Abstract
Context: Magnesium lithospermate B (MLB), an active polyphenol acid of Danshen [Radix Salviae miltiorrhizae (Labiatae)], showed renoprotective, neuroprotective and myocardial salvage effects. Previous studies demonstrated that MLB could effectively suppress the production of cytokines and their associated signaling pathways in activated human T cells.
Objective: The purpose of this study was to examine the beneficial effects of MLB on myocardial ischemia/reperfusion (MI/R) injury and to explore its potential mechanisms related to anti-inflammation.
Materials and methods: Sprague–Dawley rats were grouped as sham group, model group and MLB-treated (15, 30 and 60 mg/kg) groups. Animals were subjected to MI/R injury by the occlusion of left anterior descending artery for 30 min followed by reperfusion for 3 h. At the end of reperfusion, blood samples were collected to determine the serum levels of cardiac troponin (cTnI), creatine kinase-MB (CK-MB), tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β) and interleukin 6 (IL-6). Hearts were harvested to assess infarct size, histopathological changes and the activity of myeloperoxidase (MPO). The expression of phosphor-IkB-α and phosphor-nuclear factor kappa B (NF-κB) were assayed by western blot.
Results: MLB administration significantly (p < 0.05) reduced: (1) ST-segment elevation (0.23 mv), (2) the infarct size (22.5%), (3) histological scores of myocardial injury (1.67 score), (4) myocardial injury marker enzymes: cTnI (5.64 ng/ml) and CK-MB (49.57 ng/ml) levels, (5) pro-inflammatory cytokines: TNF-α (97.36 pg/ml), IL-1β (93.35 pg/ml) and IL-6 (96.84 pg/ml) levels, (6) MPO activity (1.82 U/mg), (7) phosphor-NF-κB (0.87) and phosphor-IkB-α (0.96) expression.
Discussion and conclusion: Our study provided evidence that MLB ameliorated the inflammatory process associated with MI/R injury via NF-κB inactivation.
Introduction
Myocardial reperfusion therapy, the optimal therapeutic strategy for ischemic heart disease (IHD), can preserve myocardial viability and function by reversing myocardial ischemia and limiting the infarct size. However, the subsequent myocardial ischemia/reperfusion (MI/R) injury may reduce the therapeutic benefit. Therefore, therapeutic strategies aimed at preventing MI/R injury may be a reasonable choice for the treatment of related heart disease. Previous studies have shown that MI/R injury is a complicated process involving multiple pathways (Yellon & Hausenloy, Citation2007), including free radical injury, calcium overload, inflammation and injury of vascular endothelial cells (Yellon & Hausenloy, Citation2007). However, more and more evidence is found from animal experiments and clinical studies that the inflammatory responses play a vital role in MI/R injury (Di Paola et al., Citation2011; Jiang et al., Citation2010; Vinten-Johansen et al., Citation2007). Myocardial cell damage is thought to occur secondary to an intense inflammatory response initiated by the infiltration of leukocytes and the production of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) (Frangogiannis et al., Citation2000). It is well known that TNF-α triggers a cascade of nuclear factor kappa B (NF-κB) activation in response to postischemic reperfusion (Ke et al., Citation2004, Citation2011). Indeed, inhibiting NF-κB activation diminishes MI/R damage and potentially provides myocardial protection (Fischer et al., Citation2006; Gao et al., Citation2009; Kim et al., Citation2009; Zhang et al., Citation2011). It has also been reported that MI/R injury involves the generation of oxygen free radicals and elicits apoptosis along with an inflammatory response (Jennifer et al., Citation2009). Therefore, searching for anti-inflammatory compounds with minimal side effects from natural sources such as herbs or plants probably represent an ideal strategy to develop safe and effective agents for MI/R injury treatment.
