Abstract
Context: The detrimental effects of arsenic on female reproductive functions may involve overt oxidative stress. Casein and pea [Pisum sativum Linn. (Fabaceae)] proteins have antioxidant properties.
Objective: To investigate the role of casein- and pea-supplemented high-protein diet (HPD) in utero-ovarian protection from arsenic toxicity.
Materials and methods: Adult female Wistar rats were orally gavaged with vehicle (Gr-I) or arsenic at 3 ppm/rat/d (Gr-II and Gr-III) for 30 consecutive days, when they were maintained on either regular diet containing 18% protein (Gr-I and Gr-II), or HPD containing 27% protein in the form of casein (20%) and pea (7%) (Gr-III). Reproductive functions were evaluated using a battery of biochemical and histological techniques.
Results: As compared to Gr-I, the Gr-II rats suffered from loss of estrous cyclicity, reduction in weight (mg/100 g body weight) of ovary (Gr-I: 54.3 ± 4.2 versus Gr-II: 35.8 ± 1.6; p < 0.001) and uterus (Gr-I: 161.7 ± 24.6 versus Gr-II: 94.44 ± 13.2; p < 0.05), utero-ovarian degeneration, attenuated ovarian activities (unit/mg tissue/h) of Δ5, 3β-hydroxysteroid dehydrogenase (Gr-I: 3.41 ± 0.12 versus Gr-II: 2.31 ± 0.09; p < 0.01) and 17β-hydroxysteroid dehydrogenase (Gr-I: 3.82 ± 0.57 versus Gr-II: 1.24 ± 0.19; p < 0.001), and decreased serum estradiol level (pg/ml) (Gr-I: 61.5 ± 2.06 versus 34.1 ± 2.34; p < 0.001). Ovarian DNA damage was preponderant with blatant generation of malondialdehyde (nM/mg tissue; Gr-I: 15.10 ± 2.45 versus Gr-II: 29.51 ± 3.44; p < 0.01) and attenuated superoxide dismutase activity (unit/mg tissue) (Gr-I: 2.18 ± 0.19 versus Gr-II: 1.33 ± 0.18; p < 0.05). The Gr-III rats were significantly protected from these ill effects of arsenic.
Discussion and conclusion: HPD, by way of antioxidant properties, may find prospective role in the protection of reproductive damage caused by arsenic.
Introduction
Arsenic is widely distributed in the environment and available to the human population through sources including drinking water, food and air. Significant human exposure to arsenic occurs through both anthropogenic and natural sources. Occupational exposure to arsenic is common in the smelting industry, microelectronics industry and in occupational settings where arsenic is used to manufacture pesticides, wood preservatives and superfund sites where industrial waste are disposed. However, human exposure to arsenic occurring mainly through drinking water has become an important global public health concern. Population exposed to arsenic-contaminated drinking water includes those in Taiwan, China, Europe, United States, Bangladesh and India (Rahman et al., Citation2005). The maximum permissive contaminant level of arsenic in drinking water, as set by the United States Environment Protection Agency (USEPA) and the World Health Organization (USEPA, Citation2001; WHO, Citation1981), is 10 μg/L. But contamination from natural sources in different geographically distant areas of the globe can reach hundreds of micrograms/liter. In certain areas of the Indian subcontinent, the maximum arsenic concentration in ground water was found to be several hundred times the permissible limit (Chakraborti et al., Citation2002).
High arsenic exposure is responsible for causing various human diseases including cardiovascular, diabetes and cancers of the skin, lung, bladder, kidney and liver (Huang et al., Citation2004; Rossman, Citation2003; Tapio & Grosche, Citation2006). The detrimental impact of arsenic also includes dysfunction of endocrine (Tseng et al., Citation2000) and reproductive systems (Singh & Rana, Citation2007). Limited evidence suggests that adverse human reproductive effects of arsenic may include higher risk of low birth weight, spontaneous abortion, preeclampsia, congenital malformation, infant mortality (Ahmad et al., Citation2001) and carcinoma incidence to the new born (Shen et al., Citation2007). In experimental rodent model, arsenic has been reported to produce toxic effects on female reproductive system (Chattopadhyay et al., Citation1999).
Reproductive tract function in the female is primarily controlled by ovarian sex steroids, estradiol (E2) and progesterone, which are again controlled by the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Arsenic exposure poses a serious threat to the ovary, the main source of E2 (Chatterjee & Chatterji, Citation2010; Chattopadhyay & Ghosh, Citation2010). The levels of E2, LH and FSH are reported to diminish in arsenic-intoxicated rats (Chattopadhyay & Ghosh, Citation2010). Disrupted circulating level of E2 is held responsible for the gross histological alteration of the uterus; however, a recent study demonstrated that arsenic also disrupts the estrogen signaling pathway (Chatterjee & Chatterji, Citation2010). All these effects may be involved in imposing serious troubles in maintaining normal reproductive functions and pregnancy outcome. However, the precise mechanism still remains unknown.
