Abstract
Context: Semen Strychni is the seed of Strychnos nux-vomica L. (Loganiaceae). Its quality control procedure remains an issue since previous reports only focused on Strychnos alkaloids. To the best of our knowledge, chlorogenic acid (a phenolic acid) and loganin (an iridoid glycoside) are selected for the first time as marker constituents of quality control for Semen Strychni because of their bioactive activity correlating with therapeutic effects.
Objective: This study aimed to develop a simple and comprehensive quantity control method for Semen Strychni.
Materials and methods: The optimal ultrasonic extraction procedure was carried out for 45 min using 50% aqueous methanol with 1% formic acid. The satisfactory chromatographic separation was achieved on an Ultimate LP-C18 column with gradient elution using acetonitrile and water containing 30 mmol/L ammonium acetate and 1% formic acid. The high performance liquid chromatography method with diode array detector was validated for linearity, limit of detection and quantification (LOQ), precision, repeatability, accuracy and stability.
Results: All the calibration curves showed good linearity (r2 ≥ 0.999). The LOQ values for chlorogenic acid, loganin, strychnine, brucine, strychnine N-oxide and brucine N-oxide were 0.54, 0.83, 0.48, 0.50, 0.52 and 0.54 μg/mL, respectively. The method was reproducible with good accuracy in the range 95.6–104.4% and relative standard deviation (RSD) values less than 4.55%. The method was then applied to determine the components of the seed coat, seed leaf, endosperm and whole seed of Semen Strychni.
Conclusion: This newly established method is validated as a simple and practical tool for authentication and quality control of Semen Strychni.
Introduction
Semen Strychni, a known poisonous traditional Chinese medicine (TCM), is the seed of Strychnos nux-vomica L. (Loganiaceae). The powdered seeds have strong effects for alleviating pain, reducing swelling, improving blood circulation and removing blood stasis and are usually used to treat rheumatoid arthritis, paralysis, carcinoma pain as well as bone fracture in clinical use (Chinese Pharmacopoeia Committee, Citation2010). In general, the therapeutic effect of herbal medicines is attributed to the synergistic effect of their multi-constituents, which improve the therapeutic efficacy or/and minimize adverse reactions (Li et al., Citation2011). Strychnos alkaloids are generally considered to be the main active components of Semen Strychni. Strychnine and brucine together comprises more than 80% of the total Strychnos alkaloids (Xiao, Citation2002). Both alkaloids have excellent anti-inflammatory, analgesic and central nervous system stimulant effects, but they are also deadly poisonous drugs. Fortunately, their contents would be obviously reduced after processing, partly oxygenized into strychnine N-oxide and brucine N-oxide, apparently decreasing the toxicity, while the curative effect partly remains. However, the biological activity of a phenolic acid (chlorogenic acid) and an iridoid glycoside (loganin) was also found to be directly correlated to the therapeutic effect of Semen Strychni in recent years. Chlorogenic acid has anti-tumor (Jiang et al., Citation2000), anti-inflammatory (Kwon et al., Citation2009), anti-apoptotic and anti-oxidant activity (Yun et al., 2012). Loganin improves learning and memory impairments (Kwon et al., Citation2011; Lee et al., Citation1995). In recent years, the analysis of TCMs has begun to emphasize the integrative and holistic properties of TCMs (Jiang et al., Citation2010). Thus, simultaneously determining chlorogenic acid, loganin and four Strychnos alkaloids could reflect the consistent quality of Semen Strychni and correlate with the variation of clinical efficacy and safety.
The methods for measuring these components of Semen Strychni in existing literature include high performance liquid chromatography–ultraviolet (HPLC–UV; Chinese Pharmacopoeia Committee, Citation2010), thin-layer chromatography (TLC; Rathi et al., Citation2008), capillary electrophoresis (CE) (Li et al., Citation2006) and sweeping-micellar electrokinetic chromatography (SMEC) (Wang et al., Citation2006). Inferior accuracy of TLC, poor reproducibility of CE and non popularity of SMEC restrict their application as a practical and convenient quantity control for Semen Strychni. HPLC-UV is the most popular method for quality control of Chinese medicines, but it is not suitable to simultaneously monitor different constituents with large wavelength span. Above all, the common defect of the above-mentioned methods is that only Strychnos alkaloids were chosen as the index components. To the best of our knowledge, chlorogenic acid and loganin are selected for the first time as marker constituents of quality control for Semen Strychni because of their bioactive activities correlating with therapeutic effects. Therefore, a simple and accurate method determining chlorogenic acid, loganin and the four Strychnos alkaloids could be a better strategy for authentication and quality control of Semen Strychni.