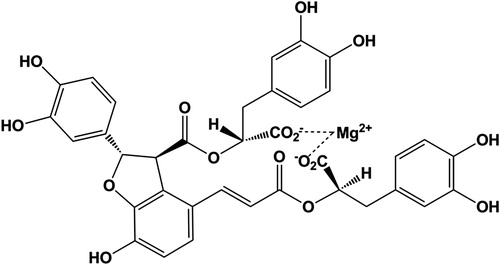
Danshen [Radix Salviae miltiorrhizae (Labiatae)], a well-known traditional Chinese medicine (TCM), has been used extensively for the treatment of coronary artery diseases, angina pectoris, myocardial infarction and celebrovascular diseases (Li et al., Citation2008; Zhong et al., Citation2009). Magnesium lithospermate B (MLB, ), an active polyphenol acid extracted from the dried root of Danshen (Tzen et al., Citation2007), is known to have antioxidative (Qu et al., Citation2011) and antifibrotic (Paik et al., Citation2011) effects. In addition, numerous studies have reported that MLB has renoprotective (Chen & Wang, Citation2006; Yokozawa et al., Citation1998) and neuroprotective (Tzen et al., Citation2007) effects. Notably, its anti-inflammation has drawn more attention in recent years. A recent study provides support for efficacy of MLB in inflammatory diseases and raises its therapeutic potential in activated T cell-mediated pathologies (Cheng et al., Citation2012).
In light of the previous findings, we hypothesize that anti-inflammatory activity of MLB might play an important role in cardioprotective effects against MI/R injury. However, up to now, few studies of MLB on cardioprotective effects have been carried out, especially the anti-inflammatory mechanisms of MLB are far from clear in the process of MI/R injury. Therefore, the aim of this study was to investigate the effects of MLB against MI/R injury and to explore its potential mechanisms of cardioprotection related to anti-inflammation.
Materials and methods
Animals
Adult male Sprague–Dawley rats (250 ± 20 g) were supplied by the animal research center at Fourth Military Medical University, Xi’an, China [Animal Certificate of Conformity: SCXK-(Army) 2007-007]. Rats were housed individually under constant temperature (23 ± 3 °C) and humidity with a 12 h light/dark cycle and had free access to chow and water. All animal protocols were approved by the Fourth Military Medical University Committee on Animal Care.
Chemical reagents
MLB (molecular formula: C36H28MgO16, molecular weight: 740.90, batch number: 1109181) was provided by Green Valley Company (Shanghai, China) and prepared using the method mentioned in the previous report (Tzen et al., Citation2007). EB (Evans blue) and TTC (triphenyltetrazolium chloride) were purchased from Sigma Chemical (St. Louis, MO). CK-MB (creatine kinase-MB), cTnI (cardiac troponin), IL-1β (interleukin 1β), IL-6 (interleukin 6) and TNF-α (tumor necrosis factor-α) ELISA (enzyme linked immunosorbent assay) kits were purchased from Westang Reagent Company (Shanghai, China). BCA Protein Assay and MPO (myeloperoxidase) ELISA kits were purchased from Jiancheng Reagent Company (Nanjing, China). Western blot reagents were purchased from Sigma (St. Louis, MO). Anti-phospho IkB-α, anti-phospho NF-κB and anti-β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Experimental protocols
The MI/R surgical procedure was used as described previously (Zhu et al., Citation2008). Briefly, Sprague–Dawley rats were intubated and artificially ventilated with a rodent ventilator (HX-100E, Taimeng, China) under anesthesia with 3% pentobarbital sodium (40 mg/kg, i.p.). The normal electrocardiogram was recorded after electrodes were placed subcutaneously and connected to an electrocardiograph (BL-420S, Taimeng, China). Coronary artery ligation was achieved with a gab occluder fixed onto the LAD coronary artery. A 7-0 silk suture was passed under neath the LAD (2–3 mm inferior to the left auricle) and tied. MI/R was induced by 30 min of ischemia followed by 3 h of reperfusion. Rats were divided into five groups: (I) Sham group, the silk suture crossed without ligation; (II) MI/R + vehicle group (model group), rats received saline alone; (III) MI/R + MLB (15 mg/kg) group, rats received MLB 15 mg/kg; (IV) MI/R + MLB (30 mg/kg) group, rats received MLB 30 mg/kg; (V) MI/R + MLB (60 mg/kg) group, rats received MLB 60 mg/kg. MLB was dissolved in saline to give a final concentration. MLB groups received MLB intravenously (i.v.) injection at the onset of reperfusion.
