Abstract
Context: Polydatin, also named piceid (3,4′,5-trihydroxystilbene-3-β-d-glucoside, PD), is a monocrystalline compound isolated from Polygonum cuspidatum Sieb. et Zucc. (Polygonaceae), but is also detected in grape, peanut, hop cones, red wines, hop pellets, cocoa-containing products, chocolate products and many daily diets. There are numerous investigations reported of PD in the past 22 years, but they are usually scattered across various publications, which may block further research and clinical use of PD.
Objective: The article summarizes and evaluates the published scientific information of PD pharmacological effects and pharmacokinetics since 1990.
Materials and methods: The information from 98 cases included in this review was compiled using major databases such as MEDLINE, Elsevier, Springer, PubMed, Scholar and CNKI.
Results: Numerous pharmacological investigations of PD mainly focus on cardiovascular effects, neuroprotection, anti-inflammatory and immunoregulatory effects, anti-oxidation, anti-tumor, liver and lung protection, etc.
Conclusion: A great number of pharmacological and pharmacokinetic investigations in the past 22 years have demonstrated that PD has favorable therapeutic properties, indicating its potential as an effective material. However, further research is needed to explore its molecular mechanisms of action and definitive target proteins.
Introduction
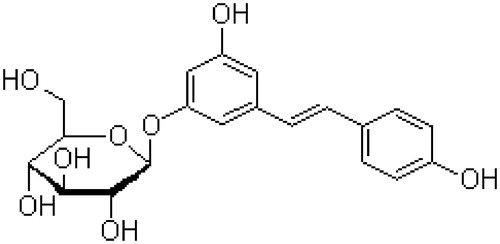
Plants have been the most important source of traditional medicines throughout the world for thousands of years and provide continuously new remedies for humankind. Great efforts have been made to identify natural active ingredients from plants using various techniques. Polydatin (PD, also named pieceid, (E)-piceid, (E)-polydatin, trans-polydatin, 3,4′,5-trihydroxystilbene-3-β-d-glucoside) is a monocrystalline compound originally isolated from the root and rhizome of Polygonum cuspidatum Sieb. et Zucc. (Polygonaceae), a traditional Chinese medicine that has long been used in China as an analgesic, anti-pyretic, diuretic and expectorant. It is a glucoside of resveratrol (3,4′,5-trihydroxystilbene) in which the glucoside group bound to the position C-3 substitutes a hydroxyl group, belonging to stilbene phytoalexins (). There are four main derivatives of PD in nature, including trans-polydatin, trans-resveratrol, cis-polydatin and cis-resveratrol. The bioactivity of trans-isomers is higher than that of cis-isomers (Mikulski & Molski, Citation2010).
PD can also be detected in grape, peanut, hop cones, red wines, hop pellets, cocoa-containing products, chocolate products and many daily diets. PD is the most abundant form of resveratrol in nature (Reqev-Shoshani et al., Citation2003). Previous studies have demonstrated that PD has many biomedical properties such as anti-platelet aggregation, anti-oxidative action of low-density lipoprotein (LDL), cardioprotective activity, anti-inflammatory and immune-regulating functions. In this review, we tried to present and assess the pharmacological and pharmacokinetic studies of PD.
Pharmacological effects
Effects on cardiac muscle cells
PD can protect myocardial cells (MCs) against injury elicited by oxygen and glucose deprivation (OGD) and chlorpromazine (Luo et al., Citation1990), increase Ca2+ in MCs with enhancement of MC contraction extent (Zhao et al., Citation2003). Zhao et al. (Citation2010) observed the effect of PD on adriamycin-injured myocardial ultra-structure of rats and discovered that PD significantly reduces the toxicity of adriamycin on cardiomyocytes (CMs), showing an evident protective action. In myocardial infarction, using the canine model established by coronary left anterior descending branch ligation, injection of PD markedly reduces the severity of ischemia, diminishes the ischemic and infarcted area, lowers the activities of serum lactate dehydrogenase (LDH) and creatine kinase, and thus alleviates the ischemic injury of CMs (Zhang et al., Citation2006). PD can significantly decrease the number of TdT-mediated dUTP nick end labeling-positive cells (apoptotic cells) and apoptosis rate in ischemia/reperfusion (I/R)-induced myocardial injury of rats through regulation of Bcl-2 and Bax protein expression (Zhang et al., Citation2009).
