Abstract
Context: A number of Blumea (Asteraceae) species are being used in traditional Chinese and Indian folklore medicines to cure various diseases including cancer, fungal and bacterial infections.
Objective: To evaluate the in vitro antiplasmodial potential and cytotoxicity of various extracts and fractions of B. membranacea DC and B. eriantha DC and high performance liquid chromatography (HPLC) chemical fingerprinting of their crude extracts.
Materials and methods: The aerial parts and roots of B. membranacea and B. eriantha were extracted with ethanol and the extracts were successively partitioned with n-hexane, ethyl acetate and n-butanol, which were later evaluated for their in vitro antiplasmodial activity against Plasmodium falciparum NF-54 and in vitro cytotoxicities against non-cancerous Vero cell line. HPLC chemical fingerprinting was performed on extracts of B. membranacea and B. eriantha.
Results: The n-hexane (MA1), ethyl acetate (MA2) fractions of aerial parts and n-butanol (MR3) fraction of roots of B. membranacea showed IC50 values of 17.4, 19.0 and 3.3 µg/mL respectively, while the n-hexane (EA1), ethyl acetate (EA2) fractions of aerial parts and ethyl acetate (ER2) fraction of roots of B. eriantha showed IC50 values of 25.0, 26.5 and 15.6 µg/mL, respectively, against P. falciparum NF-54. All these fractions were non-toxic to Vero cells.
Discussion and conclusion: Both B. membranacea and B. eriantha possesses a high degree of selective antiplasmodial activity (selectivity index up to >60) and hence, may find their use in antimalarial phytopharmaceuticals as well as in discovery of a safer and novel antimalarial lead.
Introduction
Malaria is one of the most common infectious diseases in the world, especially the tropical areas (Snow et al., Citation2005). However, as a result of intensive efforts to control malaria, mortality rates have fallen by more than 25% globally since 2000 and by 33% in the African region (WHO, Citation2011), but the increasing resistance of Plasmodium falciparum to common antimalarial drugs, including chloroquine (Babiker et al., Citation2001) and artemisinin (Arjen et al., Citation2009), has created an urgent need for new antimalarial agents. The majority of antimalarial drugs, including quinoline- and artemisinin- based antimalarials, have been derived from medicinal plants or from structural modifications in plant lead compounds. Hence, there are ample chances of getting novel natural antimalarial leads as well as new herbal remedies.
Blumea (Asteraceae) is found in the tropical and sub-tropical zones of Asia, including the Indian sub-continent. The plants of this genus are mostly small annual weeds and are of great medicinal value. A number of species of the genus Blumea are used as folklore medicine in India (Ji et al., Citation2007; Sharma et al., Citation2010; Verma & Chauhan, Citation2007). One species of this genus, B. balsamifera, is a component of various herbal formulations in traditional Chinese medicine (TCM) for treating cancer (Wang et al., Citation2011), cold, dysentery (Hu & Huang, Citation2010), influenza (Huang, Citation2009), gynecological diseases (Pang et al., Citation2009) and viral infections (Fan & Fan, Citation1997). Recently, B. balsamifera has also shown antiplasmodial activity (Noor et al., Citation2007). In India, B. membranacea and B. eriantha are commonly found along with other species of Blumea. The B. eriantha is commonly known as “Nimurdi” in Marathi and “Kukronda” in Hindi (Singh et al., Citation2012). The essential oil of B. membranacea produces a marked and long-lasting fall in blood pressure in anaesthetized dogs, exerts a direct depressant action on frog heart and a spasmolytic effect on rabbit ileum (Mehta et al., Citation1986) and has also shown significant antifungal activity (Geda & Bokadia, Citation1979). A warm infusion of leaves of B. eriantha is given as a sudorific, while a cold infusion is considered diuretic and emmenagogue (Chen et al., Citation2009). The essential oil of B. eriantha has shown antibacterial (Jain & Kar, Citation1971) and antifungal activities (Jain & Jain, Citation1972). To the best of our knowledge, there is no previous report on the in vitro antiplasmodial activity and cytotoxicity evaluation of extracts of B. membranacea and B. eriantha. Therefore, the present study on the evaluation of in vitro antiplasmodial potential and cytotoxicity of extracts from B. membranacea and B. eriantha was undertaken as a part of our drug discovery program (Upadhyay et al., Citation2012, Citation2013).