Measurement of ST-segment elevation
In brief, needle electrodes were inserted under the anesthetized rats’ skin as limbled at position II. Electrocardiograph parameters were monitored and recorded continuously, and ST-segment elevation in experimental animals was taken into consideration as previously described (Li et al., Citation2010).
Determination of infarct size
At 3 h after reperfusion, 2 ml 3% Evans blue was perfused into the aorta and coronary arteries after the ligation of the coronary artery. The entire ventricular tissue was sliced into five sections through the transverse axis from the apex to the atrioventricular groove. Tissues were incubated in 3 ml 2% TTC at 37 °C for 15 min as previously described (Gao et al., Citation2004). TTC stains viable tissue dark red while the infarct portion remains grayish-white. The area of infarction was determined by computerised planimetry (Image-ProPlus, Media Cybernetics, Bethesda, MD). The size of the risk zone (RZ) was estimated by the area as a percentage of the total left ventricle (TV). Similarly, the size of the infarct zone (IZ) was estimated by the area as a percentage of the RZ.
Determination of serum levels of CK-MB, cTnI, TNF-α, IL-1 and IL-6
At 3 h after reperfusion, carotid artery blood was collected and stored at −20 °C after centrifuging at 4000g for 15 min (TDZ4A-WS, Xiangyi, China) as previously described (Li et al., Citation2011). The levels of CK-MB, cTnI, IL-1, IL-6 and TNF-α in serum were measured using ELISA kits according to the manufacturer’s instructions.
Determination of MPO activity
Myocardial tissues from the IZ were saved. The tissues were homogenized in 5.0 ml of 0.1 M Tris-HCl buffer (pH = 7.4) in ice-cold conditions. The activity of MPO in tissue was measured using an ELISA kit according to the manufacturer’s instructions.
Histopathological examination
Heart samples were fixed in 10% formalin and embedded in paraffin (Hoffmeyer et al., Citation2000). The level of histological tissue injury was assessed by H&E staining and analyzed by light microscopy (Olympus, Tokyo, Japan). The following morphological criteria were used to determine the histopathological damage as previously described (Zingarelli et al., Citation1998): score 0, no damage; score 1 (mild), interstitial edema and focal necrosis; score 2 (moderate), diffuse myocardial cell swelling and necrosis; score 3 (severe), necrosis with the presence of contraction bands, neutrophil infiltration and the capillaries were compressed; and score 4 (highly severe), widespread necrosis with the presence of contraction bands, neutrophil infiltration, capillaries compressing and hemorrhage.
Western blot analysis
Total nuclear protein 50 μg was resolved on a 15% SDS-polyacrylamide gel. The fractionated proteins were electrophoretically transferred to an immobilon polyvinylidene difuride membrane and probed with rabbit polyclone anti-phosphor-NF-κB and antiphosphor-IkB-α at overnight 4 °C followed by incubation with the corresponding secondary antibodies at room temperature for 2 h as previously described (Li et al., Citation2011). The blots were visualized with ECL-Plus reagent (GE Healthcare, Pisca-taway, NJ). In all immunoblotting experiments, the blots were reprobed with an anti-β-actin antibody to control for protein loading.
Statistical analysis
Data were presented as mean ± S.D. and analyzed by Graphpad Prism software version 5.01 (La Jolla, CA). One-way ANOVA followed by Tukey’s test was applied for the statistical analysis of the parameters. Values at p < 0.05 were considered statistically significant.
Results
Effect of MLB on electrocardiograph parameters
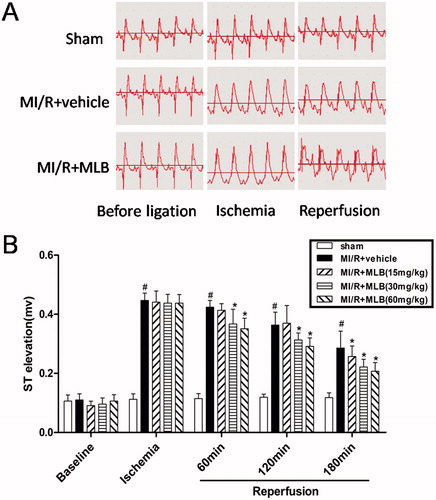
Rats in the MI/R group showed a marked elevation in ST-segments compared with rats in the sham group (p < 0.05, ). These changes were restored to normal in MLB-treated rats (p < 0.05, ).
