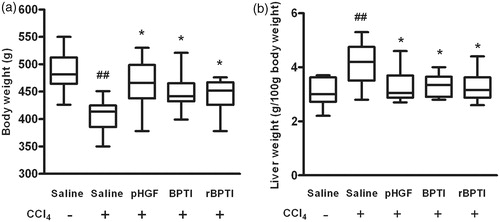
The weights of the rats and liver were measured at last administration. It displayed that the body weight significantly decreased and the liver weight significantly increased in saline/CCl4 group than those in saline group. However, this loss of body weight () significantly increased (407.20 ±29.04 versus 444.10 ± 32.07, p = 0.017) and this swelling of the liver () significantly decreased (4.12 ± 0.83 versus 3.28 ± 0.53, p = 0.023) in the rats co-treated by rBPTI.
Figure 1. Effect of rBPTI on body weight and relative liver weight in rats. rBPTI: ecombinant bovine pancreatic trypsin inhibitor; pHGF: hepatocyte growth-promoting factors. The rats were treated with 40% (v/v) CCl4 dissolved in corn oil subcutaneously at a dose of 2 ml/kg body weight twice a week for 12 weeks. In the 8th week, the rats were treated with rBPTI (80 MU/kg), BPTI (80 MU/kg) and pHGF (100 mg/kg) intraperitoneally every day for the next 4 weeks. In saline and saline/CCl4 groups, the rats received the saline (5 ml/kg) only. During the administration of rBPTI, BPTI, pHGF and saline, the rats still received the same administration of 40% (v/v) CCl4 for the next 4 weeks. Data are shown as mean ± SD (n = 10). ##p < 0.01 compared with saline group (group 1). *p < 0.05 compared with saline/CCl4 group (group 2).
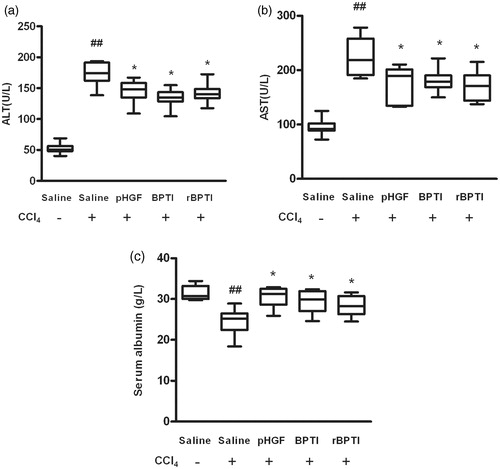
Next, the effect of rBPTI on preventing the disruption of the liver function was performed. As markers of liver function, the levels of ALT and AST were determined. The levels of ALT () and AST () significantly increased in rats treated with CCl4, which decreased in the rats co-treated by rBPTI (ALT: 172.7 ± 18.16 versus 141.2 ± 15.28, p = 0.003; AST: 225.10 ± 36.54 versus 170.06 ± 27.14, p = 0.007). The level of albumin was also determined () in the rats. The level of albumin significantly decreased in rats treated with CCl4, which increased (24.44 ± 3.26 versus 28.35 ± 2.45, p = 0.031) in the rats co-treated by rBPTI. These data suggest that rBPTI was able to preserve liver function in rats induced by CCl4.
Figure 2. Effect of rBPTI on activities of serum ALT and AST and albumin in rats. ALT, alanine aminotransferase; AST, aspartate aminotransferase; see for the treatment. Data are shown as mean ± SD (n = 10). ##p < 0.01 compared with saline group (group 1). *p < 0.05 compared with saline/CCl4 group (group 2).
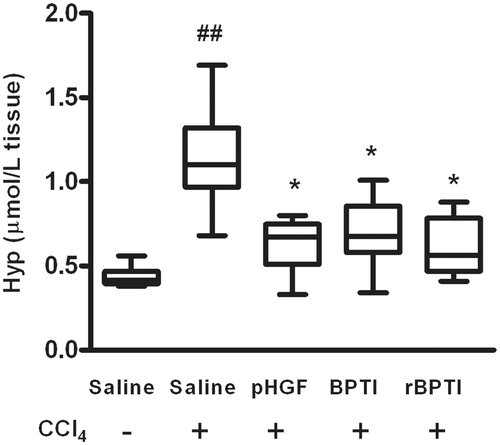
To evaluate the effect of rBPTI on liver fibrosis, we detected the Hyp content of the rats. Hyp contents were significantly higher in rats treated with CCl4, which decreased (1.14 ± 0.27 versus 0.62 ± 0.17, p = 0.001) in the rats co-treated by rBPTI ().