An extra-corporeal experiment was conducted by Zhao et al. (Citation2004) to explore the impact of lipopolysaccharide (LPS) on β-adrenergic receptor (β-AR) and prevention/treatment effects of PD. The results indicate that LPS directly induces β-AR decrease and down-regulates its affinity in CMs, while PD reverses these alterations. It may be one of the important mechanisms of PD improving myocardial contraction (Zhao et al., Citation2004). Another experiment reveals that LPS induces obvious lowering of myocardial contraction and mitochondrial injury, while PD reverses these unfavorable changes to protect CMs by regulating protein kinase C activity and protecting the ultrastructure of myocardial fibers (Xue et al., Citation2008).
Intravenous administration of PD (20 μg/kg) causes a significant decrease in the release of creatine phosphokinase and LDH from the damaged myocardium by activation of protein kinase C-ATP-sensitive K+ channel-dependent signaling (Miao et al., Citation2011, Citation2012). The electrophysiological mechanism is that PD shortens the duration of 50% repolarization (APD50) and 90% repolarization (APD90), but has no effects on resting potential, overshoot (OS), amplitude of action potential (APA) and the maximal rate of depolarization in phase 0 (Vmax) in normal papillary muscles. In partially depolarized papillary muscles, PD (50 μmol/L) not only shortens APD50 and APD90 but also decreases OS, APA and Vmax (Zhang et al., Citation2011a). The further study shows that PD down-regulates L-type Ca2+ channel activity and up-regulates ryanodine receptor activity, resulting in the moderate decrease of Ca2+ transient. Furthermore, PD increases myofilament Ca2+ sensitivity and at the same time modulates β-AR regulation of excitation–contraction (EC) coupling by remarkably alleviating β-AR stimulation-induced enhancement of Ca2+ signaling without impairing β-AR inotropic effect (Deng et al., Citation2012).
PD reduces cardiac weight indexes and the content of cyclic adenosine monophosphate and angiotensin II (Ang II) in isoproterenol-induced mice. It also decreases the size of CM and the levels of aldosterone (ALD), tumor necrosis factor-α (TNF-α), Ang II and endothelin-1 (ET-1), reduces ventricular collagen volume and depresses blood pressure in pressure-overload rats. These results demonstrate that PD has favorable effects on attenuating ventricular remodeling by inhibiting the activation of neurohormone, especially in rennin-angiotensin-ALD system (Gao et al., Citation2010). PD can also protect rats against myocardial I/R injury by up-regulating the levels of superoxide dismutase (SOD), nitric oxide synthase (NOS), constitutive NOS and nitric oxide (NO) and decreasing malondialdehyde (MDA) content (Zhang et al., Citation2008a).
Effects on endothelial cells
The structural integrality and normal function of vascular endothelial cells (VECs) are of great importance to maintain the permeability, immune defense and inflammation response of vessels, and VEC injury is the essential ring for genesis and development of atherosclerosis. The contractive response of normal rabbit aortic stripe to phenylephrine (PE) is not affected by PD or asymmetric dimethyl-arginine (ADMA), but can be weaken by PD in a dosage-dependent manner after the stripe is preconditioned by ADMA, indicating that PD dose does not put any impact on the contractive function of normal aortic stripe but noncompetitively antagonizes the contractive response of VECs to PE in the presence of ADMA (Qin et al., Citation2004).
PD can inhibit inducible cell adhesion molecule-1 (ICAM-1) expression in LPS-stimulated EC and attenuate white blood cell (WBC)-EC adhesion (Zhao et al., Citation2003). The inhibitory activities of PD on monocyte adhesion to TNF-α-activated endothelial cells are effective. PD could depress the protein and mRNA expression levels of ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1) in cultured endothelial cells through inhibition of nuclear factor-kappa B (NF-κB) pathway activation (Deng et al., Citation2011). Inducible nitric oxide synthase (iNOS) activity significantly increased in experimental hyperlipidemia rats, indicating the production of large amounts of NO, but evidently decreased by PD treatment (Zhu & Jin, Citation2005).