Materials and methods
Plant material
The plant samples were collected locally from Faizabad, Uttar Pradesh, India in November, 2010 and proper identification was done by Dr. D. C. Saini, Scientist, Birbal Shahni Institute of Palaeobotany (BSIP), Lucknow and Dr. S. C. Singh, Taxonomy & Pharmacognosy Department, Central Institute of Medicinal & Aromatic Plants (CIMAP), Lucknow, India. The voucher specimen numbers 9462 and 9457 for B. membranacea and B. eriantha, respectively, have been deposited at the CIMAP herbarium.
Extraction and fractionation
The shade-dried and pulverized plant samples (20 g each) corresponding aerial parts and roots of B. eriantha and B. membranacea were separately extracted with ethanol (250 mL × 3) and evaporated until dryness under vacuum at 40 °C, which afforded the ethanol extracts of aerial parts (EA, 2.50 g) and roots (ER, 1.80 g) of B. eriantha and the ethanol extracts of aerial parts (MA, 2.40 g) and roots (MR, 1.90 g) of B. membranacea. A small amount (∼50 mg) from each crude extract were kept for biological evaluations and the rest were separately dissolved in distilled water and successively partitioned with n-hexane, ethyl acetate and n-butanol (100 mL × 3). The combined extract fractions were separately dried under vacuum. In this way, EA (2.4 g) afforded n-hexane (EA1, 0.20 g), ethyl acetate (EA2, 0.21 g) and n-butanol (EA3, 0.95 g) fractions while ER (1.70 g) afforded n-hexane (ER1, 0.28 g), ethyl acetate (ER2, 0.25 g) and n-butanol (ER3, 0.85 g) fractions. Similarly, the MA (2.35 g) afforded n-hexane (MA1, 0.41 g), ethyl acetate (MA2, 0.25 g) and n-butanol (MA3, 0.75 g) fractions while MR (1.84 g) afforded n-hexane (MR1, 0.42 g), ethyl acetate (MR2, 0.38 g) and n-butanol (MR3, 0.84 g) fractions. The extract/fraction samples for biological activity evaluations were kept under −20 °C condition until use.
Chemicals and reagents
All the solvents used for extraction, fractionation and high performance liquid chromatography (HPLC) analysis were purchased from E. Merck Ltd., Mumbai, India. The standards, chloroquine and doxorubicin hydrochloride were purchased from Sigma-Aldrich, Bangalore, India.
Chemical finger-printing of the extracts by HPLC
The HPLC analysis of aerial part and root extracts of B. membranacea and B. eriantha were performed on a Shimadzu LC-10AD liquid chromatograph equipped with a SPD-M10A VP diode array detector, a SIL-10ADVP auto injector and CBM-10 interface module. Data were collected and analyzed using a class LC-10 work-station. A Waters Spherisorb ODS2 column (250 × 4.6 mm, i.d., 10 µm) was selected for HPLC analysis.
A 100 mg sample of each plant extract was dissolved in 1.0 mL of methanol using an ultrasonicator (Microclean 109, Oscar Ultrasonic, Mumbai, India) for 15 min and centrifuged at 10 000 rpm for 10 min. The supernatant was filtered through a 0.45 µm Millipore membrane (Millipore, Billerica, MA) and quantitatively transferred into a volumetric flask, adjusted to a final volume of 1 mL to be used for the HPLC analysis. The separation was achieved with a gradient program for pump A (water) and pump B (acetonitrile) with a linear gradient elution: 75% A (10 min), 65% A (40 min), 75% A (50 min). The flow rate was 0.6 mL/min throughout the gradient run. The column temperature was maintained at 25 ± 1 °C. Before use in HPLC, the solvents (HPLC grade) were filtered through a 0.45 µm Millipore membrane. The data acquisition was performed at 254 nm.