This research first developed a simple and accurate HPLC method coupled with a diode array detector (DAD) to determine six bioactive constituents of Semen Strychni. Influencing factors, such as the extraction method, mobile phase and gradient elution program, were carefully investigated considering the complexity of the samples. Semen Strychni samples, which are from different origins, were also determined to validate the developed method.
Materials and methods
Chemicals and standards
Chromatographic grade acetonitrile and methanol was obtained from Kemiou (Tianjin, China). Water used in the experiment was produced by an Aquapro AJY-2000-U pure system (Ever Young, Chongqing, China). Other reagents were of analytical grade. Reference standards of chlorogenic acid, loganin, strychnine and brucine were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Strychnine N-oxide and brucine N-oxide were purchased from Sigma-Aldrich (St. Louis, MO).
The three crude drug samples were purchased from Burma and Yunnan (China) and authenticated by Professor Zhaoming Xie (Hunan University of Chinese Traditional Medicine, Changsha, China). The voucher specimens of these samples were deposited at the Department of Pharmacy, Chemistry College, Xiangtan University, Xiangtan, China. Semen Strychni was processed according to the literature (Tang et al., Citation2010). The seed coat, endosperm and cotyledons were separated from the processed seed.
Instrumentation and chromatographic conditions
All analyses were performed using a Dionex liquid chromatography (Dionex, Idstein, Germany) system equipped with a P680 pump, a DAD-100 diode array detector, an ASI-100 automated sampler and a Chromeleon workstation. The separation was conducted using an Ultimate LP-C18 column (5 µm, 250 mm × 4.6 mm, Welch Materials, Potomac, MD) with a ULT5BG18 guard column (Welch Materials). The mobile phase consisted of acetonitrile (A) and water containing 30 mmol/L ammonium acetate and 1% formic acid (B). The gradient started at 90% B and followed by a linear reduction, reaching at 85% B at 30 min and then kept isocratic at 90% B from 30 to 35 min for equilibrium. The flow rate was kept at 1.0 mL/min and the injection volume was 20 μL for each run, while the column temperature was maintained at 40 °C. The monitoring wavelength was set at 240 nm for loganin, 254 nm for strychnine and strychnine N-oxide, 264 nm for brucine and brucine N-oxide and 327 nm for chlorogenic acid. Peaks were identified on the basis of comparison of retention times and UV spectra with standards of these analytes.
Preparation of standard solutions and samples
A combined standard stock solution of chlorogenic acid (0.108 mg/mL), loganin (0.220 mg/mL), strychnine (0.296 mg/mL), brucine (0.250 mg/mL), strychnine N-oxide (0.052 mg/mL) and brucine N-oxide (0.054 mg/mL) was prepared with 50% methanol containing 1% formic acid in a 50 mL volumetric flask. The stock solution was then stored at 4 °C avoiding light and brought to room temperature before it was diluted with 50% methanol for the appropriate standard solution concentrations.
The dried samples were ground using a FW 177 mini-Mill (Tianjin, China) and sieved through a No. 50 mesh (355 µm ± 13 µm). Each sample (0.5 g) was accurately weighed and transferred in a 100 mL conical flask with a stopper. Subsequently, 50 mL extracting solvent (50% methanol containing 1% formic acid) was added. Ultrasonication (44 KHz, 250 W) was performed at room temperature for 45 min after accurately weighing the samples. The same solvent was then added to compensate for the lost weight during the extraction. The samples were centrifuged at 10 000 rpm for 15 min, and the supernatant was filtered through a 0.45 µm membrane before it was injected into the HPLC system for analysis.
Analytical method validation
The method was validated following the guidelines from the International Conference on Harmonization (ICH) (Citation1997 edition).
For linearity examination, the standard stock solutions were diluted with 50% methanol to provide a series of standard solutions with the appropriate concentrations. Seven concentrations of the standard solution were analyzed in duplicates, and then the calibration curves were constructed by plotting the peak areas versus the concentration of each analyte. The limit of detection (LOD) and quantification (LOQ) under the chromatographic conditions were determined by injecting a series of standard solutions until the signal-to-noise ratio for each compound was 3 for LOD and 10 for LOQ. The intra- and inter-day precisions were investigated at three concentration levels by determining a mixed standard solution in six replicates during a single day and by duplicating the experiments on three consecutive days. Six sample solutions extracted from the entire seed of sample one were analyzed using the proposed method to evaluate repeatability. The stability of sample solution at room temperature was analyzed at 0, 2, 4, 8, 12, 24 and 36 h, respectively. A recovery test was used to evaluate the accuracy of the developed method. Known amounts of the six standards in triplicate at low, medium and high concentration levels were added to approximate 0.25 g entire seed powder of sample one. Then the spiked samples were extracted and quantified in accordance with the methods described above. The percentage recoveries were calculated according to the following equation: (detected amount − original amount)/spiked amount × 100.
