Abstract
Context: Longkong [Lansium domesticum Corr. (Meliaceae)] is a popular tropical plant producing economic edible fruits found mainly in Southeast Asia. However, limited information is available concerning anticancer activity of Longkong.
Objective: To investigate anticancer activities in human mouth epidermal carcinoma (KB) of Longkong extracts.
Materials and methods: Various parts of Longkong which was collected from Northern and Eastern of Thailand were extracted by the hot and cold processes using water, chloroform, and methanol. The extracts were tested for anti-oxidative activities and anti-proliferation as well as matrix metalloproteinase inhibition on KB cells.
Results: The hot water extract of seeds from Northern region (NSEWH), the cold water extract of old leaves from Northern region (NOLWC), and the hot chloroform extract of young leaves from Eastern region (EYLCH) showed the highest free radical scavenging, metal ion chelating, and lipid peroxidation inhibition with SC50, MC50 and IPC50 values of 0.34 ± 0.03, 0.47 ± 1.60 and 0.86 ± 0.31 mg/ml, respectively. The hot and cold chloroform extract of young fruits from Northern region (NYFCH and NYFCC) exhibited anti-proliferation effect against KB cells with IC50 values of 603.45 ± 55.35 and 765.06 ± 46.19 mg/ml, respectively. NYFCC exhibited the highest pro- and active MMP-2 inhibition at 53.03 ± 2.65 and 31.30 ± 0.43%, more than all tested standard anticancer drugs except cisplatin.
Discussion and conclusion: The cold chloroform extract of young fruits from Northern region appeared to contain anticancer active compounds against KB cells because of its high anti-proliferation and MMP-2 inhibition activities.
Introduction
Lansium domesticum Corr. (Meliaceae) is the popular tropical plant producing economic edible fruits found mainly in Southeast Asia. This plant is known locally, depending on variety, as Longkong, Langsat, and Duku in Thai. The activities of L. domesticum have been investigated for medical application. The tetranortriterpenoids from the seeds of L. domesticum show antimalarial activity against Plasmodium falciparum with IC50 values of 2.4–9.7 µg/ml (Saewan et al., Citation2006). Its skin and aqueous leaf extracts were found to reduce parasite populations equally of the drug sensitive strain (3D7) and the chloroquine-resistant strain (T9) of P. falciparum (Yapp & Yap, Citation2003). However, no work has been performed on anticancer activity of its extracts, especially on human mouth epidermal carcinoma. Cancer is the major public health burden both in the developed and developing countries. The discovery of novel anticancer agents from natural sources was largely based on the testing of cytotoxic activity against cancer cell lines in vitro and animal cancer models in vivo. In our previous study, the extract of Longkong young fruit by hot chloroform (YFCH) demonstrated apoptosis against KB and HT-29 cell lines of 13.84 ± 4.21 and 8.68 ± 1.85%, respectively (Manosroi et al., Citation2012). MMPs (matrix metalloproteinases) can promote cancer progression by increasing cancer-cell growth, migration, invasive, metastasis, and angiogenesis. Thus, the study of the role of MMPs in cancer metastasis has been greatly expanded. The two metalloproteinases including MMP-2 and MMP-9 (72 kDa and 92 kDa type IV collagenases or gelatinase A and gelatinase B, respectively) have been associated with the malignant phenotype of tumor cells because of their unique ability to degrade type IV collagen, which is a major component of the basement membrane. Usually, high levels of the active form of MMPs in almost all carcinomas was observed. Hrabec et al. (Citation2002) have demonstrated that the MMP-9 levels in lung cancer were over two-fold higher than in normal lung tissues. However, there are no reports on the anti-proliferative and the matrix metalloproteinase inhibition activities of the Longkong extracts.
In the present study, the in vitro anticancer activities in human mouth epidermal carcinoma cell lines (KB) including anti-proliferation and matrix metalloproteinase inhibition of the extracts from various parts of Longkong were investigated in order to evaluate their potential for cancer treatment.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin, and streptomycin were obtained from GIBCO (Grand Island, NY). Acrylamide (minimum 99%) and glycerol were purchased from Sigma Chemicals Co. (St. Louis, MO). Ammonium sulfate and glycine from BDH Limited, Poole, England and trifluoroactic acid and sodium chloride from Merck, Darmstadt, Germany were used. Tris (hydroxymethyl) methylamine was purchased from Fisher Scientific UK Limited, Loughborough, UK. N,N′-methylenebisacrylamide and TEMED (N,N,N′,N′-tetramethyl ethylenediamine) from Fluka (Virginia, VA), sodium dodecyl sulfate and Coomassie brilliant blue R-250 from Bio-Rad Laboratories, Hemel Hempstead, UK, and bromophenol blue dye and ammonium persulfate from Amersco Inc., Solon, OH were used. Methanol, ethanol, chloroform, and gracial acetic acid were analytical grade solvents.