In vitro antiplasmodial assay
The in vitro inhibitory assays were performed against P. falciparum (NF54 strain) cultured in human type O-positive red blood cells according to an earlier described method (Rieckmann et al., Citation1978) with minor modifications. Briefly, 180 μL of synchronized ring stage parasite culture with 1% parasitaemia and 2.5% hematocrit were transferred into a 96-well plate containing 20 μL of graded concentrations (in duplicate) of test extracts or standard drugs (Lambros & Vanderberg, Citation1979). The plate was incubated for 48 h and Geimsa-stained thin smears were prepared from each well and examined microscopically to record percentage parasitemia. The inhibition of parasite multiplication in relation to control was calculated for each concentration of the extract. The 50% inhibitory concentration (IC50) values were calculated from a dose–response curve prepared from percentage inhibition values verses concentration. Four dilutions (200, 50, 10 and 1 μg/mL) were evaluated for plant extracts. Chloroquine was used as standard drug and served as the positive control while only parasitized red blood cells in medium with same volume of DMSO served as negative control.
In vitro cytotoxicity assay
Vero cells (VERO C1008; ATCC CRL-1586, kidney epithelial cells from African vervet monkey) were cultured in 75 cm2 flasks in RPMI-1640 medium (Invitrogen BioServices Ltd., Bangalore, India) supplemented with 0.2% NaHCO3, 1X antibiotic-antimycotic solution (Gibco BRL, Mumbai, India), and 10% fetal bovine serum at 37 °C in an atmosphere of 95% humidity and 5% CO2. The assay was performed in a 96-well plate using the neutral red uptake method as described previously (Weniger et al., Citation2001). Doxorubicin hydrochloride was used as standard drug and served as the positive control while only Vero cells with same volume of DMSO served as negative control.
Determination of selectivity index (SI)
Selectivity index was determined as the ratio of IC50 for cytotoxicity against Vero cells with IC50 for growth inhibition of P. falciparum (Wright & Phillipson, Citation1990).
Statistical analysis
The IC50 values were calculated from a dose–response curve prepared from percentage inhibition values verses concentration using Graph Pad Prism (Version 4.0). Tests were carried out in duplicate and the data are means of three independent experiments.
Results and discussion
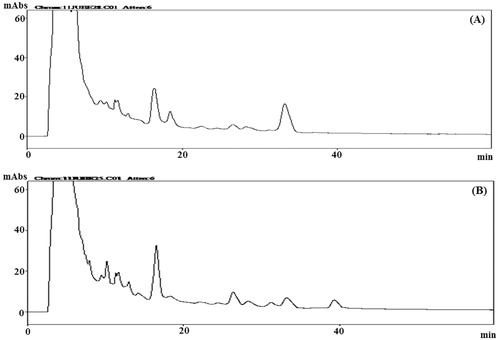
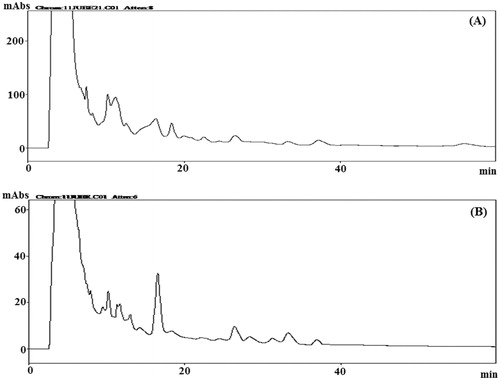
For quality control, the crude ethanol extracts corresponding to aerial part and roots of B. membranacea and B. eriantha were separately subjected to HPLC analysis and the chemical profiles of the extracts were obtained as chromatograms (, ). Several peaks were observed in all the chromatograms which suggest the presence of many phytomolecules in these extracts.
The results (IC50) of in vitro antiplasmodial potential and the safety profile of various extracts of aerial parts and roots of B. membranacea and B. eriantha are shown in .
Table 1. In vitro antiplasmodial activity and safety profile of various extracts of B. membranacea and B. eriantha.
