Abstract
Context: Saussurea lappa Dence (Compositae) is used as a traditional herbal medicine to treat abdominal pain and tenesmus in East Asia. Current studies have shown that S. lappa has anticancer activity in divergent of cancer cells. However, the effects of S. lappa on oral cancer and its mechanisms of action have yet to be elucidated.
Objective: To explore its potential chemotherapeutic effects and mechanism of cell growth inhibition on human oral cancer cells.
Materials and methods: The dried roots of S. lappa were used in this study. Cell viability of KB cells was evaluated by 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide assay after treatment with 30 µg/ml of methanol extract from the dried roots of S. lappa. To understand whether its effect on cell death is related with apoptosis pathway, we performed DNA fragmentation assay, western blot, caspase activity assay and fluorescence-activated cell sorting (FACS) analysis.
Results: Treatment of S. lappa extract onto KB cells reduced cell viability significantly with an IC50 value of 30 µg/ml. The formation of a DNA ladder was observed starting at the 24 h treatment. In western blotting analysis, the S. lappa extract induced the proteolytic processing of caspase-3, -9 and poly (ADP-ribose) polymerase, a significant increase of Bax and marked reduction of Bcl-2. We also confirmed the activation of caspase-3/-7 in living KB cells by fluorescence microscopy.
Conclusion: These results suggested that S. lappa extract inhibited cell proliferation through the apoptosis pathway in KB human oral cancer cells.
Introduction
Oral squamous cell carcinoma is the most common cancer of the oral and maxillofacial region, with more than 300 000 new cases reported annually worldwide. Based on currently available clinical assessment and treatment methods, patients are often diagnosed at a late stage of the disease, and the 5-year survival rate has remained relatively low (50 ∼ 0%; Schliephake, Citation2003). Surgical treatment for oral cancer can cause functional and aesthetic impairment, leading to withdrawal and social isolation (Hopper et al., Citation2004). Complications of radiotherapy can impair wound healing and further complicate surgical salvage after a failed procedure (Bodin et al., Citation2004). Conventional chemotherapeutic agents have been associated with numerous significant clinical complications, including nausea, hair loss and pancytopenia; thus, alternative and less toxic chemical treatments for oral cancer are required (Yamachika et al., Citation2004). Therefore, in recognition of nature’s potential, several plants screenings have performed since 1960 to replace drugs that have side effects. Today, the taxanes are often the most powerful compounds among all chemotherapeutic drugs, exhibiting a wide range of activity and are of special benefit in fighting metastatic breast, ovarian and lung cancer (Crown et al., Citation2004; Gligorov & Lotz, Citation2004).
The dried root of Saussurea lappa Dence (Compositae) has been traditionally used for abdominal pain and tenesmus as a traditional medicine in Asia, including Korea and China. S. lappa root extract contains resinoids, essential oil, alkaloid, inulin, a fixed oil and other minor constituents such as tannins and sugars (Madhavi et al., Citation2001). The essential oil of the roots has strong antiseptic, disinfectant and anti-inflammatory properties (Madhavi et al., Citation2001). Previous study has shown that the ethanol extract of S. lappa has anticancer activity (Ko et al., Citation2004) in AGS gastric cancer cells. Recently, studies reported that the methanol extract of Gracilaria tenuistipitata (Yeh et al., Citation2012) and the hexane extract of Rheum undulatum L inhibited oral cancer cell proliferation through apoptosis and the down-regulation of specificity protein 1 and surviving (Choi et al., Citation2011), respectively. However, the effects of S. lappa on oral cancer and its mechanisms of action have yet to be elucidated.
One of the hallmarks of cancer is the deregulation of apoptosis (Hanahan & Weinberg, Citation2000), a universal and efficient cellular suicide pathway. Therefore, increasing apoptosis in cancers can be an effective method for chemopreventive and chemotherapeutic intervention in many types of cancers. Apoptosis, which is a major form of programmed cell death, plays an important role in the regulation of tissue development and homeostasis in eukaryotes (Green & Reed, Citation1998; Hengartner, Citation2000; Kaufmann & Hengartner, Citation2001). Apoptosis can occur via a death receptor-dependent extrinsic or a mitochondria-dependent intrinsic pathway and can be induced by various chemotherapeutic agents (Kaufmann & Earnshaw, Citation2000).
In this study, to explore the possibility that S. lappa functions as a chemotherapeutic agent in human oral cancer, we tested the effects of a S. lappa extract in inhibiting cell proliferation in KB oral cancer cells and characterized the its underlying mechanisms of action.