Plant materials
From the screening test, parts of Longkong were selected from the two regions of Thailand (the Northern region form Uttaradit province and the Eastern region from Chantraburi province) during October to November 2010. The specimen was authenticated by a botanist (Ms. Suda Saowakon) at NPRDC and deposited at Chiang Mai University in Thailand (voucher specimen no. NPRDC2010LK9-24). Eight parts of Longkong including old fruits (OF), young fruits (YF), old leaves (OL), young leaves (YL), seeds (SE), peels (PE), stalks (ST), and branches (BR) were collected.
Preparation of the extracts
All Longkong parts were washed, dried at 50 ± 2 °C, ground into powder and extracted by the hot and cold processes using three different solvents (water, methanol, and chloroform). For the hot process, the dried powder was boiled with solvents at the boiling points [water (100 ± 2 °C), methanol (65 ± 2 °C) and chloroform (60 ± 2 °C)] for 1 h. For the cold process, the dried powder was mixed with solvents and sonicated in a sonicator bath (Crest ultrasonics corp., Ewing Township, NJ) at ambient temperature (27 ± 2 °C) for 1 h. Each extraction was repeated three-times. The mixture from both extraction processes (hot and cold methods) was filtered, pooled, and dried with a rotary evaporator (Rotavapor R210, Buchi, Flawil, Switzerland) under vacuum at 50 °C and lyophilized. The percentage yield was calculated on a dry weight basis. The obtained crude extracts were kept at 4 °C in a tight container until use. Descriptions and the extract codes of each Longkong sample are presented in .
Table 1. Comparison of the percentage yields of the 85 extracts from 8 parts of Longkong prepared by water, methanol and chloroform with cold and hot processes from the Northern and Eastern region of Thailand.
Determination of the total phenolic and flavonoid contents
The total phenolic contents of the extracts were determined by the modified Folin-Ciocalteu assay (Tepe & Sokman, Citation2007). Briefly, 5 µl of each sample dissolved in DMSO at the concentration of 10 mg/ml were mixed with 50 µl of Folin-Ciocalteu reagent, and adjusted to the total volume of 200 µl with distilled water. After vigorous shaking in dark at room temperature for 2 h, 25 µl of 2% (v/v) Na2CO3 were added and mixed. The absorbance was measured at 760 nm by a well reader. The total phenolic contents were quantified by the calibration standard curve obtained from various known concentrations of gallic acid [absorbance = 0.7276 gallic acid (mg) + 0.0695; R2 = 0.9979]. The concentrations were expressed as mg of gallic acid equivalents (GAE)/g of the extracts. All measurements were done in triplicate.
The total flavonoid contents of each extract were determined by a colorimetric method as previously described with some modifications (Ardestani & Yazdanparast, Citation2007). Briefly, 20 µl of the appropriately diluted sample (20 mg/ml dissolved in MeOH) were mixed with 80 µl of distilled water and followed by the immediate addition of 6 µl of 15% (w/v) NaNO2 and 6 µl of 10% (w/v) AlCl3. After 5 min, 80 µl of 4% (w/v) NaOH were added. After 1 min, 8 µl of water were immediately added to adjust the final volume to 200 µl. Then, the mixture was thoroughly mixed with shaking for 15 min at room temperature (27 ± 2 °C). The absorbance of the mixture was determined at 510 nm with a plate reader. The total flavonoid content of the samples was expressed as mg of quercetin equivalents (QE)/g of the extracts and calculated by the linear equation based on the calibration curve [absorbance = 0.272 quercetin (mg) + 0.4006; R2 = 0.9951]. All measurements were done in triplicate.
Antioxidant activities
Free radical scavenging assay
Free radical scavenging activities of the extracts was determined by a modified DPPH assay (Manosroi et al., Citation2010b). Briefly, 50 µl of five serial concentrations of the extracts (at 0.001–10 mg/ml) dissolved in the mixture of DMSO and 70%v/v ethanol and 50 µl of ethanol solution of DPPH were put into each well of a 96-well microplate (Nalge Nunc International, Rochester, NY). The reaction mixtures were allowed to stand for 30 min at 27 ± 2 °C, and the absorbance was measured at 515 nm with a plate reader (Bio-Rad, model 680 microplate reader, Philadelphia, PA) against the blank (the mixture of DMSO and 70%v/v ethanol). Ascorbic acid (0.001–10 mg/ml) was used as a positive control. The experiments were done in triplicate. The percentages of free radical scavenging activity were calculated as follows: scavenging (%) = [(A − B)/A] × 100, where A was the absorbance of the control and B was the absorbance of the sample. The sample concentrations providing 50% of scavenging (SC50) were calculated from the graph plotted between the percentages of scavenging and sample concentrations.