Figure 2. Effect of MLB on ST elevation. (A) Electrocardiogram observation during the course from anesthesia (before ligation), ischemia to reperfusion. (B) Effect of MLB on ST elevation. Myocardial ischemia/reperfusion; MLB, magnesium lithospermate B. n = 8; values are expressed as the mean ± S.D. Significance was determined by ANOVA followed by Tukey’s test. #p < 0.05 versus Sham group; *p < 0.05 versus MI/R + vehicle group.
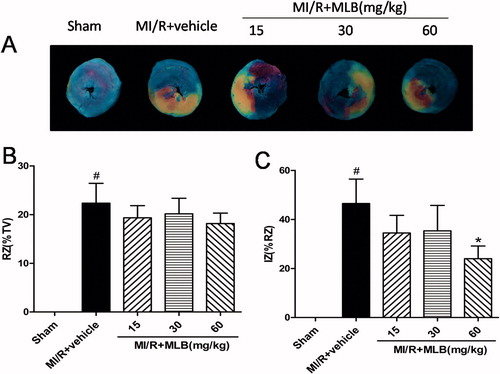
Effect of MLB on myocardial infarct area
The RZ was not different in the groups (). Myocardial IZ was 46.5 ± 9.93% in the model group. Treatment with MLB (60 mg/kg) was followed by lower IZ values (24.0 ± 5.18%, p < 0.05, ).
Figure 3. Effect of MLB on myocardial infarct size. (A) Myocardial risk and infarct area were determined by Evans blue and TTC method. (B) The effect of MLB administration on RZ (% TV) in rat after MI/R. (C) The effect of MLB administration on IZ (% RZ) in rat after MI/R. TV, total LV; RZ, risk zone; IZ, infarct zone; MI/R, myocardial ischemia/reperfusion; MLB, magnesium lithospermate B. n = 8; values are expressed as the mean ± S.D. Significance was determined by ANOVA followed by Tukey’s test. *p < 0.05 versus MI/R + vehicle group.
Effect of MLB on CK-MB, cTnI levels in serum
Compared with the sham group, CK-MB and cTnI levels in the serum of the model group markedly increased (p < 0.05, ), but decreased significantly when treated with MLB (60 mg/kg) (p < 0.05, ).
Table 1. Effect of MLB on the levels of CK-MB and cTnI in serum.
Effect of MLB on IL-1, IL-6 and TNF-α levels in serum
The levels of IL-1, IL-6 and TNF-α in serum were significantly elevated in the model group (p < 0.05, ), but markedly decreased by administration of MLB (60 mg/kg) (p < 0.05, ).
Table 2. Effect of MLB on the levels of IL-1β, IL-6 and TNF-α in serum.
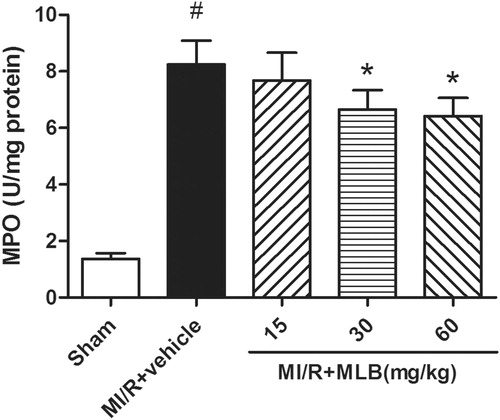
Effect of MLB on MPO activity in tissue
MPO activity was very low in the sham group, but markedly increased in the model group (8.24 ± 0.85, p < 0.05, ). MLB attenuated MPO activity in a dose-dependent manner (p < 0.05, ).