Materials and methods
Materials
3-[4, 5-Dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) was purchased from Sigma (St. Louis, MO). Bax, Bcl-2, caspase-3, caspase-9, cleaved caspase-3, cleaved caspse-9 and poly(ADP-ribose) polymerase (PARP) antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Cell-permeable fluorogenic substrate PhiPhiLux-G1D2 was purchased from OncoImmunin, Inc. (Gaithersburg, MD).
Plant material and extract preparation
The dried roots of S. lappa were purchased from Jeonnam herbal medicine farmer’s cooperative (Jeollanam-do, Korea) and identified by Prof. Su-In Cho, School of Oriental Medicine, Pusan National University, Korea. The roots of S. lappa (100 g) were ground with a Wiley mill to pass a 1 mm screen and were extracted with 95% methyl alcohol at 40 °C for 5 h. The collected filtrate was dried by evaporation under vacuum at 40 °C using a rotary evaporator (Eyela, Tokyo, Japan). After evaporation, the concentrated extract (3 g) was freeze-dried at −40 °C for 3 days and stored at −20 °C until used. For the treatment, freeze-dried extract was dissolved in 70% dimethyl sulfoxide (DMSO).
Cell culture and reagents
The KB human oral cancer cells were provided by the American Type Culture Collection (Rockville, MD). The cells were incubated in minimum essential medium containing 5% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin (Invitrogen, Carlsbad, CA) at 37 °C in an atmosphere containing 5% CO2.
MTT assay
The cell viability test was performed as described previously with minor modifications (Ahn et al., Citation2008; Park et al., Citation2008). KB cells were seeded at a density of 5 × 104 cells per well in 12-well plates. After 24 h growth, the cells were treated with methanol extract of S. lappa at various concentrations for 24 h and incubation times.
Before testing, the MTT solution (Sigma) was added, and the cells were incubated at 37 °C for 3 h. The culture medium was aspirated, and an acid–isopropanol mixture was added to dissolve the dark blue crystals. The optical density value of the dissolved solute was measured using a microplate autoreader (Winooski, VT) at a wavelength of 570 nm. Three separate experiments were performed for each concentration/exposure time combination.
DNA fragmentation analysis
Approximately 5 × 106 cells were collected and transferred to lysis buffer containing 100 mM NaCl, 10 mM ethylenediaminetetraacetic acid (EDTA), 300 mM Tris-HCl, pH 7.5, 200 mM sucrose, 0.5% sodium dodecyl sulfate (SDS) and 0.5 mg/ml proteinase K and incubated at 65 °C for 1 h. DNA was extracted with an equal volume of phenol/chloroform/isoamyl alcohol (25:24:1, v/v) and precipitated with ethanol. Genomic DNA was resuspended in Tris-EDTA buffer (pH 8.0) containing 5 μg/ml DNase-free RNase and incubated at 37 °C for 1 h. Genomic DNA was visualized using gel electrophoresis in 1.5% agarose gel (Hayashi et al., Citation2006).
RNA isolation and reverse transcription polymerase chain reaction analysis
Total RNA was isolated from cell lines using Trizol (Invitrogen) according to the manufacturer’s instructions and was reverse-transcribed using the Reverse Transcription System (Promega, Madison, WI). The reverse transcription reaction was performed sequentially for 10 min at 25 °C, for 60 min at 42 °C and for 5 min at 95 °C. Glyceraldehyde 3-phosphate dehydrogenase was used as an internal control (Liu et al., Citation2009). PCR Master Mix reagent (Applied Biosystems, Foster, CA) was used for quantitative polymerase chain reaction (PCR). Primers were designed by Applied Biosystems according to the complementary DNA sequences as follows: Bax (5′-CAG CTG ACA TGT TTT CTG ACG GC-3′, 5′-CTC CCG CCA CAA AGA TGG TCA CG-3′) and Bcl-2 (5′-AGT TCG CCG AGA TGT CCA GGC A-3′, 5′-ACT TGT GGC CCA GAT AGGCAC C-3′). The PCR products were analyzed using gel electrophoresis in 1.2% agarose gel.