Results and discussion
Optimization of HPLC conditions
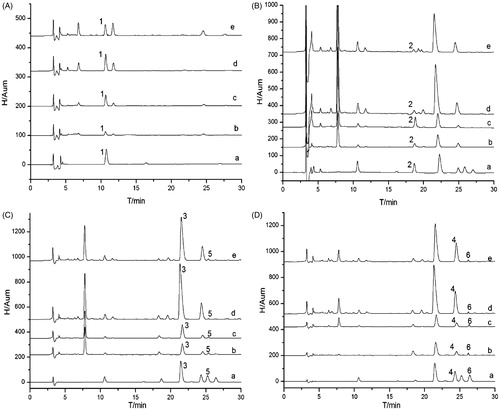
Acetonitrile was chosen as the organic phase since it gave better peak shape and more stable baseline compared with methanol. Triethylamine is known to reduce the peak-tailing of alkaloids in a C18 column through bonding with the exposed silanol (Si-OH) that can interact with the sample. At the beginning, although an end-capped Ultimate LP-C18 column was used, we still added triethylamine and formic acid in the aqueous phase to suppress the peak-tailing and improve the peak shapes of the alkaloids. However, the relative positions of loganin and strychnine in the chromatograms would change dramatically with any slight variation in the ratio of mobile phase consisting of acetonitrile and water (1% formic acid and 0.3% triethylamine), making this elution system unsuitable. This may be largely due to the fluctuation of pH when the mobile phase is in lack of buffer salt, or maybe it is because triethylamine could change the surface properties of alkyl-bonded silica phases (Park et al., Citation1999). Consequently, we referred to an alternative mobile phase with more stable pH, which is composed of acetonitrile (A) and water containing 30 mmol/L ammonium acetate and 1% formic acid (B). When the mobile phase consisting of 12.5% A and 87.5% B was used in an isocratic mode, the six components could be completely separated, but the peak shape was still unsatisfactory. For that reason, gradient elution was further applied to improve the peak shape because gradient peaks are usually compressed and tend to be narrower than isocratic peaks. Based on the results obtained from isocratic separation, we decided to employ a gentle gradient elution with the ratio of 87.5% B in its middle. The optimum chromatograms were obtained when the gradient began at 90% B and accompanied by a slow linear reduction, reaching at 85% B at 30 min ().
Optimization of sample extraction conditions
Ultrasonic extraction proves to be a good strategy to treat solid samples and shows promise to speed up and simplify sample treatment. Parameters, such as extraction solvents and time for extraction, were thoroughly investigated to obtain optimal extraction efficiency using the univariate approach. The extraction solvent used for alkaloids was usually basic chloroform, acidic methanol or water. Since chlorogenic acid and loganin can only dissolve in water and methanol, various formic solvents including water, methanol and 25, 50 and 75% (v/v) methanol–water solutions were investigated to obtain the most suitable extraction solvent. The optimal extraction solvent was 50% aqueous methanol with 1% formic acid, which allowed extraction of all the six constituents at high yields. In the selection of extraction time, four aliquots of the powdered sample were extracted with this optimized extraction solvent for 15, 30, 45 and 60 min, respectively. The assay results suggest that the six constituents can be almost completely extracted from the powdered sample within 45 min.
Validation of HPLC assay
The results of linearity, LOD and LOQ are given in . The concentrations of different constituents are related to the amounts of the extraction solvent and Semen Strychni powder. The optimal ratio of solvent to powder we chose is 50 mL/0.5 g = 100 (mL/g). Therefore, the measured ranges of the content in Semen Strychni for quality control (mg/g) need to be converted from linear range (µg/mL) by an index of 0.1, as also shown in . All the calibration curves showed good linearity (r2 ≥ 0.999). The lowest concentration of the calibration curve is usually used to define the LOQ value because researchers usually want to quantify the analyte as low as possible. In our study, the lowest concentrations in calibration of strychnine N-oxide and brucine N-oxide were exactly their LOQ values, which is consistent. But for strychnine, brucine, loganin and chlorogenic acid, we had to establish the calibration with their starting concentrations much higher than their LOQs in order to cover the quite high concentration ranges of the analytes in the real samples, which is therefore no longer identical. The precisions, repeatability and stability results are shown in , indicating that the relative standard deviation (RSD) values of the six compounds were all less than 4.55%. also shows that the HPLC method was reproducible with good accuracy in the range of 95.6–104.4% (RSD < 4.65%).
Table 1. Linear regression data, LODs and LOQs of the investigated compounds.