rBPTI reduces oxidative stress in rats
CCl4 has been shown to induce oxidative stress, leading to lipid peroxidation and free radical production, which plays an important pathological role in liver injury (Korabiowska et al., Citation2004). The effect of rBPTI on lipid peroxidation products, measured as TBARS in the liver, is shown in . The level of TBARS was significantly higher in rats treated with CCl4, which decreased (1.15 ± 0.16 versus 0.87 ± 0.15, p = 0.003) in rats co-treated with rBPTI ().
Figure 4. Effect of rBPTI on liver TBARS and SOD activity in rats. TBARS, thiobarbituric acid reactive substance; SOD, superoxide dismutase; see for the treatment. Data are shown as mean ± SD (n = 10). ##p < 0.01 compared with saline group (group 1). *p < 0.05 compared with saline/CCl4 group (group 2).
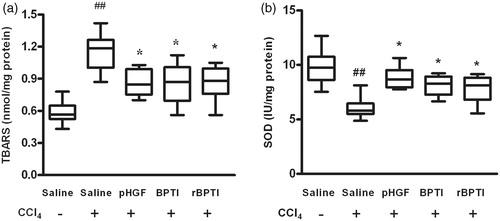
SOD is a scavenger of peroxide anion radicals and could inhibit the initiation of lipid peroxidation by free radicals. To evaluate oxidative status, we detected the activity of SOD. The activity of SOD in the homogenate significantly decreased in rats treated with CCl4 and this decrease was prevented (6.07 ± 0.95 versus 7.75 ± 1.12, p = 0.007) in rats co-treated by rBPTI ().
rBPTI reduces the production of pro-inflammatory cytokine
Some pro-inflammatory cytokines play an important role in the pathophysiology of liver diseases, so we investigated the mRNA expression level of some cytokines such as IL-1β, TGF-β and TNF-α. The mRNA expression levels of IL-1β and TGF-β significantly increased in rats treated with CCl4, which decreased in the rats co-treated by rBPTI (). The rats treated with CCl4 demonstrated a modest increase in mRNA expression of TNF-α and this increase was partially prevented in the rats co-treated by rBPTI. However, there was no significance (p > 0.05; ).
Table 1. Effects of rBPTI on cytokines levels in rats treated with carbon tetrachloride.
rBPTI prevented CCl4-induced chronic liver injury
To determine the hepatoprotective effect of rBPTI, we investigated the histological changes of the liver. CCl4 treatment induced various histological changes in the liver, including liver fibrosis, gross hepatocyte necrosis, massive fatty degeneration and broad inflammatory cell infiltration (). To access these histopathological changes, liver injury and inflammation were scored by pathologists blinded to the treatment (). CCl4 induced mild to severe liver injury, including hepatocellular necrosis, fatty degeneration and inflammatory cell infiltration, in 100% of rats. Co-treatment with rBPTI significantly increased the grade of the scores compared with the rats in saline/CCl4 group.
Figure 5. rBPTI reduces carbon tetrachloride-induced liver damage in rats. Paraffin-embedded liver tissue sections were stained with H&E for light microscopic assessment of liver damage (200 × magnification). Images shown are representative of 10 rats in each group.
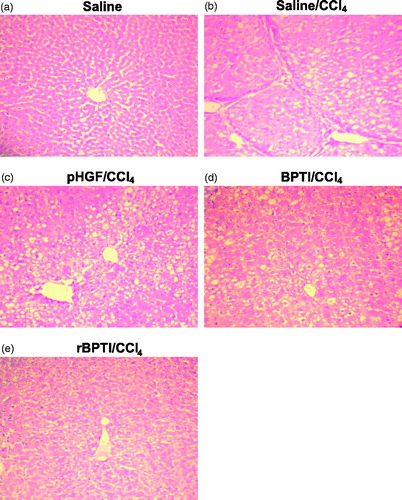
Table 2. rBPTI protects carbon tetrachloride-induced chronic liver damage.
Discussion
The present study demonstrated that CCl4 induced severe chronic liver damage and disrupted liver function in rats, which was significantly alleviated by rBPTI administration. It indicated that rBPTI may exert a hepatoprotective effect in chronic liver fibrosis.