Lipid peroxidation inhibition
The antioxidant activity of the extracts was assayed by the modified ferric-thiocyanate method (Manosroi et al., Citation2010b). A total of 50 µl of 5 serial concentrations of the extracts (at 0.01–100 mg/ml) dissolved in DMSO were added to 50 µl of linoleic acid in 50% (v/v) DMSO. The reaction was initiated by the addition of 50 µl of NH4SCN (5 mM) and 50 µl of FeCl2 (2 mM). The mixture was incubated at 37 ± 2 °C in a 96-well microplate for 1 h. During the oxidation of linoleic acid, peroxides are formed leading to the oxidation of Fe2+ to Fe3+. A complex formed with thiocyanate can be detected at 490 nm. The solution without the sample was used as a negative control. Vitamin E (at 0.001–10 mg/ml) was used a positive control. All determinations were performed in triplicate. The inhibition percentages of lipid peroxidation of linoleic acid were calculated by the following equation: Inhibition of lipid peroxidation (%) = [(A − B)/A] × 100, where A was the absorbance of the control and B was the absorbance of the sample. The sample concentrations providing 50% inhibition of lipid peroxidation (IPC50) were calculated from the graph plotted between the percentages of lipid peroxidation inhibition and sample concentrations.
Metal ion chelating assay
The metal ion chelating activity of the extracts was assayed by the modified ferrous ion chelating method (Decker & Welch, Citation1990). Briefly, 100 µl of 5 serial concentrations of the samples (0.001–10 mg/ml) dissolved in DMSO were added to the solution of 2 mM FeCl2 (50 µl) in distilled water. The reaction was initiated by the addition of 5 mM ferrozine (50 µl) and the total volume was adjusted to 300 µl by distilled water. Then, the mixture was left at room temperature (27 ± 2 °C) for 15 min. Absorbance of the resulting solution was then measured at 570 nm with a microplate reader. EDTA (0.001–10 mg/ml) was used as a positive control. FeCl2 and ferrozine, which was the complex formation molecules were used as a negative control. All experiments were performed in triplicate. The percentages inhibition of ferrozine-Fe2+ complex formation were calculated by the following equation: Metal chelating activity (%) = [(A − B)/A] × 100, where A was the absorbance of the negative control and B was the absorbance of the sample. The sample concentration providing 50% metal chelating activity (MC50) was calculated from the graph plotted between the percentages of metal chelating activity and the sample concentrations.
Anti-proliferation of the extracts on human mouth epidermal carcinoma cell lines (KB)
Cell culture
The human epidermal carcinoma cell line (KB) was cultured under standard conditions in complete culture medium containing DMEM supplemented with 10% (v/v) fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml). Cells were incubated in a temperature-controlled, humidified incubator (Shel Lab, model 2123TC, Cornelius, OR) with 5% CO2 at 37 °C.
Anti-proliferation by the SRB assay
The cells were seeded in 96-well plates at an amount of 10 000 cells/well and allowed to attach overnight in 5% CO2 at 37 °C. Then, the cells were exposed to various concentrations of the extracts for 24 h. After incubation, the adherent cells were fixed by adding cold 50%w/v trichloroacetic acid and further incubated for 1 h at 4 °C. Then, the cells were rinsed with distilled water, air-dried and stained with 0.4% SRB in 1% glacial acetic acid for 30 min at room temperature (27 ± 2 °C). The unbound SRB was removed by washing with 1% glacial acetic acid solution for 4 times. After air-drying, 100 µl per well of 10 mM Tris base were added to dissolve the bound stain (Papazisis et al., Citation1997). After mixing, the absorbance was measured at 540 nm with a microplate reader (Biorad, Milan, Italy). The untreated cells were used as a negative control. Anti-proliferation activity (%) was calculated by the following equation (Bouquet et al., Citation2009; Han et al., Citation2009):
The IC50 values were obtained by extrapolating the sample concentrations that inhibit 50% cell viability from the graph plotted between cell viability and the sample concentrations.
Gelatinolytic activity on MMP-2 inhibition zymography
The cells were seeded in 6-well plates at an amount of 5 × 105 cells/well. The monolayer of the cells was maintained in the culture medium without FBS for 24 h, treated with the samples and incubated for 48 h. The culture supernatants were collected to assess to the gelanolytic activities of MMP-2 stimulation.
SDS-PAGE zymography using gelatin as a substrate was performed according to the method previously described with some modifications (Balitaan et al., Citation2010; Kobayashi et al., Citation2003). Briefly, the cell culture supernatant was suspended in the loading buffer [0.125 M Tris (pH 6.8), 4% SDS and 0.04% bromophenol blue, without prior denaturation] and run on the 10% SDS polyacrylamide gel in the presence of 0.1% w/v gelatin. After electrophoresis, gels were washed to remove SDS and incubated for 20 min in the renaturing buffer (50 mM Tris, 5 mM CaCl2, 0.02% NaN3, 2.5% Triton X-100). The gels were then incubated for 24 h at 37 °C in the developing buffer [50 mM Tris (pH 7.5), 5 mM CaCl2, 0.02% NaN3, and 1% Triton X-100]. Gels were subsequently stained with 0.5% Coomassie brilliant blue G-250 and de-stained in 30% methanol and 10% acetic acid (v/v) at room temperature (27 ± 2 °C) to visualize the bands. The gelanolytic activity was then detected as a white band against a blue background (Kim et al., Citation2008). Electrophoretic data were determined by the gel documentation system (Bio-Rad Laboratories, UK) and analyzed by the Quantity 1-D analysis software (Bio-Rad Laboratories, Hemel Hempstead, UK). The area multiplied by the intensity (mm2) of the bands on the gel was determined as the relative MMP-2 content (Carmeliet et al., Citation1997; Manosroi et al., Citation2010a). The percentages of MMP-2 inhibition compared the control (the untreated systems) were calculated by the following equation:
The assays were done in three independent separate experiments. The potency of MMP-2 inhibition of the samples was compared with the standard ascorbic acid.