The metabolism of arsenic contributes significantly to the adverse health consequences in arsenic-exposed population. In humans, inorganic arsenic is oxidatively methylated to monomethylarsonic acid and finally to dimethylarsinic acid, followed by renal excretion. This biomethylation of arsenic is considered as the primary detoxification mechanism, since the inorganic arsenic is more toxic to the living organism (Yamauchi & Fowler, Citation1994).
Arsenic is known to produce oxidative stress (Kitchin, Citation2001). Exposure to inorganic arsenic in vitro sets on a wide array of molecular events that are often associated with production of free radicals and reactive oxygen species (ROS) (Tabacova et al., Citation1992). The source of the ROS is not precisely known but repeated oxidation and reduction of arsenic during its metabolism possibly contribute. The deleterious effects of arsenic are believed to be mediated largely by exaggerated ROS generation that can lead to DNA damage (single-strand breaks; Kitchin, Citation2001) and contribute significantly to the induction of gonadal toxicity (Akram et al., Citation2010; Chatterjee & Chatterji, Citation2010). It has been demonstrated that arsenic-induced increased generation of ROS causes oxidative damage to the proteins involved in the estrogen signaling pathway, thus, disrupts estrogen signaling (Chatterjee & Chatterji, Citation2010).
Association between nutritional status and arsenic toxicity is well established. Some of the dietary proteins are known to have antioxidant properties of which pea [Pisum sativum Linn. (Fabaceae)] seed proteins and milk protein casein are very remarkable. The antioxidant activities of pea and casein, and their potential effectiveness behind the minimization of lipid peroxidation along with the ability to prevent free radical-mediated oxidative damages have been demonstrated in different liposomal models (Maiti & Chatterjee, Citation2001; Mukherjee et al., Citation2003; Wong & Kitts, Citation2003). Pea proteins in addition, can act as potent methyl transferring agents in the methylation process (Joshi & Handler, Citation1960) and thereby contribute a major role in detoxification of inorganic arsenic (Mukherjee et al., Citation2003). Recent studies suggest that the simultaneous use of casein and pea could ameliorate arsenic-induced gonadal toxicity in male rats (Mukherjee & Mukhopadhyay, Citation2009), and all-trans retinoic acid could also reverse the arsenic-induced structural, endocrinological and molecular damages of female reproductive system of rats, which provide the presumptive evidence for the protective effect of antioxidant substances in arsenic toxicity (Chatterjee & Chatterji, Citation2011). However, to date, no report is available to suggest if supplementation of these dietary proteins, by virtue of their potential antioxidative properties, can protect from arsenic-induced utero-ovarian toxicity.
This study, therefore, uses a suitable rat model that emphasizes two major objectives: (a) to explore the relationship between arsenic and utero-ovarian oxidative damages and (b) to investigate if strategic use of high dietary proteins in the form of casein and pea can protect animals from the arsenic-mediated reproductive damages.
Materials and methods
Animal selection and care
Twenty-four adult female albino rats of Wistar strain having regular estrous cycle of 4–5 d length and weighing from 113–127 g were selected for this experiment. The animals were maintained under standard laboratory conditions (14 h light:10 h dark, 25 ± 2 °C temperature) with free access to food and water. All animal experiments were performed according to the ethical guidelines suggested by the Institutional Animal Ethical Committee and Committee for the Purpose of Control of Supervision of Experiments on Animals, Ministry of Environment and Forest, Government of India.
Preparation of high-protein diet
The standard diet was composed of 71% carbohydrate, 18% protein, 7% fat, 4% salt mixture and vitamins (Chatterjee et al., Citation1970). High-protein diet (HPD), which was isocaloric with respect to the normal diet, was prepared by increasing the protein content from 18 to 27% with the addition of pea and excess casein.
Preparation of aqueous solution of arsenic trioxide
Arsenic trioxide (75 mg/ml) was dissolved in 1 ml 10% NaOH, and the volume of the solution was made up to 100 ml with distilled water after adjusting the pH at 6.8 with 1.0 N H2SO4 (Chappell et al., Citation1997).
Treatment protocol
The rats were allocated to three groups, each comprising eight rats. They were treated with the vehicle (Group I: Control) or aqueous solution of arsenic trioxide at a dose level of 3 ppm/rat/d (Groups II and III: Experimental) for 30 consecutive days and fed standard diet (Groups I and II) or HPD (Group: III).
Study of estrous cycle and sample collection
Vaginal lavage of the rats were collected at 10.00 h and estrous cyclicity was checked using a conventional microscopic method (Marcondes et al., Citation2002). The respective stage of the cycle was identified on the basis of relative abundance of nucleated epithelial cells (pro-estrus), cornified cells (estrus) and leukocytes (diestrus). Cycles with duration of 4–5 d were considered regular. Continuation of diestrus phase for more than 3 d was considered as persistent diestrus. Feeding habits of the rats were noticed throughout the experimental schedule, and the body weights were recorded before and after the experimental period. All the animals were subjected to light anaesthesia by intramuscular injection of ketamin (22 mg/kg body wt) on the day of appearance of the first diestrus phase following cessation of treatment, usually within 1–3 d. Immediately, blood was collected from the dorsal aorta, and the serum was separated (using standard protocol), frozen and stored at −20 °C for hormone assay. The rats were autopsied, and the ovaries and uteri were dissected out and precisely weighed. One ovary and one uterine horn from each rat were processed for the histological examination, while the remaining ovary and uterine horn were stored frozen (–20 °C) for biochemical and DNA damage studies.