Table 2. Precision, repeatability, stability and accuracy data of the analytes.
Application the developed methods to determine Semen Strychni from different areas and batches
The established HPLC–DAD method was subsequently applied to simultaneously determine the content of the six compounds in the samples of seed coat, seed leaf, endosperm and whole seed yield from Burma and Yunnan, as shown in . The content of these active components in the seed coat, seed leaf and endosperm of Semen Strychni are obviously different. Chlorogenic acid, strychnine and brucine contents were the highest in the endosperm and the lowest in the seed coat, whereas loganin content was the highest in the seed leaf and is much higher than loganin content in the seed coat and the endosperm. Strychnine N-oxide and brucine N-oxide were transformed from strychnine and brucine via thermal reaction during the processing of Semen Strychni, which may explain why the content of both N-oxidized compounds were the highest in the seed coat. Although the content of six active components would significantly alter, the established HPLC–DAD method could accurately determine these active components.
Table 3. Contents of the six active compounds in different parts of Semen Strychni.
Conclusion
A selective HPLC–DAD method for the simultaneous determination of chlorogenic acid, loganin and four Strychnos alkaloids has been developed. To the best of our knowledge, chlorogenic acid and loganin are selected for the first time as marker constituents for quality control of Semen Strychni. The established method was applied to determine the content of the six compounds in the samples of seed coat, seed leaf, endosperm and whole seed yield from Burma and Yunnan. The results show that the HPLC–DAD method is a convenient and comprehensive quantity control for Semen Strychni.
Declaration of interest
The authors do not have any conflicts of interest. No other conflict of interest exits in the submission of this manuscript. We confirm that all the listed authors seen and approved the submitted manuscript.
Acknowledgements
We gratefully acknowledge the financial support from the Hunan Provincial Science and Technology Department (No. 2010FJ3107), the Hunan Provincial Traditional Chinese Medicine Bureau (No. 2009103), the National Natural Science Foundation of China (No. 81202985) and the Fundamental Research Funds for the Central Universities of China (Central South University, No. 2012QNZT151).
References
- Chinese Pharmacopoeia Committee. (2010). Pharmacopoeia of Peoples Republic of China. Beijing, China: Chemical Industry Press, 47--8
- International Conference on Harmonization (ICH). (1997). Q2b: Validation of analytical procedures: Methodology. US FDA Federal Register 62:27463--7
- Jiang Y, David B, Tu P, et al. (2010). Recent analytical approaches in quality control of traditional Chinese medicines – A review. Anal Chim Acta 657:9–18
- Jiang Y, Kusama K, Satoh K, et al. (2000). Induction of cytotoxicity by chlorogenic acid in human oral tumor cell lines. Phytomedicine 7:483–91
- Kwon SH, Kim JA, Hong S, et al. (2011). Loganin protects against hydrogen peroxide-induced apoptosis by inhibiting phosphorylation of JNK, p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem Int 58:533–41
- Kwon SH, Kim HC, Lee SY, et al. (2009). Loganin improves learning and memory impairments induced by scopolamine in mice. Eur J Pharmacol 619:44–9
- Lee SJ, Shin EJ, Son KH, et al. (1995). Anti-inflammatory activity of the major constituents of Lonicera japonica. Arch Pharm Res 18:133–5
- Li SP, Zhao J, Yang B. (2011). Strategies for quality control of Chinese medicines. J Pharm Biomed Anal 55:802–9
- Li YQ, He XJ, Qi SD, et al. (2006). Separation and determination of strychnine and brucine in Strychnos nux-vomica L. and its preparation by nonaqueous capillary electrophoresis. J Pharm Biomed Anal 41:400–7
- Park JH, Ryu YK, Lim HJ, et al. (1999). Effect of triethylamine in the mobile phase on the retention properties of conventional polymeric and horizontally polymerized octadecylsilica in RPLC. Chromatographia 49:635–42
- Rathi A, Srivastava N, Khatoon S, et al. (2008). TLC determination of strychnine and brucine of Strychnos nux vomica in ayurveda and homeopathy drugs. Chromatographia 67:607–13
- Tang HB, Wu P, Hu H. (2010). Experimental study on innovation of semen strychni stir-baked in sand. Central South Pharm 8:461–4
- Wang C, Han DD, Wang Z, et al. (2006). Analysis of Strychnos alkaloids in traditional Chinese medicines with improved sensitivity by sweeping micellar electrokinetic chromatography. Anal Chim Acta 572:190–6
- Xiao PG. (2002). Modern Chinese Material Medica. Beijing, China: Chemical Industry Press, 81–7
- Yun N, Kang JW, Lee SM. (2012). Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J Nutr Biochem 23:1249–55