Statistical analysis
Data were expressed as mean ± SD. Statistical analysis was carried out using the ANOVA using the software SPSS 13.0 (Chicago, IL) for Windows and the p value at less than 0.05 was considered as a statistical significance.
Results and discussion
Percentage yields of the extracts prepared by the six extraction processes
The percentage yields of the 85 extracts prepared by the six extraction processes (CC, CH, MC, MH, WC, and WH) by various raw material sources, solvents, temperatures and parts of the Longkong plant are summarized in . The highest percentage yield of the extracts was from the hot methanol method of the young fruits from the Northern region (NYFMH) at 35.00%, whereas the lowest percentage yield was from the hot chloroform process of the old fruits from the Eastern region (EOFCH) at 0.16%. shows the summation of the percentage yields depending on the sources of Longkong, parts used, the extraction solvents and temperatures of the 85 extracts. The methanol and water extracts shows the significantly higher (p < 0.05) summation of the % yields (15.63 ± 8.75 and 11.83 ± 7.26, respectively) than the chloroform extracts (the summation of the % yields was 5.72 ± 4.68). This indicated that Longkong may contain more polar compounds than the non-polar compounds. The extracts from the hot processes also showed significantly (p < 0.05) higher percentage yields (the summation of the % yields was 12.01 ± 9.32) than those from the cold processes (the summation of the % yields was 9.96 ± 6.61). In fact, the heated solvents have been reported to be able to release the cell wall bioactives by breaking down the cellular constituents and resulting in high percentage yields (Tachibana et al., Citation2001). For the parts of Longkong, the young fruits gave higher yields (the summation of the % yields was 20.20 ± 11.42) than other plant parts. The high yields of the L. domesticum fruit extracts may be from the carbohydrate contents which were the constituents mainly found in the fruit (Morton, Citation1987). However, the percentage yields of the extracts showed no difference between Longkong from the Northern and the Eastern regions.
Total phenolic and flavonoid contents in the extracts
presents the total phenolic and flavonoid contents of the 85 extracts from 8 plant parts of Longkong. The total phenolic content (µg of GAE/g) varied from 0 to 315.67, whereas the total flavonoid content (µg of QE/g) varied from 0 to 171.10. The highest phenolic and flavonoid content were observed in the cold water extract of the branches from the Northern region (NBRWC) and the hot methanol extract of the peels from the Northern region (NPEMH) with the GAE and QE values of 315.67 ± 2.36 and 171.10 ± 0.45 µg, respectively. shows the summation of the total phenolic and flavonoid content depending on the sources of Longkong, parts used, the extraction solvents and temperatures of the 85 extracts. The mean total phenolic content of the extracts was decreased in the order of water > methanol > chloroform. It has been reported that less phenolic amounts can be extracted by chloroform due to the polar nature of the phenolic compounds, but the low-molecular weight phenolic compounds can be readily extracted by methanol (Sun et al., Citation2006). On the contrary, the mean total flavonoid content of the extracts was decreased in the order of chloroform > methanol > water. As known, flavonoids usually exist in two forms of the lipophilic aglycone (without a sugar moiety such as quercetin, apigenin, and luteolin) and the hydrophilic glycoside (with a sugar moiety such as rutin, naringin, and hesperidin). The lipophillic flavonoids in Longkong may be more than the hydrophillic flavonoids and can be extracted more by chloroform than methanol and water. For the extraction temperatures, the extracts by the cold process gave significantly higher (p < 0.05) total phenolic content (the summation of GAE was 106.04) than those by the hot process (the summation of GAE was 75.99). However, there was no significant difference in the total flavonoid content between the cold and hot extracts. The high temperature appeared not to destroy the flavonoid aglycone which agreed with a previous report (Rossi et al., Citation1986). However, the degradation of some compounds in the Longkong extract including phenolics may be accelerated by high temperature (Burg & Fraile, Citation1995; Tomaino et al., Citation2005), thereby possibly changing their structures and activities.
Table 2. Total phenolic and flavonoid contents of the 85 extracts from 8 parts of Longkong.
Antioxidant activities
The biological activities including free radical scavenging, lipid peroxidation inhibition, and metal ion chelating of the 85 extracts from 8 plant parts of Longkong are shown in .