Histological and histomorphometric studies of ovarian and uterine tissues
Bouin’s fixed ovarian and uterine tissues were embedded in paraffin, serially sectioned at 5 µ and stained with hematoxylin/eosin for different microscopic observations. The follicular developmental pattern along with the status of corpus luteum and nature of the stroma were studied from the ovarian sections. The diameter of the uterine horn, height of the uterine luminal epithelium, thickness of endometrium and myometrium were measured microscopically (Zeiss, Thornwood, NY) using Progress CapturePro 2.5 software (GENOTYPIC Optical Systems GmBH, Jena, Germany).
Assay of ovarian Δ5, 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase activities
A fraction of one frozen (−20 °C) ovary from each rat of all groups was homogenized separately in 20% spectroscopic-grade glycerol containing 5.0 mM potassium phosphate and 1.0 mM ethylenediamine tetra acetic acid (EDTA) at a tissue concentration of 15 mg/ml homogenizing mixture. The homogenate was centrifuged at 10 000 × g for 30 min at 4 °C, and the supernatant was used to assess ovarian Δ5, 3β-hydroxysteroid dehydrogenase (Δ5, 3β-HSD) and 17β-hydroxysteroid dehydrogenase (17β-HSD) activities. The Δ5, 3β-HSD activity was measured after addition of 0.5 µM of nicotinamide adenosine dinucleotide (NAD) and 30 µg of dehydroepiandrosterone in the tissue supernatant using a spectrophotometer (JASCO v-530, Easton, MD) at 340 nm against reagent blank (without NAD; Talalay, Citation1962). The residual supernatant of the homogenizing mixture was used to measure the 17β-HSD activity. The supernatant was mixed with 25 mg of crystalline bovine serum albumin, 0.3 µM testosterone and 1.1 µM of nicotinamide adenosine dinucleotide phosphate. The activity was measured at 340 nm against a reagent blank (without nicotinamide adenosine dinucleotide phosphate; Jarabak, Citation1969). One unit of enzyme activity is equivalent to a change in absorbance of 0.001/min at 340 nm.
Assay of serum E2
Serum concentration of E2 was assayed by radioimmunoassay using commercial kits purchased from Bio-Line, Brussels, Belgium, following the procedure as elaborated in the kit manual. The antisera used, had the maximum cross-reactivity (1.8%) with estrone. The minimum detection limit of E2 was 8 pg/ml serum. The intra- and inter-assay coefficients of variation were 4.9 and 10.1%, respectively.
Measurement of ovarian malondialdehyde content
Malondialdehyde (MDA) levels in the ovarian tissues from rats of all groups were measured biochemically (Ohkawa et al., Citation1979). Ovarian tissue samples were homogenized in 0.1 M phosphate buffer (pH 7.4) at tissue concentration of 20 mg/ml and centrifuged at 15 000 × g at 4 °C for 5 min. The supernatant (0.5 ml) was used for the measurement of MDA. The supernatant was mixed with 0.5 ml saline (0.9%) and 2 ml of thiobarbituric acid--trichloroacetic acid (TBA–TCA) mixture (0.392 g TBA in 75 ml of 0.25 N HCl with 15 g TCA, volume up to 100 ml by 95% ethanol) and boiled for 10 min. The mixture was then cooled to room temperature and centrifuged at 4000 rpm for 10 min. The whole supernatant was taken in spectrophotometric cuvette and read at 535 nm.
Ovarian superoxide dismutase activity
Superoxide dismutase (SOD) activity in ovarian tissues was measured biochemically (Martin et al., Citation1987). The tissue was homogenized in an ice cold medium containing 0.1 M phosphate buffer (pH 7.2) at a tissue concentration of 20 mg/ml. The homogenate was centrifuged at 10 000 × g for 20 min at 4 °C. The enzyme activity was assessed in a medium containing 0.1 M phosphate buffer with 0.5 mM EDTA by measuring the auto oxidation of hematoxylin with or without adding the supernatant of tissue homogenate. The spectrophotometric readings were taken at 560 nm.