Hepatoprotective effects
The hepatoprotective effects of PD are closely related to the anti-inflammatory and anti-oxidative activities. Series studies indicate that PD is capable of alleviating liver injury induced by carbon tetrachloride (CCl4) and high-fat food feeding (HFD). PD (10−7–10−4 mol/L) can protect primarily cultured rat hepatocytes against CCl4 injury via reducing glutamic pyruvic transaminase release, MDA formation and glutathione (GSH) content (Huang et al., Citation1999). PD (0.05–4 mmol/L) decreases NO and MDA contents, inhibits NOS activity, increases SOD, GSH-Px and GSH activities, and suppresses alanine aminotransferase (ALT) release in pyrogallic acid induced hepatocyte culture medium (Mo et al., Citation1999). The increases in serum aspartate aminotransferase (AST), ALT and hepatic MDA, TNF-α, interleukin (IL)-1β, cyclooxygenase (COX)-2, iNOS and NF-κB in CCl4-induced liver injury mice are significantly reversed, and the content of GSH, activities of GSH transferase, SOD, catalase (CAT), GSH peroxidase and mRNA and protein expression levels of hepatic transforming growth factor-β1 (TGF-β1) are also modulated after PD preadministration for 5 continuous days (Zhang et al., Citation2012a).
PD can alleviate hepatic steatosis and reduce plasma and liver concentrations of TG, total cholesterol (TC) and free fatty acid significantly in HFD-induced chronic liver damage rat. In addition, the levels of TNF-α, MDA and 4-hexanonenal are markedly suppressed by PD in the liver of HFD-fed rats. PD also decreases the gene expression of sterol-regulatory element binding protein and its target genes involved in lipogenesis, including fatty acid synthase and stearoyl-CoA desaturase 1 in HFD-fed rats (Zhang et al., Citation2012b). Another in vivo test reveals that PD obviously reduces serum fasting insulin, fasting blood glucose, insulin resistance index and TNF-α level, and improves the insulin sensitivity index level, whose effects at high dose are better than those of fenofibrate (Zhang & Lv, Citation2010).
Fulminant hepatic failure (FHF) is a devastating clinical syndrome with extremely poor prognosis and high mortality, which can be induced by LPS in d-galactosamine (d-GalN)-sensitized mice. Pretreatment with PD (10, 30 and 100 mg/kg) exerts the evident protective effects on LPS/d-GalN-induced FHF mice, which reduces serum ALT and AST activities, diminishes liver histopathological injury and decreases mortality in a dose-dependent manner. In addition, pretreatment with PD also suppresses TNF-α production, myeloperoxidase (MPO) activity, intercellular adhesion molecule-1 (ICAM-1) and endothelial cell adhesion molecule-1 expression, caspase-3 activation and NF-κB activity in model mice. PD probably reduces TNF-α production via inhibition of NF-κB activation (Wu et al., Citation2012).
Neuroprotective activity
Previous studies have proved that PD has an obvious neuroprotective activity, especially in the cerebral ischemic pathogenesis. PD protects against the brain damage caused by permanent middle cerebral artery occlusion (MCAO) through up-regulating the expression of glioma-associated oncogene homolog1, patched-1 and SOD1, down-regulating the expression of NF-κB p65, and ameliorating blood–brain barrier permeability (Ji et al., Citation2012). Intravenous injection of PD can reduce the volume of brain infarction, improve neurological deficits, inhibit the expression of ICAM-1, VCAM-1, E-selectin, L-selectin and integrins after the operation of MCAO for 1 h (Cheng et al., Citation2006c). PD protects against learning and memory impairments, markedly attenuates cognitive deficits, decreases the production of MDA, meanwhile significantly increases the activities of SOD and CAT in the rat model of vascular dementia induced by chronic cerebral hypoperfusion (Li et al., Citation2012). PD treatment significantly enhances the cell viability, reduces the levels of LDH, NO, MDA and increases SOD activity of pheochromocytoma cells injured by OGD (Xu et al., Citation2010) and effectively alleviates OGD-induced neuron injury (Li et al., Citation2012). Additionally, PD can up-regulate the expression of brain-derived neurotrophic factors in cerebral cortex of neonatal rats (Sun et al., Citation2012).