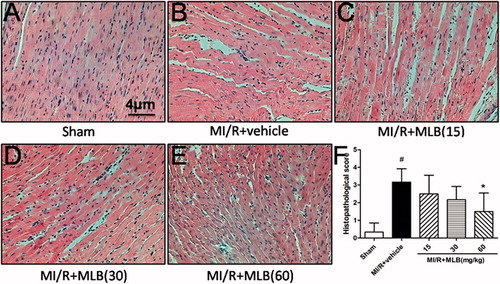
Effect of MLB on the morphology of heart muscle
The sham group showed normal histoarchitecture of myocardium without edema, myonecrosis and inflammatory infiltration (). However, the model group showed marked myocardial necrosis, membrane damage, infiltration of inflammatory cells, edema and inflammation compared to the sham group (). Animals treated with MLB (60 mg/kg) showed normal myocardial histoarchitecture resembling the sham group (). In addition, the histopathological score in group 60 mg/kg was 1.50 ± 1.05 (p < 0.05, ) and was lower than that in the model group (3.17 ± 0.75, p < 0.05, ).
Figure 5. Histopathological changes in rat cardiac tissue (H&E × 400). (A) Sham group, (B) MI/R + vehicle group, (C) MI/R + MLB (15 mg/kg) group, (D) MI/R + MLB (30 mg/kg) group, (E) MI/R + MLB (60 mg/kg) group, (F) Histopathological scores. MI/R, myocardial ischemia/reperfusion; MLB, magnesium lithospermate B. n = 8; values are expressed as the mean ± S.D. Significance was determined by ANOVA followed by Tukey’s test. # p < 0.05 versus Sham group; *p < 0.05 versus MI/R + vehicle group.
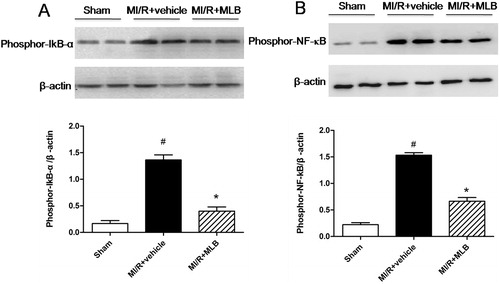
Effect of MLB on phosphorylated proteins that are involved in inflammation
Phosphor-IkB-α and phosphor-NF-κB were expressed highly after MI/R (1.36 ± 0.10; 1.53 ± 0.04, p < 0.05, ), but were inhibited, respectively, by treatment with MLB (0.40 ± 0.08, p < 0.05, ; 0.66 ± 0.06, p < 0.05, ).
Figure 6. Effect of MLB on protein expression of phosporylation of IkB-α and NF-κB in MI/R injury rats. (A) Phosporylation of IkB-α was analyzed by western blot. Graphs showing the protein ratio of phospor-IkB-α/β-actin. (B) Phosporylation of NF-κB was analyzed by western blot. Graphs showing the protein ratio of phospor-NF-κB/β-actin. MI/R, myocardial ischemia/reperfusion; MLB, magnesium lithospermate B. Values are expressed as the mean ± S.D. Significance was determined by ANOVA followed by Tukey’s test. # p < 0.05 versus Sham group; *p < 0.05 versus MI/R + vehicle group.
Discussion
MI/R injury is an essentially and strong process of inflammatory injury. In recent years, increasing evidence has shown that there is a close relationship between inflammatory response and MI/R injury (Jiang et al., Citation2010; Vinten-Johansen et al., Citation2007). In the present study, we demonstrate for the first time that MLB protects the heart from MI/R injury in a rat model, which is probably duo to its anti-inflammatory actions.
We assessed the therapeutic efficacy of MLB against MI/R injury by focusing on the correlated eletrocardiographic and infarct size. Electrocardiograph-abnormalities are the main criteria generally used for the definite diagnosis of myocardial injury (Zhou et al., Citation2008), and ST-segment elevation is considered as a proof of induced infarction (Agelaki et al., Citation2007). These alterations could be due to the consecutive loss of cell membrane in injured myocardium (Zhou et al., Citation2008). In the present study, MLB treatment markedly inhibited ST-segment elevation, suggesting its cell membrane protecting effects. The infarct size is considered as a key criterion in evaluating MI/R injury, and is related to the prognosis of patients in the clinic (Friedrich et al., Citation2008). It is worthy of noting that MLB could significantly reduce infarct size. In addition, during a reperfusion period, myocytes release a variety of intracellular enzymes into blood. CK-MB, which serves as the diagnostic marker of myocardial tissue damage, leaks out from the damaged tissues to the blood stream when the cell membrane becomes permeable or ruptured (Gürgün et al., Citation2008). cTnI is a protein that is specific to the myocardium. It is not found in the serum of healthy people, but can be detected in the serum only if myocardial injury has occurred. Therefore, cTnI has been shown to be specific for myocardial injury and is used as a sensitive and specific biochemical marker for clinically detecting myocardial damage (Fishbein et al., Citation2003). In our study, CK-MB and cTnI elevations were markedly blunted, respectively, when treated with MLB. This could be due to its action on maintaining membrane integrity thereby restricting the leakage of these enzymes. On histopathological examination, widespread myocardial structure disorder, extensive cloudy swelling, hyperemia, sinusoidal distension and focal vacuolar degeneration were observed in the model group. MLB restored most of these heart pathological lesions, showing the cardioprotective action of it. The overall pharmacodynamic results were rational to understand the beneficial effects of MLB on cardioprotection against MI/R injury in rats.