Ovarian single cell gel electrophoresis
Small ovarian tissues were minced and suspended (2 mg/ml) in cold Hank's balanced salt solution (HBSS) (0.137 M NaCl, 5.4 mM KCl, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 1.3 mM CaCl2, 1.0 mM MgSO4 and 4.2 mM NaHCO3) containing 20 mM EDTA and 10% dimethyl sulfoxide (DMSO; Singh et al., Citation1988). The tissues were allowed for 15 min to settle down, then 5–10 µL of the cell suspension was taken and mixed with 75 µL 1% low melting point agarose. It was embedded in a thin 1% normal melting point agarose on a microscope slide. All cellular proteins are then removed from the cells by lysing with a lysis buffer (containing 2.5 M NaCl, 0.1 M Na2EDTA, 10 mM Trizma base, 1% Triton X and 10% DMSO) and refrigerated overnight. DNA was allowed to unwind under alkaline condition. Electrophoresis was performed for 20 min under alkaline condition (300 mM NaOH and 1 mM EDTA, pH > 13) at 280 mA and 24 V (∼0.74 V/cm), the broken DNA fragments or damaged DNA undergoing electrophoresis migrate away from the nucleus. In this process, the length of migration is inversely proportional to the size of fragment. The slides were then neutralized using neutralizing buffer (0.4 M Tris, pH 7.5) followed by staining with a fluorescent dye (ethidium bromide 2 μg/mL), and DNA “comet” was visualized by fluorescent microscope (Leica DM 3000). The head and tail lengths were measured in 50 randomly selected cells per slide using Leica QWinPlus digital image processing and analysis software, Leica Microsystems, Heerbrugg, Switzerland. The DNA damage was evaluated by the length of the Comet’s tail by “CometScore” software (TriTek Corp., Sumerduck, VA).
Statistical analysis
The data were expressed as mean ± standard error of mean. One-way ANOVA followed by Bonferroni test was used to compare the means between the different treatment groups. For all instances, p < 0.05 was considered to be statistically significant. Statistical analyses were carried out by GraphPad statistical software (GraphPad Software Inc., La Jolla, CA).
Results
Food consumption and body and organ weights
There were no differences in food consumption between the groups throughout the experimental schedule. The body weight of the arsenic-treated groups with and without HPD supplementation also did not differ significantly from that of the control (). Arsenic treatment caused significant reduction in the wet weight of the ovary (p < 0.001) and uterus (p < 0.05) in comparison to that of the control. Arsenic-exposed group with HPD supplementation, by contrast, experienced no such decrease in the ovarian and uterine weight that were significantly (p < 0.05) higher than that of the arsenic-treated group without HPD supplementation ().
Table 1. Changes in body weight, ovarian and uterine somatic indices and pattern of estrous cycle in arsenic trioxide exposed rats co-administered with/without HPD.
Estrous cycle
The regular course of the estrous cycle was significantly (p < 0.001) interrupted in the arsenic-treated group. Their estrous cycles were characterized by an initial increase in cycle length that reduced the number of cycles; but subsequently after 22 ± 2 d of arsenic administration, the cycle was replaced by a constant diestrus phase. In the HPD-supplemented group, no major estrous cycle irregularities were observed (p < 0.001).
Ovarian histology
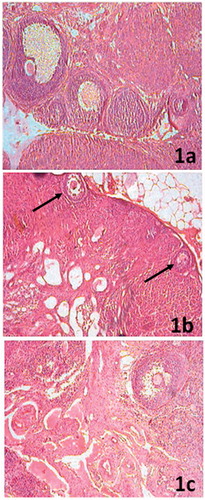
Ovarian sections of control rats showed follicles belonging to different stages of maturation lying somewhat deeper into the cortex. There were few functional corpora lutea scattered throughout the periphery that document recent ovulation (). Scarce number of follicles was evident with arrested growth at preantral stage. Follicles were surrounded by a large number of pyknotic granulosa cells arranged in symmetric rings beneath the basement membrane offering the structure of a string of bids that marked the preponderance of atretic follicles. Lutein bodies comprising dense collagenous tissues characterized the old scar of corpora lutea. A significant part of the stroma was filled with fibrous connective tissue (). More than 60% of the supplemented rat ovaries exhibited grossly normal follicular growth and maturation. A number of late-stage secondary follicles were present. Presence of few fully functional corpora lutea marked recent past ovulation ().
Figure 1. (a) Ovarian section of control rats showing follicles at different stages of maturation and recent ovulation is documented by few functional corpora lutea scattered throughout the periphery (100×). (b) Ovarian section of arsenic-treated rats exhibiting scarce number of follicles and atretic cells with arrested growth at preantral stage (arrow) and a significant part of the stroma is filled by fibrous connective tissue (100×). (c) In greater than 60% of the HPD-supplemented group ovaries, there is major restoration of follicular growth and maturation. Presence of few fully functional corpora lutea marks recent past ovulation (100×).
Uterine histology
The arsenic intoxicated rats demonstrated significant thinning of the uterine myometrium (p < 0.001), endometrium (p < 0.05) and epithelial layer (p < 0.001) with consequent reduction in the diameter of the uterine horn (p < 0.001; ). The HPD-supplemented group exhibited no degenerative changes in the uterine layers. The uterine horn diameter in this group, however, was significantly higher (p < 0.001) than that of the arsenic-treated group, but still lower (p < 0.001) as compared to the control (, ).
Figure 2. (a) Uterine section from control rats showing normal histological features (100×). (b) Significant thinning of uterine luminal epithelium, myometrium and endometrium with a consequent reduction in the diameter of the uterine horn in arsenic intoxicated rats (100×). (c) Restoration of normal histological features of the uterus in HPD-supplemented group (100×).