Preparation of cell lysates and western blot analysis
The cell pellet was dissolved in lysis buffer (1% Triton-X 100, 0.5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 5 µg/ml aprotinin and 5 µg/ml leupeptin) and centrifuged for 10 min at 12 000 rpm. Protein concentrations were determined using a BCA Protein Assay Kit (Rockford, IL). The proteins were separated on a 10% SDS-polyacrylamide gel electrophoresis gel and transferred to a polyvinylidene fluoride membrane followed by western blot analysis. A solution of 5% nonfat dried milk in Tris-buffered saline (TBS) containing 0.1% Tween-20 was used to block nonspecific binding. The membrane was subsequently incubated with antibodies against Bax, Bcl-2, caspase-3, caspase-9, cleaved caspase-3, cleaved caspse-9 and PARP. After incubation, the blots were extensively washed in TBS containing 0.1% Tween-20. For detection, an ECL kit (Arlington Heights, IL) was used according to the manufacturer’s instructions.
Determination of caspase-3/7 activity
The activity of caspase-3/-7 was determined using the cell-permeable fluorogenic substrate PhiPhiLux-G1D2 (OncoImmunin), which was used according to the manufacturer’s instructions. The cells were incubated for 1 h with PhiPhiLux-G1D2. This substrate molecule contains a peptide homo-doubly labeled with a fluorophore. The cleaved substrate has the following excitation and emission peak: excitation = 505 nm and emission = 530 nm. The activity of caspase-3/-7 was visualized by fluorescence microscopy (IX71, Olympus, Tokyo, Japan), as described in the previous study (Kim et al., Citation2012).
Flow cytometric cell cycle analysis and annexin V-FITC/propidium iodine double staining
The cells were fixed in chilled 75% methanol and stained with a propidium iodine (PI) solution (100 µg/ml RNase and 10 µg/ml PI in phosphate buffered saline) for cell cycle analysis. The cells were stained using a Vybrant® apoptosis assay kit (Molecular Proves, Eugene, OR) followed by labeling with Alexa Fluor® 488 Annexin V and PI for apoptosis analysis. Data acquisition and analysis were carried out using the Cell Lab Quanta™ SC flow cytometer and software (Beckman Coulter Inc., Miami, FL).
Statistical analysis
Data are expressed as the mean ± SEM of at least three individual experiments. Statistical significance was analyzed by using Student’s t-test for two groups and one way analysis of variance for multi-group comparisons. p < 0.05 is considered statistically significant.
Results
The methanol extract of S. lappa inhibited the cell proliferation of KB cells
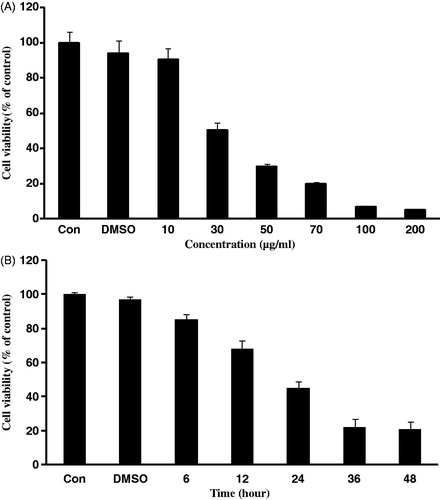
The objective of this study was to investigate potential cytotoxic effect of S. lappa extract on KB human oral cancer cells. When treated to KB cells, the methanol extract prepared from the dried root of S. lappa showed the growth inhibitory effect in a concentration-dependent manner exhibiting IC50 value of 30 μg/ml, approximately (). Time course study at 30 µg/ml concentration revealed that the S. lappa extract dramatically decreased cell viability in a time-dependent manner as shown in .
Figure 1. Effect of Saussurea lappa extract on cell viability in KB cells. Cells were treated with various concentrations of Saussurea lappa extract for 24 h in KB cells (A). 30 µg/ml of Saussurea lappa extract treated into cells for different time periods (B). Cell viabilities were determined by the MTT assay. The percentage of cell viability was calculated as a ratio of A570 nm. Results were expressed as percent of the control. Each data point represents the mean ± SEM from three experiments.
S. lappa extract induces apoptosis in KB oral cancer cells
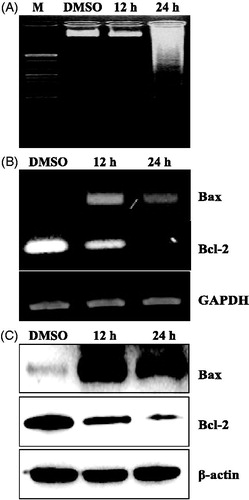
To investigate whether S. lappa extract causes cell death by apoptosis in KB cells, we evaluated two biochemical events specific for apoptosis; genomic DNA laddering, relatively late event in apoptotic pathway and activation of effector molecules. For analyzing the DNA laddering event, KB cells were treated with S. lappa extract for 12 and 24 h before preparing genomic DNA. When compared with the control group, 24 h treatment of S. lappa extract induced significant fragmentation of genomic DNA ().