Table 3. Antioxidative activities of the 85 extracts from 8 parts of Longkong.
Free radical scavenging activity
The hot water extract of the seeds from the Northern region (NSEWH) exhibited the highest free radical scavenging activity (SC50 values of 0.34 ± 0.03 µg/ml), but lower than ascorbic acid (SC50 values of 0.08 ± 0.02 µg/ml) by 4.25-times. From , the extracts from the Northern region gave higher free radical scavenging activity than those from the Eastern region. For the parts of Longkong, the stalks (the summation of SC50 values = 1.19 µg/ml) gave higher radical scavenging activity than other plant parts. The mean free radical scavenging activity of the solvents used to prepare extracts were decreased in the order of water > methanol > chloroform. The high phenolic content in the extracts may be responsible for this scavenging ability. The water extracts showed high free radical scavenging activity according to their high phenolic content. The phenolic compounds which have been reported to scavenge DPPH include flavonoids, anthraquinones, anthocyanidins, xanthones and tannins. They also scavenged superoxide and hydroxyl radicals by single electron transfer (Choi et al., Citation2002).
Table 4. Summations of the percentage yields, total phenolic content, total flavonoid content and antioxidative activities depending on the sources of Longkong, parts used, the extraction solvents and temperatures of the 85 extracts.
Lipid peroxidation inhibition activity
The hot chloroform extract of the young leaves from the Eastern region (EYLCH) exhibited the highest lipid peroxidation inhibition with IPC50 values of 0.86 ± 0.31 µg/ml, whereas the IPC50 value of α-tocopherol was 0.03 ± 0.01 µg/ml. From , the extracts from the Northern region gave higher lipid peroxidation inhibition than those from the Eastern region. The lipid peroxidation inhibition of the extracts might be from the existing phenolic and flavonoid compounds in the extracts that can inhibit peroxidation formation. For the parts of Longkong, the seeds (the summation of SC50 values = 1.18 µg/ml) gave higher lipid peroxidation inhibition than other plant parts. Most compounds containing in the seed of Longkong are tetranorterpenoids, including domesticulide A--E, with 11 known triterpenoids. Six classes of limonoids have been isolated from the seed extracts of L. domesticum, including andirobin derivates, methyl angolensates, mexicanolides, azadiradiones, onoceranoids, and dukunolides (Tanaka et al., Citation2002). Sassa et al. have demonstrated the inhibitory effect of the dienone-phenolic triterpene on lipid peroxidation in rat liver mitochondrial membranes induced by ADP and Fe2+ (Sassa et al., Citation1990). The mean lipid peroxidation inhibition of the extracts decreased in the order of chloroform > water > methanol. Terpenes which are the lipophillic compounds in the seed can be extracted by chloroform more than by methanol and water.
Metal ion chelating activity
The cold water extract of the old leaves from the Northern region (NOLWC) gave the highest metal ion chelating (MC50 values of 0.47 ± 1.60 µg/ml), but lower than EDTA (MC50 values of 0.06 ± 0.03 µg/ml) by 7.83-times. From , the extracts from the Northern region gave higher metal ion chelating than those from the Eastern region. The phenolic contents found in the extracts of the Northern region which are more than in the Eastern region could serve as a chelator, by chelating with Fe2+ instead of ferrozine to prevent the formation of the ferrozine complex. For the parts of Longkong, the seed (the summation of MC50 values was 1.52 µg/ml) gave higher metal ion chelating activity than other plant parts. This high chelating activity of the extracts might be from the high phenolic and flavonoid contents. Phenolic acids and flavonoids have been shown to complex with iron and copper ions to provide antioxidant effects. Metal chelating effects of phenolics due to their catechol, galloyl, or 1,3 positioned hydroxyl and carbonyl moieties, also imparts antioxidant effects by inactivating metals. The major flavonoid in liquorice, the isoflavone glabridin, is known to have metal chelating capacity. The mean metal ion chelating of the solvents used to prepare the extracts were decreased in the order of methanol > water > chloroform. Alcoholic solvents have been commonly employed to extract phenolic compounds from plants, because of obtaining high yield although they were not highly selective for phenols (Spigno & Faveri, Citation2007). However, there was no difference in the metal ion chelating activities between the extracts prepared by the cold and hot processes.
Anti-proliferative of the extracts on human mouth epidermal carcinoma cell lines (KB)
Only 2 extracts from 85 extracts including the hot and cold chloroform young fruit extracts from the Northern region (NYFCH and NYFCC) exhibited an anti-proliferation effect against KB cells with the IC50 values of 603.45 ± 55.35 and 765.06 ± 46.19 mg/ml, respectively. However, they gave lower activity than standard anticancer drugs including cisplatin, fluorouracil, doxorubicin and vincristine, which showed IC50 values of 12.72, 12.94, 0.82 and 0.03 μg/ml, respectively. The nonpolar compounds containing especially in the chloroform extracts may be related to the cytotoxic activity. Triterpenoids which are the major nonpolar secondary metabolite constituents found in L. domesticum may be responsible for these cytotoxic effects since the significant inhibitory activity of skin-tumor promotion on Epstein-Barr virus activation of these compounds have been reported (Nishizawa et al., Citation1989). However, there were quite a few reports on the effects of L. domesticum extracts or their isolated compounds on cancer cell lines. The hot and cold chloroform extracts of the young fruits of Longkong from this study which gave the highest cytotoxic activity on KB cells were further investigated for the inhibition of cancer promotion progression by the gelatinolytic activity.