PD can improve the abilities of learning and memory in chronic alcoholism mice. Alcohol induces the mRNA expression of cyclin-dependent kinases 5 and N-methyl-d-aspartate in the prefrontal cortex of chronic alcoholism mice, but PD markedly reverses these alterations (Xu et al., Citation2011, Citation2012). Although the molecular mechanism is not fully understood, PD significantly inhibits cerebral edema and increases the content of Asp and Glu in cerebral hemorrhage rats (Liu et al., Citation2010). Amyloid-β peptide (Aβ) accumulation is one of the major neurodegenerative processes occurring during the progression of pathogenesis in Alzheimer’s disease. Rivière et al. (Citation2010) tested 20 stilbenes derivatives against amyloid-β peptide (Aβ) aggregation in vitro by ultraviolet (UV)-visible measurements and electron microscopy. The results indicate that PD effectively and dose-dependently inhibits Aβ polymerization and has the best inhibitory activity among these compounds. The inhibitory rate is 63% and EC50 6 ± 2 μM (Rivière et al., Citation2007, Citation2010). PD probably destabilizes fibrils and oligomers to give back monomers induced by Aβ25–35 that can open the hydrophobic zipper and shift the reversible equilibrium “random coil ↔ β-sheet” to the disordered structure (Rivière et al., Citation2009).
Lung protective effects
The protective effects of PD against acute or chronic lung disease are supported by numerous studies. PD has a weak activity in inducing relaxation of isolated pulmonary arteries in vitro. Glycosylation markedly diminishes the biological effects of stilbene derivatives possibly because of decreased lipophilicity and/or target accessibility (Waffo-Téquo et al., Citation2001). The prophylactic and therapeutic effects of PD on acute lung injury in rats with endotoxic shock are carried out by inhibiting phospholipase A2 activity and gene expression of secretory phospholipase A2 type IIA. The mechanism is possibly that PD up-regulates Clara cell secretory protein mRNA expression and down-regulates cPLA2 mRNA expression in lung (Shu et al., Citation2004, Citation2011).
On the one hand, PD alleviates lung I/R injury in rabbits by down-regulating TLR4 and NF-κB expression and inhibiting the release of mediators of inflammation as ICAM-1 (Jin et al., Citation2009). On the other hand, PD also ameliorates SOD activity and injured alveoli rate and reduces MDA content to protect against lung I/R injury (Wang et al., Citation2008). Moreover, PD regulates the levels of NO, Ang II and ET, which are closely related to pulmonary hypertension remodeling system, and abates the forced activation of PKC signaling by thymeleatoxin (Miao et al., Citation2012). PD significantly protects against rat pulmonary micro VECs injury in vitro induced by hypoxia through preventing the increases of LDH activity and vascular endothelial growth factor (VEGF) expression (Wang et al., Citation2001). After PD intraperitoneal injection, the activity of PLA2, the concentration of hydroxyproline in lung homogenate and the levels of prostaglandin E2 (PGE2), leukotriene C4 and TGF-β1 in bronchoalveolar lavage fluid are significantly reduced. However, PD does not completely block the process of pulmonary fibrosis (Zhang et al., Citation2011b).
Anti-arteriosclerosis
Zhu and Jin (Citation2006) observed the effect of PD on blood lipids metabolism in the experimental rat model of hyperlipidemia established by HFD. The results show that oral administration of PD for four successive weeks significantly decreases the serum levels of TC, triglyceride (TG), LDL cholesterol (LDL-C) and apolipoprotein A1 (ApoA1) in the model rats but evidently increases the ratios of high-density lipoprotein cholesterol (HDL-C)/TC and ApoA1/ApoB (Zhu & Jin, Citation2006). Although there is no statistically significant difference observed in the serum HDL-C levels, the ratios of LDL-C/HDL-C and TC/HDL-C remarkably decrease in PD-treated high-fat/cholesterol hamsters (Du et al., Citation2009). Similar effects are displayed in rabbits, and administration of PD can significantly reduce the rabbit serum levels of TC, TG and LDL-C in a dose-dependent manner (Xing et al., 2009).
Anti-inflammatory activity
PD has several beneficial effects attributed to its anti-inflammatory properties, such as nephroprotective, hepatoprotective and lung protective activities. Endometriosis, an estrogen-dependent inflammatory disease, is defined as the presence of endometrial-like tissue outside the uterus, which induces a chronic inflammatory reaction (Kennedy et al., Citation2005). PD exhibits anti-hyperuricemic activity through regulating renal organic ion transporters in hyperuricemic mice (Shi et al., Citation2012).