Figure 2. The expression levels of apoptosis-related proteins by treatment with Saussurea lappa extract in KB cells. KB cells were seeded at 1 × 106 and were then treated with 30 µg/ml of Saussurea lappa extract for the indicated time point (12 h and 24 h). Fragmentation of internucleosomal DNA by Saussurea lappa extract treatment in KB cells. Genomic DNA was subjected to 1.5% agarose gel electrophoresis (A). After 12 h, and 24 h Saussurea lappa extract treatment, mRNA was determined by RT-PCR (B), and Bax and Bcl-2 protein levels were determined by Western blot analysis (C). Whole cell lysates were separated by 12% SDS-PAGE and probed for Bax, Bcl-2 and β-actin as a loading control.
In the next series of experiments, we addressed the potential mechanism by which S. lappa extract was causing decreased cell viability in KB cells.
Bcl-2 is a member of a large family of cell survival-regulating proteins consisting of both pro- and anti-apoptotic regulators. We therefore determined the level of Bcl-2 (anti-apoptotic) and Bax (pro-apoptotic) expression in KB cells following treatment with S. lappa extract. For both transcriptional () and translational () levels, treatment of S. lappa extract decreased levels of Bcl-2 in cancer cells, while the level of Bax expression is highly induced after 12-h treatment.
It has been known that the caspase family activation represents one of the earliest known steps in the cell death process. We next explored the mechanism of apoptosis induction by the treatment of S. lappa extract in the cancer cells. KB cancer cells exposed to S. lappa extract for 12 h exhibited marked activation of caspase-3 and -9 activities ().
Figure 3. Saussurea lappa extract caused caspase-3-dependent apoptosis. Caspase-9, cleaved caspase-9, caspase-3, cleaved caspase-3, PARP and cleaved PARP protein levels were determined by western blot analysis. Whole-cell lysates (50 µg/lane) were subjected to immunoblotting for the indicated proteins. Probing with β-actin was used to show equal protein loading (A). Activation of caspase-3/7 by Saussurea lappa extract treatment in living KB cells. The cells were treated with 30 μg/ml of Saussurea lappa extract for 24 h and followed by adding specific cell-permeable substrate Phiphilux G1D2. Caspase-3/7 activity was visualized by fluorescence microscopy (B).

One of the targets for activated caspase-3 is the DNA repair enzyme PARP. So, we tested the effect of S. lappa extract treatment on caspase-3 activity by western blot using a monoclonal antibody against PARP that detects the full length and the cleaved forms of PARP. As expected, the cleavage of PARP protein was evident in S. lappa extract-treated cells after 12-h treatment ().
In addition, we also confirmed that the S. lappa extract treatment activated the caspase-3/7 in living KB cells by fluorescence microscopy using a cell-permeable fluorogenic caspase-3/7 substrate PhiPhiLux-G1D2 ().
Altogether, these findings demonstrate that S. lappa extract induces apoptotic cell death in KB oral cancer cells through caspase-3 - and caspase-9-dependent pathways.
Loss of cell membrane asymmetry, detectable by Annexin V staining, represents one of the earliest events in apoptosis. Thus, we performed an Annexin V-PI staining to further demonstrate the S. lappa extract-mediated apoptosis. As shown in , there was an increase in the number of cell undergoing apoptosis (Annexin V-positive) after treating the cells with 30 µg/ml of S. lappa extract. In the DMSO-treated control cells, 8.2% was positive for Annexin V-FITC staining, while S. lappa extract treatment resulted in increases of 31.4 and 32.6% for 12 - and 24 h treated cells, respectively.
Figure 4. Saussurea lappa extract induced apoptosis in KB cells. Cells were treated with 30 µg/ml of Saussurea lappa extract for 12 h and 24 h. The cells were stained with Annexin V-FITC and propidium iodine (PI). The apoptotic cells were then analyzed by fluorescence-activated cell sorting (FACS) analysis. This apoptotic data was determined by FACS analysis showing the percentages of lower right quadrant for early and upper right quadrant for late apoptotic cells.