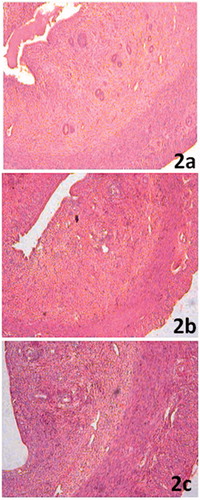
Table 2. Changes in uterine histometry in arsenic trioxide exposed rats co-administered with/without HPD.
Ovarian steroidogenic enzyme activities
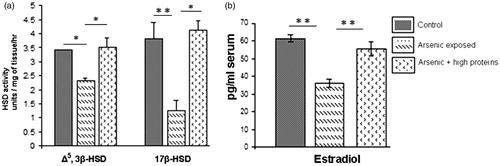
A notable inhibition in the activities of ovarian Δ5, 3β-HSD (p < 0.01) and 17β-HSD (p < 0.001) was observed in arsenic-treated rats compared with that in control. The HPD supplementation significantly protected these steroidogenic enzyme activities (p < 0.05 and <0.001, respectively; ).
Figure 3. (a) Comparative ovarian Δ5, 3β-HSD and 17β-HSD activities in the control and arsenic-treated groups with or without HPD supplementation (mean ± SEM), n = 8. (b) Comparative estradiol levels in the control and arsenic-treated groups with or without HPD-supplementation (mean ± SEM), n = 8. *p < 0.05 and **p < 0.001.
Serum E2 level
A significant decrease of serum E2 level (p < 0.001) was observed in the arsenic-treated group as compared to that in control. A significant elevation (p < 0.001) towards the normal level was observed with the co-administration of HPD ().
Ovarian MDA level
There was significant elevation in the ovarian MDA level (p < 0.01) in the arsenic-treated group compared with the control group. However, supplementation with HPD in arsenic intoxicated rats made a significant correction in MDA level (p < 0.01; ).
Table 3. Effect of HPD co-administration on ovarian MDA level, SOD activity and DNA damage in arsenic trioxide-treated rats.
Ovarian SOD activity
Diminution in ovarian SOD activity (p < 0.05) was observed in the arsenic induced state, but a noticeable alteration (p < 0.05) was resulted with HPD supplementation. Ovarian SOD activity was seen to be totally protected (p < 0.05) in the HPD co-treated group ().
Ovarian DNA damage
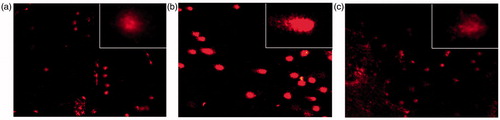
Comet assays revealed significant (p < 0.001) DNA strand breaks in ovarian cells of the arsenic-treated rats. As evaluated by the mean length of comets, co-administration of HPD significantly prevented (p < 0.001) arsenic-mediated DNA damage, though the recovery was not to the extent that of control (p < 0.001; ). The photomicrographs of comets, as visualized under fluorescence microscope, are presented in .
Discussion
Arsenic, a potent heavy metal toxicant widely distributed through drinking water and food, is considered a serious health threat worldwide. Arsenic toxicity is reported to jeopardize female reproductive functions in diverse ways (Akram et al., Citation2010; Chatterjee & Chatterji, Citation2010). This study demonstrates that strategic use of high dietary proteins in the forms of casein and pea may help in preventing arsenic-induced female reproductive toxicity to a significant extent. Human exposure to arsenic occurs mainly through drinking water. Oral route of administration was therefore preferred. There exists a wealth of literature on experimental laboratory models of arsenic toxicity (Chatterjee & Chatterji, Citation2010; Chattopadhyay & Ghosh, Citation2010). Taking the reported doses into account, we have previously optimized the dose and duration of arsenic treatment that could effectively develop arsenic toxicity in male rats (Mukherjee & Mukhopadhyay, Citation2009). This remains the basis of selecting the arsenic dose for the present communication.
Exposure to arsenic did not impact the general body growth of rats. However, reproductive functions were grossly affected. The treated rats lost estrous cyclicity and displayed persistent diestrus phase. There was a significant decrease in ovarian and uterine weight. Histologically, the ovary demonstrated the presence of follicles arrested at preantral stage and virtual absence of antral follicles, while the uterine architecture reflected absence of estrogenic support. These findings are in good agreement with earlier reports (Chatterjee & Chatterji, Citation2010; Chattopadhyay & Ghosh, Citation2010) pertaining to arsenic effects on female reproduction.
Growth and proliferation of uterine layers are primarily regulated by E2 (Patil et al., Citation1998), while progesterone mainly differentiates. Thus, attenuated follicular maturation with consequent decrease in E2 level under arsenic-intoxicated state may be held responsible for the cellular degeneration in uterine layers.