Gelatinolytic activity on MMP-2 inhibition zymography
presents the gelatinolytic activity on MMP-2 inhibition of the Longkong extracts in comparison to standard anticancer drugs. The selected two Longkong extracts which showed the highest anti-proliferation effect exhibited MMP-2 inhibition on KB cells. The cold chloroform young fruit extracts from the Northern region (NYFCC) gave pro- and active MMP-2 inhibition at 53.03 ± 2.65 and 31.30 ± 0.43%, while the hot chloroform young fruit extracts from the Northern region (NYFCH) showed 49.40 ± 0.87 and 21.72 ± 1.06%, respectively. The MMP-2 inhibition activities of the standard anticancer drugs including cisplatin, fluorouracil, doxorubicin, and vincristine, were 57.94 ± 2.50, 25.13 ± 1.73, 22.07 ± 0.42 and 22.25 ± 2.71% for pro-MMP-2; 32.06 ± 2.53, 20.11 ± 1.13, 10.33 ± 1.04, and 5.71 ± 1.53% for active MMP-2, respectively. Both extracts showed both pro- and active MMP-2 inhibition higher than all standard anticancer drugs, except cisplatin. These extracts may inhibit the MMP-2 synthesis and secretion steps or interrupt the activation processes by converting the latent form of MMP-2 (pro MMP-2) to an active form (active MMP-2), resulting in the decrease of the area and intensity of the active MMP-2 on the zymogram.
Figure 1. MMP-2 inhibition of the two selected Longkong extracts in comparing to the standard anticancer drugs on KB cells. NYFCC: Cold chloroform young fruit extracts from the Northern region and NYFCH: Hot chloroform young fruit extracts from the Northern region. *Significant difference (p < 0.05) in comparing to all standard anticancer drugs. †Significant difference (p < 0.05) in comparing to all standard anticancer drugs, except cisplatin. χSignificant difference (p < 0.05) in comparing to all standard anticancer drugs, except fluorouracil.
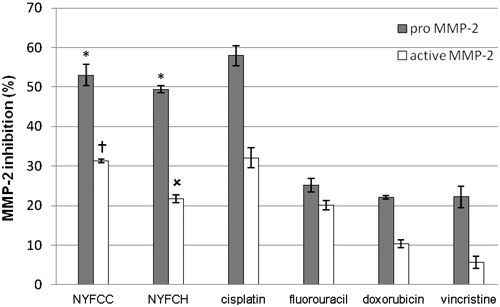
Both MMP-2 and MMP-9 are the major MMPs involved in invasion and metastasis because of their capacity to degrade type-IV collagen, an important component of basement membranes. Our experiments showed only MMP-2 activity. MMP-2 appears to have prognostic significance in early forms of cancer. Also, its strong expression is associated with shorter survival (Wadowska-Jaszczyńska et al., Citation2011). Multivariate analyses showed that only MMP-2 overexpression by cancer cells in peritoneal implants was associated with a significant risk of death by the disease (Périgny et al., Citation2008). In fact, it is still unknown for the mechanism of the extracts on the MMP-2 inhibition. One possible mechanism is anti-oxidative activity. It has been reported that exogenous hydrogen peroxide and endogenous ROS can induce MMP expression in endothelial cells, cardiac fibroblasts, macrophages, and breast cancer cells (Zhang et al., Citation2002). ROS can affect gene expression including the induction of MMPs through the signal transduction pathway (Brenneisen et al., Citation1998). Since MMPs are upregulated by an increased formation of the reactive oxygen species (ROS), antioxidant approaches can thus decrease MMP-2 upregulation (Castro et al., Citation2009). Phenolic compounds including sesquiterpene lactones, triterpenes, phytosterols, and cinnamic acid derivatives have been reported to inhibit MMP-2 and -9 (Löser et al., Citation2000). Several studies have explained that the anti-oxidative activity of some plant extracts can inhibit ROS from their reducing power by breaking the free radical chain and donating a hydrogen atom (Shahidi & Wanasundara, Citation1992). Actually, pro-MMP-2 activation does not only depend on ROS. The membrane type-1 MMP (MT-1 MMP) and tissue inhibitor of metalloproteinase (TIMP-2) can also play a critical role to activate the pro-MMP-2. In addition, ROS suppression is a bypassed way to inhibit pro-MMP-2 activation. However, even the young fruit extract which showed low free radical scavenging gave high MMP-2 inhibition. Thus, the free radical scavenging may not be the only factor to suppress MMP-2. A triterpene-dependent effect may be another factor to regulate MMPs (Slaton et al., Citation2006). Triterpenes are known to be inhibitors of matrix metalloproteases (MMP) which regulate cellular motility and invasion. It has been demonstrated that mushroom extracts with high triterpene content showed a great inhibitory effect on in vitro growth of tumor cells and inhibited expression of MMP-2. Ganoderic acid Me, which is triterpene isolated from various parts of G. lucidum, effectively inhibited tumor growth, lung metastasis (Wang et al., Citation2007), and tumor invasion through down-regulating matrix metalloproteinase 2/9 (MMP2/9) gene expression (Chen et al., Citation2007). It was found that GA-Me depressed the viability of tumor cells at much lower concentrations than against normal cells (Chen & Zhong, Citation2009), making it an effective potential therapeutic drug for the prevention and treatment of tumors in the clinic. Thus, the MMP-2 inhibition mechanism of Longkong young fruit extract may be from the down-regulating matrix metalloproteinase by its triterpene contents.