Discussion
Many plant-derived bioactive constituents, including paclitaxel (from Taxus brevifolia), camptothecin (from Camptotheca acuminata), podophyllotoxin (from Podophyllum emodi) and vinblastine (from Catharanthus roseus), have been developed as potential sources of anticancer agents (Hsu et al., Citation2011). Recent scientific efforts have focused on the potential roles of traditional herbs extracts as alternative and complementary medications for cancer treatment (Kim et al., Citation2012; Lee et al., Citation2010; Moon, Citation2012). Currently, Compelling evidence reported by several investigators suggested different anticancer activities of S. lappa extract in both in vitro and in vivo (Ko et al., Citation2004, Citation2005; Sun et al., Citation2003). However, the effect of the S. lappa extract on the growth of KB human oral cancer cell has not been investigated. As shown , S. lappa extract inhibited the growth of KB cells in a dose- and time-dependent manner. Therefore, in this study, we intended to elucidate the mechanism of cytotoxic activity of S. lappa extract in KB human oral cancer cells.
Apoptosis is characterized by the presence of distinct morphological features and formation of a ladder of genomic DNA fragments (Hu & Kavanagh, Citation2003). We showed the appearance of a DNA ladder in S. lappa extract treated KB cells (). This result suggested that S. lappa extract could induce apoptosis in KB cells. Therefore, we performed a series of experiments to identify the mechanism of S. lappa extract-induced apoptosis in KB cells. First, we determined the effects of S. lappa extract on the level of Bcl-2 family proteins. In a previous study, the cytotoxic effects of S. lappa extract on AGS gastric cancer cells were attributed to the regulation of pro-apoptotic factors including Bax, Bad and anti-apoptotic factor including Bcl-2 and Bcl-xl (Ko et al., Citation2005). In this study, we showed that the treatment of S. lappa extract to KB cancer cells induces Bax in both transcriptional () and translational () levels. In contrast, Bcl-2 level was gradually decreased in a time-dependent manner. The pro-apototic Bcl-2 family increases mitochondrial membrane permeability thereby induces the activation of initiator caspase. The activation of a family of intracellular cysteine proteases (caspases) is known to play an important role in the initiation and execution of apoptosis induced by various stimuli (Datta et al., Citation1997; Liu et al., Citation1997). Among the caspases identified in mammalian cells, caspase-3, -7 and -9 may serve as effectors of apoptotic cells (Cohen, Citation1997). Thus, we evaluated the effect of S. lappa extract on the regulation of caspase activity. The results strongly suggest that its anti-proliferative effect on KB cancer cells is through the induction of initiator capase-9, which in turn activates the executioner caspase-3 ( and ). Moreover, we showed that PARP, a cellular substrate of activated caspase-3, is efficiently cleaved, and the Annexin V-FITC positive cells are increased upon treatment with S. lappa extract. Altogether, our findings demonstrate that S. lappa extract exhibits anti-proliferative effect by inducing caspase-3-dependent apoptosis pathway in KB oral cancer cells
In line with our findings, a previous study showed that costunolide, isolated from the root of S. lappa, is a potent inducer of apoptosis, and facilitates its activation via reactive oxygen species generation in HL-60 human leukemia cells (Lee et al., Citation2001). Other investigators also reported that S. lappa extract induces apoptotic cell death in AGS human gastric cancer cells (Ko et al., Citation2004). The powder formulation of S. lappa extract is being used for the treatment of gastric cancers either by traditional herbal therapy or combinational therapy (Ko et al., Citation2005). Therefore, current findings should facilitate further work to examine the beneficial effects of S. lappa extract on cancer therapy and to elucidate its mechanism of action.
Conclusions
This study demonstrates that methanol extract of S. lappa inhibits cell growth and induces apoptosis in KB human oral cancer cells. The results imply that S. lappa extract could be a promising traditional herbal medicine for potential use in oral cancer therapy and that search for new compounds capable of inducing apoptosis is a promising strategy for oral cancer treatment.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This study was supported by research fund from Chosun University 2010.