Interrupted ovarian steroidogenesis under arsenic toxicity was perhaps a secondary consequence of attenuated activity of Δ5, 3β-HSD and 17β-HSD, the two key enzymes involved in ovarian steroidogenesis. Steroidogenic function of ovary is under the direct stimulatory influence of pituitary gonadotropins, and earlier studies have demonstrated that arsenic exposure causes a decrease of serum gonadotropins and the E2 levels (Tagatz et al., Citation1970). Arrested folliculogenesis therefore may be due to the low plasma levels of gonadotropins and E2, which are the prime regulators involved in follicular maturation process (Gore-Langton & Daniel, Citation1990).
There are earlier reports that ovotoxic effects of arsenic perhaps involve a loss of balance between oxidant and antioxidant systems leading to oxidative stress (Cassano et al., Citation1999; Hochstein & Atallah, Citation1988; Sun, Citation1990). As for many other aspects of human health, the accumulation of damage exerted by increased levels of ROS is claimed to be involved in ovarian aging (Tarin, Citation1996). ROS play a role in the modulation of an entire spectrum of physiological reproductive functions including oocyte maturation, ovarian steroidogenesis, corpus luteal function and luteolysis (Agarwal et al., Citation2005). Exposure to inorganic arsenic in vitro also demonstrates production of free radicals and ROS (Kitchin, Citation2001). Several studies in both animal models and humans suggest that primordial and preovulatory follicles may suffer from oxidative stress due to impairment of antioxidant enzymatic defenses (Tarin, Citation1996). Arsenic toxicity triggered ovarian oxidative stress is supported by the decreased ovarian SOD activity along with the overproduction of MDA. It may be significant in this context to note that FSH and E2 belong to antiapoptotic factors, which contribute significantly to follicular survival (Quirk et al., Citation2004), and the antiapoptotic effect of FSH is mediated, in part, by suppression of ROS (Tsai-Turton & Luderer, Citation2006). Since arsenic toxicity decreased gonadotropin levels and inhibited follicular E2 synthesis, the resultant follicular environment had to suffer from deficiency of two major antiapoptotic factors.
Notwithstanding, uterine degenerative changes in the arsenic exposed population were a withdrawal effect of estrogenic support due to inhibited ovarian production of E2, possible involvement of a direct antiestrogenic effect of arsenic at the endometrial level may not be ruled out. Arsenic has been reported to inhibit E2-induced proliferation of MCF-7 cells at low concentrations (0.25–1 μM) and stimulate apoptosis at higher concentration (Chow et al., Citation2004). Arsenic possibly competitively binds to estrogen receptor (ER) and inhibits E2 binding to the ERα (Du et al., Citation2012). Thus, apart from lowering the E2 level, another possible mechanism of uterine effects of arsenic may involve attenuation of estrogen effect at the receptor level. The precise mechanism remains unknown, but arsenic has been shown to induce endometrial carcinogenicity or cellular toxicity leading ultimately to cell death (Akram et al., Citation2010). We did not evaluate the effect of arsenic on endometrial ROS scavenging system, but endometrium is known to be a potential site of superoxide anion generation (Sugino et al., Citation2001), while arsenic is reported to produce oxidative stress by promotion of endometrial ROS (Chatterjee & Chatterji, Citation2010). Thus, the low level of E2 along with increased generation of ROS perhaps plays roles in arsenic-led disruption of endometrial cycle (Akram et al., Citation2010; Beltran-Garcia et al., Citation2000).
HPD supplementation prevented the arsenic-induced suppression of ovarian and uterine weight and normalized ovarian steroidogenesis and follicular growth. The precise mechanism remains yet to be ascertained. However, circumstantial evidence suggests that the high dietary proteins perhaps prevented the oxidative stress by preservation of ovarian SOD activity as well as diminution of MDA level as observed in the protein-supplemented group.
Pea seed is a rich source of protein, which is approximately 25% by weight (Aykroyd et al., Citation1963). Evidence suggests that pea protein hydrolysate displays a greater H2O2 scavenging activity and optimum antioxidant enzyme response status (Pownall et al., Citation2010). On the other hand, there is direct evidence that casein or casein-derived peptides inhibit enzymatic and nonenzymatic lipid peroxidation, most likely by being a preferred target over fatty acid free radical intermediate (Rival et al., Citation2001). Taken together, it is envisaged that HPD has the potential to effectively deal with the ROS threat and as ovarian steroidogenesis is diminished by oxidative injuries, mitigation of oxidative injuries by these proteins in arsenic-exposed rats helped in preserving the ovarian steroidogenesis and other ROS-mediating ill effects. Relevant to this are reports that administration of both casein and pea successfully minimized the arsenic-induced pancreatic (Mukherjee et al., Citation2003) and testicular (Mukherjee & Mukhopadhyay, Citation2009) damage and helped in restoring normal functions.