Conclusions
The present study has demonstrated in vitro anticancer activities in human epidermal carcinoma cells (KB) including anti-proliferation and matrix metalloproteinase inhibition of Longkong extracts cultivated in the Northern and Eastern region of Thailand. Only 2 of the 85 extracts including the hot and cold chloroform young fruit extracts from the Northern region (NYFCH and NYFCC) of Thailand exhibited anti-proliferation effects with IC50 values of 603.45 ± 55.35 and 765.06 ± 46.19 mg/ml, respectively. These two extracts showed the pro- and active MMP-2 inhibition at 53.03 ± 2.65 and 31.30 ± 0.43% (NYFCC); 49.40 ± 0.87 and 21.72 ± 1.06% (NYFCH), respectively, higher than all standard anticancer drugs except cisplatin. Thus, the cold chloroform extract appeared to have superior anticancer activity in comparing to the hot extract. The antioxidative activity of triterpenes in hot chloroform extract may inhibit matrix metalloproteases (MMPs), which regulate cellular motility and invasion of cancer cell. This study suggests the hot chloroform extract from the young fruits of Longkong has the potential to be further developed as an oral anticancer agent.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Acknowledgements
This work was supported by the Agricultural Research Development Agency (Public Organization) the investment funds following the Royal Decree (ARDA) in Thailand and Natural Products Research and Development Center (NPRDC), Science and Technology Research Institute (STRI), Chiang Mai University, Thailand.
References
- Ardestani A, Yazdanparast R. (2007). Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem 104:21–9
- Balitaan JN, Steinbrenner H, Ramos MC. (2010). Zymography-based assay for screening potential anti-gelatinase agents using Serratia marcescens serralysin. Philipp Sci Lett 3:100–8
- Bouquet W, Boterberg T, Ceelen W, et al. (2009). In vitro cytotoxicity of paclitaxel/[beta]-cyclodextrin complexes for HIPEC. Int J Pharm 367:148–54
- Brenneisen P, Wenk J, Klotz LO, et al. (1998). Central role of ferrous/ferric iron in the ultraviolet B irradiation-mediated signaling pathway leading to increased interstitial collagenase (matrix-degrading metalloprotease (MMP)-1) and stromelysin-1 (MMP-3) mRNA levels in cultured human dermal fibroblasts. J Biol Chem 273:5279–87
- Burg P, Fraile P. (1995). Vitamin-C destruction during the cooking of a potato dish. Food Sci Technol 18:506–14
- Carmeliet P, Moons L, Herbert J-M, et al. (1997). Urokinase but not tissue plasminogen activator mediates arterial neointima formation in mice. Circ Res 81:829–39
- Castro MM, Rizzi E, Rodrigues GJ, et al. (2009). Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic Biol Med 46:1298–307
- Chen NH, Liu JW, Zhong JJ. (2007). Ganoderic acid Me inhibits tumor invasion through down-regulating matrix metalloproteinases 2/9 gene expression. J Pharmacol Sci 108:212–16
- Chen NH, Zhong JJ. (2009). Ganoderic acid Me induces G1 arrest in wild-type p53 human tumor cells while G1/S transition arrest in p53-null cells. Process Biochem 44:928–33
- Choi CW, Kim SC, Hwang SS, et al. (2002). Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci 163:1161–8
- Decker EA, Welch B. (1990). Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem 38:674–7
- Han Y, Tian H, He P, et al. (2009). Insulin nanoparticle preparation and encapsulation into poly(lactic-co-glycolic acid) microspheres by using an anhydrous system. Int J Pharm 378:159–66
- Hrabec E, Strek M, Nowak D, et al. (2002). Activity of type IV collagenases (MMP-2 and MMP-9) in primary pulmonary carcinomas: A quantitative analysis. J Cancer Res Clin Oncol 128:197–204
- Kim S, Kim Y, Kim JE, et al. (2008). Berberine inhibits TPA-induced MMP-9 and IL-6 expression in normal human keratinocytes. Phytomedicine 15:340–7
- Kobayashi T, Hattori S, Shinkai H. (2003). Matrix metalloproteinases-2 and -9 are secreted from human fibroblasts. Acta Derm Venereol 83:105–7