References
- Ahn EM, Han JT, Kwon BM, et al. (2008). Anti-cancer activity of flavonoids from Aceriphyllum rossii. J Korean Soc Appl Biol Chem 51:309–15
- Bodin I, Jäghagen EL, Isberg A. (2004). Intraral sensation before and after radiotherapy and surgery for oral and pharyngeal cancer. Head and Neck 26:923–9
- Choi ES, Cho SD, Jeon JG, et al. (2011). The apoptotic effect of the hexane extract of Rheum undulatum L. in oral cancer cells through the down-regulation of specificity protein 1 and survivin. Lab Anim Res 27:19–24
- Cohen GM. (1997). Caspases: The executioners of apoptosis. Biochem J 326:1–16
- Crown J, O'Leary M, Ooi WS. (2004). Docetaxel and paclitaxel in the treatment of breast cancer: A review of clinical experience. Oncologist 2:24–32
- Datta R, Kojima H, Yoshida K, et al. (1997). Caspase-3-mediated cleavage of protein kinase C theta in induction of apoptosis. J Biol Chem 272:20317–20
- Gligorov J, Lotz JP. (2004). Preclinical pharmacology of the taxanes: Implications of the differences. Oncologist 2:3–8
- Green DR, Reed JC. (1998). Mitochondria and apoptosis. Science 281:1308–12
- Hanahan D, Weinberg RA. (2000). The hallmarks of cancer. Cell 100:57–70
- Hayashi K, Hibasami H, Murakami T, et al. (2006). Induction of apoptosis in cultured human stomach cancer cells by potato anthocyanins and its inhibitory effects on growth of stomach cancer in mice. Food Sci Technol Res 12:22–6
- Hengartner MO. (2000). The biochemistry of apoptosis. Nature 407:770–6
- Hsu HF, Huang KH, Lu KJ, et al. (2011). Typhonium blumei extract inhibits proliferation of human lung adenocarcinoma A549 cells via induction of cell cycle arrest and apoptosis. J Ethnopharmacol 135:492–500
- Hopper C, Kübler A, Lewis H, et al. (2004). mTHPC-mediated photodynamic therapy for early oral squamous cell carcinoma. Int J Cancer 111:138–46
- Hu W, Kavanagh JJ. (2003). Anticancer therapy targeting the apoptotic pathway. Lancet Oncol 4:721–9
- Kaufmann SH, Earnshaw WC. (2000). Induction of apoptosis by cancer chemotherapy. Exp Cell Res 256:42–9
- Kaufmann SH, Hengartner MO. (2001). Programmed cell death: Alive and well in the new millennium. Trends Cell Biol 11:526–34
- Kim SY, Kim SG, Oh JS, et al. (2012). Anticancer effects of quercetin on KB human oral cancer cells. Oral Biol Res 36:113–22
- Ko SG, Kim HP, Jin DH, et al. (2005). Saussurea lappa induces G2-growth arrest and apoptosis in AGS gastric cancer cells. Cancer Lett 220:11–19
- Ko SG, Koh SH, Jun CY, et al. (2004). Induction of apoptosis by Saussurea lappa and Pharbitis nil on AGS gastric cancer cells. Biol Pharm Bull 27:1604–10
- Lee BH, Lee GW, Kim DK. (2010). The effect of bibangtalmyungsan on the cell growth in the KB human oral squamous. Oral Biol Res 34:1–4
- Lee MG, Lee KT, Chi SG, et al. (2001). Costunolide induces apoptosis by ROS-mediated mitochondrial permeability and cytochrome c release. Biol Pharm Bull 24:303–6
- Liu X, Zou H, Slaughter C, et al. (1997). DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89:175–84
- Liu Z, Liu M, Niu G, et al. (2009). Genome-wide identification of target genes repressed by the Zinc finger transcription factor REST/NRSF in the HEK 293 cell line. Acta Biochim Biophys Sin 41:1008–17
- Madhavi M, Mallika G, Lokanath N, et al. (2001). A review on phytochemical and pharmacological aspects of Saussurea lappa. Int J Rev Life Sci 2:24–31
- Moon YH. (2012). Induction of apoptosis by extract of Phlox paniculata L in KB human oral cancer cells. Oral Biol Res 36:139–43
- Park SK, Lee CW, Lee MY. (2008). Inhibitory effect of ore minerals on the allergic inflammation in mouse. J Korean Soc Appl Biol Chem 51:269–75
- Schliephake H. (2003). Prognostic relevance of molecular markers of oral cancer – A review. Int J Oral Maxillofac Surg 32:233–45
- Sun CM, Syu WJ, Don MJ, et al. (2003). Cytotoxic sesquiterpene lactones from the root of Saussurea lappa. J Nat Prod 66:1175–80
- Yamachika E, Habte T, Oda D. (2004). Artemisinin: An alternative treatment for oral squamous cell carcinoma. Anticancer Res 24:2153–60
- Yeh CC, Yang JL, Lee JC, et al. (2012). Anti-proliferative effect of methanolic extract of Gracilaria tenuistipitata on oral cancer cells involves apoptosis, DNA damage, and oxidative stress. BMC Complementary Altern Med 12:142. doi: 10.1186/1472-6882-12-142