Biomethylation of arsenic in the process of its metabolism represents a primary detoxification mechanism. However, in this process, constant depletion of methyl groups causes DNA hypomethylation. Elevated levels of ROS and oxidative modifications of DNA have been observed in arsenic-exposed cells (Barchowsky et al., Citation1999; Gebel, Citation2002). Although arsenic does not cause point mutations, it has been reported to have genotoxic effects in vivo and in cultured cells (Huang et al., Citation2004; Rossman, Citation2003). This study also attests to the genotoxic effects of arsenic at the ovarian level, which could significantly be ameliorated by HPD supplementation. This detoxification effect is perhaps attributed to the potential of pea proteins as a methyl transferring agent in the methylation process (Joshi & Handler, Citation1960; Mukherjee et al., Citation2003). These findings, taken together with earlier reports, may be interpreted to mean that a diet rich in casein and pea proteins, possibly by means of their respective antioxidative and methyltransferase potential, may effectively counteract the damaging effects of arsenic on ovarian steroidogenic activity and female reproductive functions.
Conclusion
These findings propose that casein- and pea protein-enriched diets may effectively attenuate the arsenic-induced array of female reproductive dysfunctions. The protective effects of the referred proteins possibly involve their antioxidative and methyltransferase potential that inhibit enzymatic and non-enzymatic lipid peroxidation and reduce oxidative stress. However, further in-depth studies should substantiate these findings before the envisaged protective effects be explored in managing human health and diseases with reference to arsenic toxicity.
Declaration of interest
The authors report no declarations of interest.
Acknowledgements
The authors gratefully acknowledge financial assistance from the Minor Research Project No. F.PSW-074/09-10 (ERO) provided by the University Grants Commission, New Delhi, India.
References
- Agarwal A, Gupta S, Sharma RK. (2005). Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 3:28
- Ahmad SA, Sayed MH, Barua S, et al. (2001). Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect 109:629–31
- Akram Z, Jalali S, Shami SA, et al. (2010). Adverse effects of arsenic exposure on uterine function and structure in female rat. Exptl Toxicol Pathol 62:451–9
- Aykroyd WR, Gopalan C, Balasubramanian SC. (1963). The nutritive value of Indian foods and the planning of satisfactory diets. Spec Rep Ser Indian Counc Med Res 42:1–255
- Barchowsky A, Roussel RR, Klei LR, et al. (1999). Low levels of arsenic trioxide stimulate proliferative signals in primary vascular cells without activating stress effector pathways. Toxicol Appl Pharmacol 159:65–75
- Beltran-Garcia MJ, Espinosa A, Herrera N, et al. (2000). Formation of copper oxychloride and reactive oxygen species as causes of uterine injury during copper oxidation of Cu-IUD. Contraception 61:99–103
- Cassano E, Tosto L, Balestrieri M, et al. (1999). Antioxidant defense in the follicular fluid of water buffalo. Cell Physiol Biochem 9:106–16
- Chakraborti D, Rahman MM, Paul K, et al. (2002). Arsenic calamity in the Indian subcontinent. What lessons have been learned? Talanta 58:3–22
- Chappell WR, Beck BD, Brown KG, et al. (1997). Inorganic arsenic: A need and an opportunity to improve risk assessment. Environ Health Perspect 105:1060–7
- Chatterjee A, Chatterji U. (2010). Arsenic abrogates the estrogen-signaling pathway in the rat uterus. Reprod Biol Endocrinol 8:80
- Chatterjee A, Chatterji U. (2011). All-trans retinoic acid protects against arsenic-induced uterine toxicity in female Sprague-Dawley rats. Toxicol Appl Pharmacol 257:250–63
- Chatterjee AK, Jamdar SC, Ghosh BB. (1970). Effect of riboflavine deficiency on incorporation in vivo of [14C] amino acid into liver proteins of rats. Br J Nutr 24:635–40
- Chattopadhyay S, Ghosh D. (2010). Role of dietary GSH in the amelioration of sodium arsenite-induced ovarian and uterine disorders. Reprod Toxicol 30:481–8
- Chattopadhyay S, Ghosh S, Chaki S, et al. (1999). Effect of sodium arsenite on plasma levels of gonadotrophins and ovarian steroidogenesis in mature albino rats: Duration-dependent response. J Toxicol Sci 24:425–31
- Chow SK, Chan JY, Fung KP. (2004). Suppression of cell proliferation and regulation of estrogen receptor alpha signaling pathway by arsenic trioxide on human breast cancer MCF-7 cells. J Endocrinol 182:325–37
- Du J, Zhou N, Liu H, et al. (2012). Arsenic induces functional re-expression of estrogen receptor alpha by demethylation of DNA in estrogen receptor-negative human breast cancer. PLoS One 7:e35957
- Gebel TW. (2002). Arsenic methylation is a process of detoxification through accelerated excretion. Int J Hyg Environ Health 205:505–8