- Löser B, Kruse SO, Melzig MF, Nahrstedt A. (2000). Inhibition of neutrophil elastase activity by cinnamic acid derivatives from Cimicifuga racemosa. Planta Med 66:751–3
- Manosroi A, Jantrawut P, Akihisa T, et al. (2010a). In vitro anti-aging activities of Terminalia chebula gall extract. Pharm Biol 48:469–81
- Manosroi A, Jantrawut P, Sainakham M, et al. (2012). Anticancer activities of the extract from Longkong (Lansium domesticum) young fruits. Pharm Biol 50:1397–407
- Manosroi A, Ruksiriwanich W, Abe M, et al. (2010b). Biological activities of the rice bran extract and physical characteristics of its entrapment in niosomes by supercritical carbon dioxide fluid. J Supercrit Fluids 54:137–44
- Morton J. 1987. Langsat. In: Morton JF, ed. Fruits of Warm Climates. Miami, FL: Florida Flair Books, 201–3
- Nishizawa M, Emura M, Yamada H, et al. (1989). Isolation of a new cycloartanoid triterpene from leaves of Lansium domesticum novel skin-tumor promotion inhibitors. Tetrahedron Lett 30:5615–18
- Papazisis KT, Geromichalos GD, Dimitriadis KA, Kortsaris AH. (1997). Optimization of the sulforhodamine B colorimetric assay. J Immunol Methods 208:151–8
- Périgny M, Bairati I, Harvey I, et al. (2008). Role of immunohistochemical overexpression of matrix metalloproteinases MMP-2 and MMP-11 in the prognosis of death by ovarian cancer. Am J Clin Pathol 129:226–31
- Rossi M, Rickles LF, Haplin WA. (1986). The crystal and molecular structure of quercetin: A biologically active and naturally occurring flavonoid. Bioorg Chem 14:55–69
- Saewan N, Sutherland JD, Chantrapromma K. (2006). Antimalarial tetranortriterpenoids from the seeds of Lansium domesticum Corr. Phytochemistry 67:2288–93
- Sassa H, Takaishi Y, Terada H. (1990). The triterpene celastrol as a very potent inhibitor of lipid peroxidation in mitochondria. Biochem Biophys Res Commun 172:890–7
- Shahidi F, Wanasundara PKJPD. (1992). Phenolic antioxidants. Crit Rev Food Sci Nutr 32:67–103
- Slaton J, Sloper D, McKenna D, et al. 2006. Down regulation of matrix metalloproteases and tumor inhibition of Ganoderma lucidum (Reishi) mushroom depends upon triterpene content. The North American research conference on complementary and integrative medicine. Edmonton, Alberta, Canada, 24--27 May
- Spigno D, Faveri DMD. (2007). Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J Food Eng 78:793–801
- Sun T, Xu Z, Godber JS, Prinyawiwatkul W. (2006). Capabilities of oat extracts in inhibiting cholesterol and long chain fatty acid oxidation during heating. Cereal Chem 83:451–4
- Tachibana Y, Kikuzaki H, Hj-Lajis N, Nakatani N. (2001). Antioxidant activity of carbazoles from Murraya koenigii leaves. J Agric Food Chem 49:5589–94
- Tanaka T, Ishibashi M, Fujimoto H, et al. (2002). New onoceranoid triterpene constituents from Lansium domesticum. J Nat Prod 65:1709–11
- Tepe B, Sokman A. (2007). Screening of the antioxidative properties and total phenolic contents of three endemic Tanacetum subspecies from Turkish flora. Bioresource Technol 98:3076–9
- Tomaino A, Cimino F, Zimbalatti V, et al. (2005). Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem 89:549–54
- Wadowska-Jaszczyńska K, Jach R, Stangel-Wócikiewicz K, et al. (2011). Results in treatment of early breast cancers and the level of selected metalloproteinases. Neuro Endocrinol Lett 32:821–30
- Wang G, Zhao J, Liu JW, et al. (2007). Enhancement of IL-2 and IFN-γ expression and NK cells activity involved in the anti-tumor effect of ganoderic acid Me in vivo. Int Immunopharmacol 7:864–70
- Yapp DTT, Yap SY. (2003). Lansium domesticum: Skin and leaf extracts of this fruit tree interrupt the lifecycle of Plasmodium falciparum, and are active towards a chloroquine-resistant strain of the parasite (T9) in vitro. J Ethnopharmacol 85:145–50
- Zhang HJ, Zhao W, Venkataraman S, et al. (2002). Activation of matrix metalloproteinase-2 by overexpression of manganese superoxide dismutase in human breast cancer MCF-7 cells involves reactive oxygen species. J Biol Chem 277:20919–26