As a broad-spectrum serine protease inhibitor, BPTI can inhibit the release of inflammatory mediators, prevent cytokine cascade reaction and inhibit leukocyte excessive activation. BPTI was observed to antagonize the activation of coagulation – fibrinolysis – complement – bradykinin system, thereby improve the metabolic state of the cell under hypoxic conditions and protect the cells (Molenaar et al., Citation2001). By inhibiting the inflammatory response of the liver, BPTI could inhibit the activation of liver fibrosis-related cells by reducing inflammatory cells to secrete inflammatory mediators, and further suppress collagen synthesis, and reduce the formation of liver fibrosis.
CCl4 is one of the best-studied hepatotoxicants, which induces liver injury in many species, including humans and non-human primates. Rats treated with CCl4 were used to serve as an in vivo halogenated hydrocarbon-induced liver injury model (Korabiowska et al., Citation2004). In the present study, CCl4 stimulated oxidative stress, as indicated by the elevated level of TBARS and the decreased level of SOD activity in the liver of the rats, which would further induce lipid peroxidation, initial free radical damage to the hepatocyte membrane and eventually lead to the liver injury (Andus et al., Citation1991). Our results demonstrated that TBARS content, an index of lipid peroxidation, increased in the CCl4 treated rats. The increased TBARS was partially attenuated by treatment with rBPTI in the rats with chronic liver injury. This result suggested that the hepatoprotective effect of rBPTI might be due to the suppression of lipid peroxidation since rBPTI can prevent the increase in TBARS content induced by CCl4. SOD is a scavenger of peroxide anion radicals and could inhibit the initiation of lipid peroxidation by free radicals. The decrease in SOD activity in CCl4-induced rats was prevented by rBPTI treatment. Our observation indicated that rBPTI treatment could reduce CCl4-induced chronic liver injury through its antioxidant activity.
CCl4 intoxication is strongly characterized by the expression of numerous cytokines, which plays an important role in the pathophysiology of liver diseases (Raghow et al., Citation1987; Roberts et al., Citation1985). We examined the mechanisms underlying the antifibrotic effect, and found that rBPTI administration reduced the mRNA level of IL-1β and TGF-β, which were profibrogenetic cytokines. IL-1β is a very important cytokine in inflammatory and growth processes and plays a significant role in the development of fibrosis. TGF-β, another important cytokines elaborated during the inflammatory phase (Roberts et al., Citation1985) and the potent fibrogenic cytokine (Varga & Jimenez, Citation1986). TGF-β can trigger a significant increase in the synthesis and secretion of extracellular matrix proteins such as collagen and fibronectin genes (Raghow et al., Citation1987). Taken together, the present study supported the involvement of rBPTI in suppression of the oxidative responses in liver cells and inhibition of systemic pro-inflammatory cytokine production as a mechanism of rBPTI preventing liver inflammation and injury.
Based on our previous publication (Yang et al., Citation2010), we used the dosages of 80 MU/kg for the rBPTI and BPTI in this study. The results showed that rBPTI performed the protective effect on chronic liver injury in rats. However, we should consider the side effects of rBPTI in clinical applications. For example, BPTI has been reported to induce anaphylactic reactions and anaphylactic shock in patients (Laxenaire et al., Citation2000; Mycek et al., Citation1998). To reduce the side effects of rBPTI in the future trials, a series of studies should be performed, such as selecting the correct dosage and evaluating the patients’ physiological conditions and combination with other medications, which may improve the efficacy of rBPTI to treatment of liver injury.
In conclusion, our study has demonstrated that rBPTI exerted a hepatoprotective effect on chronic liver injury induced by CCl4. Thus, rBPTI can serve as a potential role in the prevention of liver fibrosis induced by metabolism of drugs and toxic substances.