- Gore-Langton RE, Daniel SA. (1990). Follicle-stimulating hormone and estradiol regulate antrum-like reorganization of granulosa cells in rat preantral follicle cultures. Biol Reprod 43:65–72
- Hochstein P, Atallah AS. (1988). The nature of oxidants and antioxidant systems in the inhibition of mutation and cancer. Mut Res 202:363–75
- Huang C, Ke Q, Costa M, et al. (2004). Molecular mechanisms of arsenic carcinogenesis. Mol Cell Biochem 255:57–66
- Jarabak J. (1969). Soluble 17β-hydroxysteroid dehydrogenase of human placenta. In: Raymond BC, ed. Methods in Enzymology. Vol. 15. New York: Academic Press, 746–52
- Joshi JG, Handler P. (1960). Biosynthesis of trigonelline. J Biol Chem 235:2981–3
- Kitchin KT. (2001). Recent advances in arsenic carcinogenesis: Modes of action, animal model systems, and methylated arsenic metabolites. Toxicol Appl Pharmacol 172:249–61
- Maiti S, Chatterjee AK. (2001). Effects on levels of glutathione and some related enzymes in tissues after an acute arsenic exposure in rats and their relationship to dietary protein deficiency. Arch Toxicol 75:531–7
- Marcondes FK, Bianchi FJ, Tanno AP. (2002). Determination of the estrous cycle phases of rats: Some helpful considerations. Braz J Biol 62:609–14
- Martin JP Jr, Dailey M, Sugarman E. (1987). Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch Biochem Biophys 255:329–36
- Mukherjee S, Das D, Darbar D, et al. (2003). Dietary intervention affects arsenic generated nitric oxide and reactive intermediate toxicity in islet cell of rats. Curr Sci 85:786–93
- Mukherjee S, Mukhopadhyay PK. (2009). Studies on arsenic toxicity in male rat gonads and its protection by high dietary protein supplementation. Al Ameen J Med Sci 2:73–7
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–8
- Patil SR, Ravindra PSR, Londonka R, et al. (1998). Nicotine induced ovarian and uterine changes in albino mice. Ind J Physiol Pharmacol 42:503–8
- Pownall TL, Udenigwe CC, Aluko RE. (2010). Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J Agric Food Chem 58:4712–18
- Quirk SM, Cowan RG, Harman RM, et al. (2004). Ovarian follicular growth and atresia: The relationship between cell proliferation and survival. J Anim Sci 82:E40–52
- Rahman MM, Sengupta MK, Ahamed S, et al. (2005). Murshidabad – One of the nine groundwater arsenic-affected districts of West Bengal, India. Part I: Magnitude of contamination and population at risk. Clin Toxicol (Phila) 43:823–34
- Rival SG, Boeriu CG, Wichers HJ. (2001). Caseins and casein hydrolysates. 2. Antioxidative properties and relevance to lipoxygenase inhibition. J Agric Food Chem 49:295–302
- Rossman TG. (2003). Mechanism of arsenic carcinogenesis: An integrated approach. Mut Res 533:37–65
- Shen J, Liu J, Xie Y, et al. (2007). Fetal onset of aberrant gene expression relevant to pulmonary carcinogenesis in lung adenocarcinoma development induced by in utero arsenic exposure. Toxicol Sci 95:313–20
- Singh NP, McCoy MT, Tice RR, et al. (1988). A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–91
- Singh S, Rana SV. (2007). Amelioration of arsenic toxicity by l-ascorbic acid in laboratory rat. J Environ Biol 28:377–84
- Sugino N, Karube-Harada A, Kashida S, et al. (2001). Reactive oxygen species stimulate prostaglandin F2α production in human endometrial stromal cells in vitro. Hum Reprod 16:1797–801
- Sun Y. (1990). Free radicals, antioxidant enzymes, and carcinogenesis. Free Radical Biol Med 8:583–99
- Tabacova S, Hunter ES, Balabaeva L. (1992). Potential role of oxidative damage in developmental toxicity of arsenic. In: Abernathy CO, Calderon RL, Chappel WR, eds. Arsenic: Exposure and Health Effects. London: Chapman and Hall, 135–44
- Tagatz G, Fialkow PJ, Smith D, et al. (1970). Hypogonadotropic hypogonadism associated with anosmia in the female. N Engl J Med 283:1326–9
- Talalay P. (1962). Hydroxysteroid dehydrogenases. In: Colowick SP, Kaplan NO, eds. Methods in Enzymology. Vol. 5. New York: Academic Press, 512–16
- Tapio S, Grosche B. (2006). Arsenic in the aetiology of cancer. Mut Res 612:215–46
- Tarin JJ. (1996). Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Mol Hum Reprod 2:717–24
- Tsai-Turton M, Luderer U. (2006). Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology 147:1224–36
- Tseng CH, Tai TY, Chong CK, et al. (2000). Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: A cohort study in arseniasis-hyperendemic villages in Taiwan. Environ Health Perspect 108:847–51
- United States Environmental Protection Agency. (2001). National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring. Federal Register 66:6975–7066
- Wong PY, Kitts DD. (2003). Chemistry of buttermilk solid antioxidant activity. J Dairy Sci 86:1541–7
- World Health Organization. (1981). Environmental Health Criteria 18 – Arsenic. Geneva, Switzerland: WHO
- Yamauchi H, Fowler BA. (1994). Toxicity and metabolism of inorganic and methylated arsenicals. ln: Nriagu JO, ed. Arsenic in the Environment, Part II: Human Health and Ecosystem Effect, New York: John Wiley and Sons Inc., 35–45