Accumulating evidence indicates that inflammatory responses, including cytokines elaboration and neutrophil infiltration, play a critical role in MI/R injury (Vinten-Johansen et al., Citation2007; Yellon & Hausenloy, Citation2007). Pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6, are not constitutively expressed in the normal heart. The up-regulation and production of these cytokines represent an intrinsic or an innate stress response against myocardial injury (Frangogiannis et al., Citation1998; Ma et al., Citation2010). In rodent models of myocardial infarction from the first hours to 1 day, there are robust up-regulations of intra myocardial cytokines including TNF-α and IL-1β (Vinten-Johansen et al., Citation2007). Moreover, TNF-α, IL-1β increase the production of superoxide anion, which reacts with NO to form peroxynitrite and in turn desensitizes myofilament to calcium, leading to myocardial contractile failure (Vinten-Johansen et al., Citation2007). In addition, IL-6 is derived from ischemic/reperfused myocardium. The amount of cytokine released by hypoxic myocytes is sufficient to stimulate transendothelial migration, superoxide anion generation and cause irreversible damage to myocytes in vitro (Jakob, Citation2004). Our data showed that the levels of TNF-α, IL-1β and IL-6 in serum increased in the model group, but a significant decrease of these inflammatory cytokines was observed by MLB treatment, which suggested that the cardioprotective effects of MLB could be attributed to the suppression of the inflammatory responses via inhibition of pro-inflammatory cytokines. This notion was further supported by the alteration of MPO activity. MPO accounts for 5% of the dry weight of the neutrophils, so the number of neutrophils in the myocardium could also be estimated by MPO activity, MPO is considered as the indicator of neutrophilic infiltration (Li et al., Citation2011). In this study, we found that MPO activity was enhanced in the cardiac tissue in the model group, while decreased by MLB treatment. This change of MPO activity indicated that MLB could reduce the neutrophils and attenuate inflammation, which was consistent with pro-inflammatory cytokine results. With our previous observations and findings, an explanation could be that MLB prevented MI/R injury due to regulation of inflammatory responses.
It has been well accepted that NF-κB is activated by MI/R in myocardium and activation of NF-κB is a very early regulatory event during reperfusion (Kis et al., Citation2003; Thiemermann, Citation2004). NF-κB is sequestered in the cytoplasm in an inactive form by its association with IkB-α, which prevents NF-κB from translocating into the nucleus, in the rest status. However, the activation of NF-κB signaling results in phosphorylation-induced proteolytic degradation of IkB-α (Tuntipopipat et al., Citation2011). Furthermore, free NF-κB translocates to the nucleus, where it induces transcription of a variety of genes including TNF-α and IL-6 (Ahn & Aggrawal, Citation2005). To further investigate anti-inflammatory mechanisms of MLB, the expression of phosphor-IkB-a and phosphor-NF-κB were determined by western blots. Our study indicated that MLB could attenuate phosphor-IkB-α and phosphor-NF-κB levels in myocardial tissues, which were associated with the decrease of TNF-α and IL-6 in serum. These results provided evidence that the anti-inflammatory activity of MLB could be due to the inhibition of NF-κB activation.