Declaration of interest
This work was supported by grants from the Health Department Fund project of Tianjin, China (No. 09KZ80). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Aibiki M, Cook JA. (1997). Ulinastatin, a human trypsin inhibitor, inhibits endotoxin-induced thromboxane B2 production in human monocytes. Crit Care Med 25:430–44
- Andus T, Bauer J, Gerok W. (1991). Effects of cytokines on the liver. Hepatology 13:364–75
- Antuch W, Berndt KD, Chávez MA, et al. (1993). The NMR solution structure of a Kunitz-type proteinase inhibitor from the sea anemone Stichodactyla helianthus. Eur J Biochem 212:675–84
- Barbar E, Hare M, Makokha M, et al. (2001). NMR-detected order in core residues of denatured bovine pancreatic trypsin inhibitor. Biochemistry 40:9734–42
- Bull DA, Connors RC, Albanil A, et al. (2000). Aprotinin preserves myocardial biochemical function during cold storage through suppression of tumor necrosis factor. J Thorac Cardiovasc Surg 119:242–50
- Englberger L, Kipfer B, Berdat PA, et al. (2002). Aprotinin in coronary operation with cardiopulmonary bypass: Does “low-dose” aprotinin inhibit the inflammatory response? Ann Thorac Surg 73:1897–904
- Frei A, Zimmermann A, Weigand K. (1984). The N-terminal propeptide of collagen type III in serum reflects activity and degree of fibrosis in patients with chronic liver disease. Hepatology 4:830–4
- Hagihara Y, Shiraki K, Nakamura T, et al. (2002). Screening for stable mutants with amino acid pairs substituted for the disulfide bond between residues 14 and 38 of bovine pancreatic trypsin inhibitor (BPTI). J Biol Chem 277:51043–8
- Inoue K, Takano H, Shimada A, et al. (2005a). Urinary trypsin inhibitor protects against systemic inflammation induced by lipopolysaccharide. Mol Pharmacol 67:673–80
- Inoue K, Takano H, Yanagisawa R, et al. (2005b). Protective role of urinary trypsin inhibitor in acute lung injury induced by lipopolysaccharide. Exp Biol Med (Maywood) 230:281–7
- Korabiowska M, Schlott T, Siems N, et al. (2004). Analysis of adenomatous polyposis coli gene expression, APC locus-microsatellite instability and APC promoter methylation in the progression of melanocytic tumours. Mod Pathol 17:1539–44
- Laxenaire MC, Dewachter P, Pecquet C. (2000). Allergic risk of aprotinin. Ann Fr Anesth Reanim 19:96–104
- Molenaar IQ, Begliomini B, Grazi GL, et al. (2001). The effect of aprotinin on renal function in orthotopic liver transplantation. Transplantation 71:247–52
- Mojcik CF, Levy JH. (2001). Aprotinin and the systemic inflammatory response after cardiopulmonary bypass. Ann Thorac Surg 71:745–54
- Mycek MA, Schomacker KT, Nishioka NS. (1998). Colonic polyp differentiation using time-resolved autofluorescence spectroscopy. Gastrointest Endosc 48:390–4
- Ohta Y, Sahashi D. (2002). .-Tryptophan administration promotes the reversion of pre-established chronic liver injury in rats treated with carbon tetrachloride. J Nutr Biochem 13:550
- Pritchard L, Dufton MJ. (1999). Evolutionary trace analysis of the Kunitz/BPTI family of proteins: Functional divergence may have been based on conformational adjustment. J Mol Biol 285:1589–607
- Raghow R, Postlethwaite AE, Keski-Oja J, et al. (1987). Transforming growth factor-beta increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J Clin Invest 79:1285–8
- Roberts AB, Anzano MA, Wakefield LM, et al. (1985). Type beta transforming growth factor: A bifunctional regulator of cellular growth. Proc Natl Acad Sci USA 82:119–223
- Sarban S, Kocyigit A, Yazar M, Isikan UE. (2005). Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clin Biochem 38:981–6
- Schmartz D, Tabardel Y, Preiser JC, et al. (2003). Does aprotinin influence the inflammatory response to cardiopulmonary bypass in patients? J Thorac Cardiovasc Surg 125:184–90
- Tassani P, Augustin N, Barankay A, et al. (2000). High-dose aprotinin modulates the balance between proinflammatory and anti-inflammatory responses during coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 14:682–6
- Varga J, Jimenez SA. (1986). Stimulation of normal human fibroblast collagen production and processing by transforming growth factor-beta. Biochem Biophys Res Commun 138:974–80
- Yang L, Dong W, He J, et al. (2008). Expression and purification of natural N-terminal recombinant bovine pancreatic trypsin inhibitor from Pichia pastoris. Biol Pharm Bull 31:1680–5
- Yang L, Dong W, Yan F, et al. (2010). Recombinant bovine pancreatic trypsin inhibitor protects the liver from carbon tetrachloride-induced acute injury in mice. J Pharm Pharmacol 62:332–8