From , it is evident that aerial crude extract (MA) of B. membranacea was almost 2-times more active than the root crude extract (MR). The further careful analysis of the in vitro antiplasmodial activity results () revealed that the fractionation of these extracts into n-hexane, ethyl acetate and n-butanol fractions, respectively, resulted in further enhancement of antiplasmodial activity of these fractions. Among the MA fractions, the n-hexane fraction (MA1) was most potent with an IC50 of 17.4 µg/mL followed by the ethyl acetate fraction (MA2) with an IC50 of 19.0 µg/mL, indicating that fractionation increased antiplasmodial potential by almost 3-times. On the other hand, the n-butanol fraction (MA3) was found inactive (IC50 > 200 µg/mL). Similarly, among the MR, the n-butanol fraction (MR3) was most potent with an IC50 of 3.3 µg/mL followed by the n-hexane fraction (MR1) with an IC50 of 57.5 µg/mL, indicating that fractionation has increased antiplasmodial potential by almost 33-times and 2-times, respectively. On the other hand, the antiplasmodial activity in the ethyl acetate fraction (MR2) was reduced by one and a half times (IC50 = 141.0 µg/mL) that of MR. The crude extracts of B. eriantha, aerial (EA) and root (ER) have shown moderate antiplasmodial activities with IC50 values of 94.0 and 75.5 µg/mL, respectively. On successive fractionation of EA, the antiplasmodial potential of n-hexane (EA1) and ethyl acetate (EA2) fractions increased by almost 2-times (IC50 values 25.0 and 26.5 µg/mL, respectively) while in the n-butanol fraction (EA3) the activity was reduced to half (IC50 = 189.0 µg/mL). Similarly, among the ER, the ethyl acetate fraction (ER2) was most potent with an IC50 of 15.6 µg/mL indicating that fractionation has increased antiplasmodial potential by almost 5-times while only a moderate increase was observed in the case of the n-butanol fraction (ER3, IC50 =61.5 µg/mL). On the other hand, the antiplasmodial potential of the n-hexane fraction (ER1) was reduced to half (IC50 = 156.0 µg/mL) that of ER.
Figure 3. In vitro antiplasmodial activity of extracts and fractions of B. membranacea and B. eriantha.
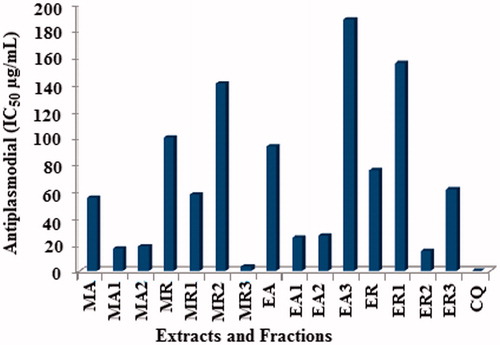
Further, the fractions MA1, MA2, MR3, EA1, EA2 and ER2, which showed potent antiplasmodial activities (IC50 < 50.0 µg/mL) were evaluated for their in vitro cytotoxicity against a mammalian cell line (Vero), and, to ascertain the safety profiles, the selectivity indices (SI) were calculated as the ratio of cytotoxicity (IC50 against Vero cells) to biological activity (IC50 against P. falciparum NF-54; ). Among the tested fractions, MR3, MA1 and MA2 showed SI, >60.606, >11.494 and >10.526, respectively. Hence, this may be considered as safe (nontoxic) antiplasmodial fractions (Wright & Phillipson, Citation1990), while rest of the active fractions EA1, EA2 and ER2 showed selectivity index (SI), >8.000, 6.792 and 6.794, respectively, and may be considered as moderately safe.
Conclusion
B. membranacea and B. eriantha both possess significant antiplasmodial activity, but among all the fractions tested, MA1, MA2 and MR3 of B. membranacea showed highest antiplasmodial activity and selectivity indices. Hence, B. membranacea may be useful as a phytopharmaceuticals for the treatment of malaria. Nevertheless, further investigations are required to ascertain the plant’s toxicity in vivo, which might result in the discovery of a safer and novel antimalarial agent/lead. Further, activity-guided isolation of antiplasmodial agents from the active fractions of B. membranacea and B. eriantha will be the focus of our future study, which might result in the discovery of a safer and novel antimalarial agent/lead from a very common and widely grown weed.
Declaration of interest
Financial support for this research by CSIR and UGC for providing fellowship to one of us (HCU) is gratefully acknowledged.