Conclusions
In summary, our study suggested that MLB possessed cardioprotection on MI/R injury, with decreasing ST-segment elevation, diminuting infarct size, alleviating myocardial damage severity, attenuating the pro-inflammatory cytokines levels and MPO activity. Furthermore, the protective effect of MLB appeared to be mediated by inhibiting the phosphorylation of IkB-α and NF-κB, and thus regulating the inflammation response in MI/R injury. These findings revealed novel evidence for the beneficial effects of MLB in treating MI/R injury, and helped to gain some insight into the therapeutic potential of MLB in MI/R.
Declaration of interest
The authors report no conflicts of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81173514; No. 81001673), the Xijing Research Boosting Program (No. XJZT10D02) and the Excellent Civil Service Training Fund of Fourth Military Medical University (No. 4138C4IDK6).
References
- Agelaki MG, Pantos C, Korantzopoulos P, et al. (2007). Comparative antiarrhythmic efficacy of amiodarone and dronedarone during acute myocardial infarction in rats. Eur J Pharmacol 564:150–7
- Ahn KB, Aggrawal BB. (2005). Transcription factor NF-{kappa} B: A sensor for smoke and stress signals. Ann NY Acad Sci 1056:218–33
- Chen CG, Wang YP. (2006). Magnesium lithospermate B ameliorates renal cortical microperfusion in rats. Acta Pharmacol Sin 27:217–22
- Cheng CC, Yang SP, Lin WS, et al. (2012). Magnesium lithospermate B mediates anti-inflammation targeting activator protein-1 and nuclear factor-kappa B signaling pathways in human peripheral T lymphocytes. Int Immunopharmacol 13:354–61
- Di Paola R, Mazzon E, Paterniti I, et al. (2011). Olprinone, a PDE3 inhibitor, modulates the inflammation associated with myocardial ischemia--reperfusion injury in rats. Eur J Pharmacol 650:612–20
- Fischer UM, Antonyan A, Bloch W, Mehlhorn U. (2006). Impact of antioxidative treatment on nuclear factor kappa-B regulation during myocardial ischemia--reperfusion. Interact Cardiovasc Thorac Surg 5:531–5
- Frangogiannis NG, Lindsey ML, Michael LH, et al. (1998). Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 98:699–710
- Frangogiannis NG, Mendoza LH, Lindsey ML, et al. (2000). IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol 165:2798–808
- Friedrich MG, Abdel-Aty H, Taylor A, et al. (2008). The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol 51:1581–7
- Fishbein MC, Wang T, Matijasevic M, et al. (2003). Myocardial tissue troponins T and I: An immunohistochemical study in experimental models of myocardial ischemia. Cardiovasc Pathol 12:65–71
- Gao HK, Yin Z, Zhang RQ, et al. (2009). GSK-3beta inhibitor modulates TLR2/NF-kappa B signaling following myocardial ischemia--reperfusion. Inflamm Res 58:377–83
- Gao F, Tao L, Yan W, et al. (2004). Early anti-apoptosis treatment reduces myocardial infarct size after a prolonged reperfusion. Apoptosis 9:553–9
- Gürgün C, Ildızlı M, Yavuzgil O, et al. (2008). The effects of short term statin treatment on left ventricular function and inflammatory markers in patients with chronic heart failure. Int J Cardiol 123:102–7
- Hoffmeyer MR, Scalia R, Ross CR, et al. (2000). PR-39, a potent neutrophil inhibitor attenuates myocardial ischemia--reperfusion injury in mice. Am J Physiol Heart Circ Physiol 279:2824–8
- Jakob VJ. (2004). Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res 61:481–97
- Jennifer LS, Kasi VR, Jennifer LS. (2009). Direct thrombin inhibition limits ischemia and reperfusion injury in rats by decreasing oxidative stress, apoptosis, and inflammation. Circulation 120:S835–6
- Jiang WL, Fu FH, Xu BM, et al. (2010). Cardioprotection with forsythoside B in rat myocardial ischemia--reperfusion injury: Relation to inflammation response. Phytomedicine 17:635–9
- Ke JJ, Wang YL, Li JG, et al. (2004). Pretreatment effect of adenosine on activation of NF-kappa B and level of TNF-alpha during myocardial ischemia and reperfusion in rats. Chin J Traumatol 7:25–7
- Ke JJ, Yu FX, Rao Y, Wang YL. (2011). Adenosine postconditioning protects against myocardial ischemia--reperfusion injury though modulate production of TNF-alpha and prevents activation of transcription factor NF-kappa B. Mol Biol Rep 38:531–8
- Kim JW, Jin YC, Kim YM, et al. (2009). Daidzein administration in vivo reduces myocardial injury in a rat ischemia/reperfusion model by inhibiting NF-kappa B activation. Life Sci 84:227–34
- Kis A, Yellon DM, Baxter GF. (2003). Role of nuclear factor-kappa B activation in acute ischemia--reperfusion injury in myocardium. Br J Pharmacol 138:894–900
- Li CM, Gao YL, Xing YL, et al. (2011). Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia--reperfusion injury in rats via regulating the inflammation response. Food Chem Toxicol 49:2090–5
- Li CM, Liu ZF, Tian LJ, et al. (2010). Protective roles of Asperosaponin VI, a triterpene saponin isolated from Dipsacus asper Wall on acute myocardial infarction in rats. Eur J Pharmacol 627:235–41
- Li MH, Chen JM, Peng Y, et al. (2008). Investigation of Danshen and related medicinal plants in China. J Ethnopharmacol 120:419–26
- Ma Y, Ni W, Zhu WZ, et al. (2010). Effect of repeated ischemic preconditioning on TLR4 and proinflammatory cytokines TNF-alpha and IL-1beta in myocardial ischemia--reperfusion injury in a rat model. Arch Med Sci 6:843–7
- Paik YH, Yoon YJ, Lee HC, et al. (2011). Antifibrotic effects of magnesium lithospermate B on hepatic stellate cells and thioacetamide-induced cirrhotic rats. Exp Mol Med 43:341–9
- Qu J, Ren X, Hou RY, et al. (2011). The protective effect of magnesium lithospermate B against glucose-induced intracellular oxidative damage. Biochem Biophys Res Commun 411:32–9
- Thiemermann C. (2004). Inhibition of the activation of nuclear factor kappa B to reduce myocardial reperfusion injury and infarct size. Cardiovasc Res 63:8–10
- Tuntipopipat S, Muangnoi C, Chingsuwanrote P, et al. (2011). Anti-inflammatory activities of red curry paste extract on lipopolysaccharide-activated murine macrophage cell line. Nutrition 27:479–87
- Tzen JT, Jinn TR, Chen YC, et al. (2007). Magnesium lithospermate B possesses inhibitory activity on Na+, K+-ATPase and neuroprotective effects against ischemic stroke. Acta Pharmacol Sin 28:609–15
- Vinten-Johansen J, Jiang R, Reeves JG, et al. (2007). Inflammation, proinflammatory mediators and myocardial ischemia--reperfusion Injury. Hematol Oncol Clin North Am 21:123–45
- Yellon DM, Hausenloy DJ. (2007). Myocardial reperfusion injury. N Engl J Med 357:1121–35
- Yokozawa T, Oura H, Nishioka I. (1998). Confirmation that magnesium lithospermate B ameliorates paraquat--induced injury in cultured renal epithelial cells. Nephron 79:373–4
- Zhang HM, Tang DL, Tong L, et al. (2011). Gua lou xie bai ban xia decoction inhibits NF-kappa B-dependent inflammation in myocardial ischemia--reperfusion injury in rats. J Tradit Chin Med 31:338–43
- Zhong GX, Li P, Zeng LJ, et al. (2009). Chemical characteristics of Salvia miltiorrhiza (Danshen) collected from different locations in China. J Agric Food Chem 57:6879–87
- Zhou R, Xu QB, Zheng P, et al. (2008). Cardioprotective effect of fluvastatin on isoproterenol -- induced myocardial infarction in rat. Eur J Pharmacol 586:244–50
- Zhu JH, Qiu YG, Wang QQ, et al. (2008). Low dose cyclophosphamide rescues myocardial function from ischemia--reperfusion in rats. Eur J Cardiothorac Surg 34:661–6
- Zingarelli B, Salzman AL, Szabo C. (1998). Genetic disruption of poly (ADP-Ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ Res 83:85–94