References
- Arjen M, Dondorp AM, Nosten F, et al. (2009). Artemisinin resistance in Plasmodium falciparum malaria. New England J Med 361:455–67
- Babiker HA, Pringle SJ, Abdel-Muhsin A, et al. (2001). High-level chloroquine resistance in Sudanese isolates of Plasmodium falciparum is associated with mutations in the chloroquine resistance transporter gene pfcrt and the multidrug resistance gene pfmdr1. J Infect Dis 183:1535–8
- Chen M, Jin H-Z, Zhang W-D, et al. (2009). Chemical constituents of plants from the genus Blumea. Chem Biodiv 6:809–17
- Fan F, Fan N. (1997). A Chinese medicinal preparation for treating viral dermatoses. Chinese Patent 1162469A 19971022
- Geda A, Bokadia MM. (1979). Antifungal activity of the essential oil of Blumea membranacea DC. Indian Drug Pharm Ind 14:21–2
- Hu H, Huang N. (2010). Traditional Chinese medicine decoction for treating cold dysentery. Chinese Patent 101905003A 20101208
- Huang Y. (2009). Anti-influenza oil, Chinese medicinal essential oil and aroma therapeutic formulation comprising the same with anti-influenza function. Chinese Patent 101612364A 20091230
- Jain SR, Jain MR. (1972). Antifungal studies on some indigenous volatile oils and their combinations. Planta Med 22:136–9
- Jain SR, Kar A. (1971). Antibacterial activity of some essential oils and their combinations. Planta Med 20:118–23
- Ji NK, Kumar RN, Patil N, Soni H. (2007). Studies on plant species used by tribal communities of Saputara and Purna forests, Dangs district, Gujrat. Indian J Trad Knowledge 6:368–74
- Lambros C, Vanderberg JP. (1979). Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65:418–20
- Mehta SC, Vardhan H, Saxena SP. (1986). Some pharmacological actions of the essential oil of Blumea membranacea. Indian J Physiol Pharmacol 30:149–54
- Noor RA, Khozirah S, Mohd-Ridzuan MAR, et al. (2007). Antiplasmodial properties of some Malaysian medicinal plants. Trop Biomedicine 24:29–35
- Pang Y, Jin D, Yuan Y, et al. (2009). A pharmaceutical composition for treating gynaecological diseases, and its preparation method. Chinese Patent 101524407A 20090909
- Rieckmann KH, Campbell GH, Sax LJ, Mrema JE. (1978). Drug sensitivity of Plasmodium falciparum: An in-vitro microtechnique. Lancet 1:22–3
- Sharma J, Painuli RM, Gaur RD. (2010). Plants used by rural communities of district Shahjahanpur, Uttar Pradesh. Indian J Trad Knowledge 9:798–803
- Singh UP, Singh AK, Sarathy RP. (2012). Effect of methanolic extracts of Blumea eriantha DC leaves on protein metabolism and marker enzymes in streptozotocin-induced hyperglycemic animals. Int J Pharm Pharm Sci 4:235–8
- Snow RW, Guerra CA, Noor AM, et al. (2005). The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214–17
- Upadhyay HC, Dwivedi GR, Darokar MP, et al. (2012). Bioenhancing and anti-mycobacterial agents from Ammannia multiflora. Planta Med 78:79–81
- Upadhyay HC, Thakur JP, Saikia D, Srivastava SK. (2013). Anti-tubercular agents from Ammannia baccifera (Linn.). Med Chem Res 22:16–21
- Verma S, Chauhan N. (2007). Indigenous medicinal plants knowledge of Kunihar forest division, district Salon. Indian J Trad Knowledge 6:494–7
- Wang Q, Qin Y, Zeng J. (2011). Medicine for treating liver cancer. Chinese Patent 102188638A 20110921
- Weniger B, Robledo S, Arango GJ, et al. (2001). Antiprotozoal activities of Colombian plants. J Ethnopharmacol 78:193–200
- WHO-Global Malaria Programme. (2011). World Malaria Report (Fact Sheet). Available from: http://www.who.int/malaria/world_malaria_report_2011/WMR2011_factsheet.pdf [last accessed 10 Jan 2013]
- Wright CW, Phillipson JD. (1990). Natural products and the development of selective anti-protozoal drugs. Phytother Res 4:127–39