Numerous studies have reported that PD modulates the expression of inflammatory cytokines and cell adhesion molecules in vivo and in vitro. PD decreases IL-17 production in activated human peripheral blood mononuclear cells through down-regulation of IL-17 mRNA expression in cells (Lanzilli et al., Citation2012). The expression of ICAM-1 and TGF-β1 in glomerular mesangial cells and high glucose-induced production are significantly suppressed by PD in vitro (Xie et al., Citation2012). Additionally, PD downregulates NF-κB p65 activity and expression, blocks the expression of TNF-α, IL-6 and IL-1β at mRNA and protein levels, decreases MPO activity and alleviates inflammatory damage of colitis in mice with ulcerative colitis, suggesting that the anti-inflammation effects of PD can be attributed, at least partially, to the blocking of the NF-κB pathway (Xie et al., Citation2012; Yao et al., Citation2011). After intraperitoneal injection of PD (20 mg/kg), NF-κB expression also significantly decreases in renal ischemia-reperfusion injury rats (Fei et al., Citation2009). PD suppresses the level of serum uric acid in vivo and in vitro by inhibition of xanthine oxidase activity and ameliorates the renal function in urate nephropathy mice induced by fructose. The nephroprotective activity is attributed to PD inhibition of the involved inflammatory cascade, including the expression of NF-κB p65, COX-2 and iNOS proteins and the production of TNF-α, PGE2 and IL-1β, which are related to the oxidative stress (Chen et al., Citation2013).
In many chronic inflammatory diseases, PD can inhibit constitutive bacterial LPS- and interferon-γ (T/I)-induced but not TGF-α-induced extracellular signal regulated kinase (ERK) phosphorylation and suppress NF-κB activity in LPS and T/I-induced primary human keratinocytes (HaCaT). PD activates aryl hydrocarbon receptor machinery in UV-exposed keratinocytes through down-regulating the gene expression of pro-inflammatory cytokines/enzymes, suppressing interferon gamma-inducible protein 10 (IP-10) release, and up-regulating IL-8 level (Potapovich et al., Citation2011), opposing enhanced monocyte chemotactic protein-1 (MCP-1) and IP-10 transcription/synthesis (Pastore et al., Citation2012). PD is also able to modulate IL-6, IL-8 and TNF-α gene expression, increase the release of human β-defensin 2 and gene expression of heat shock protein 70B′ in heat-stressed HaCaT (Ravagnan et al., 2013). PD enhances fibroblast proliferation at the concentrations of 10–5 and 10−4 mol/L but blocks the cellular cycle in S phase at 10−3 mol/L, suggesting its bidirectional regulatory effects in fibroblasts (Bian et al., Citation2012).
Anti-shock effects
Combined with hypotensive resuscitation, bolus infusion of PD (2 mg/kg) evidently prolongs the survival time of pregnant rabbits with uncontrolled hemorrhagic shock by improving capillary perfusion as indicated by increased arteriole diameter and higher functional capillary density (Sheng et al., Citation2011). PD can activate KATP channels of vascular smooth muscle cells (VSMC) and decrease pH value and Ca2+ of VSMC. PD has multiple effects on VSMC, MC, WBCs and endothelial cells (EC), which are closely related to the enhancement of heart function and improvement of microcirculatory perfusion in shock (Zhao et al., Citation2003). PD can inhibit mitochondrial swelling, increase mitochondrial membrane potential and improve intracellular adenosine triphosphate (ATP) levels. Furthermore, PD also preserves lysosomal stability, suppresses activation of KATP channels and arteriolar smooth muscle cell hyperpolarization and reduces vasoresponsiveness to norepinephrine that normally follows severe shock (Wang et al., Citation2012). Increased lipid peroxides levels, lysosomal injury and mitochondrial permeability transition pore opening cause swollen mitochondria with poorly defined cristae, decreased mitochondrial membrane potential (ΔΨ) and reduced ATP content in neurons of rats after 2 h of shock, indicating mitochondrial dysfunction. However, PD evidently inhibits these alterations, which increases the ATP level from 44.14 ± 13.81% to 89.57 ± 9.21% and prolongs survival time from 6.3 ± 5.9 h to 31.6 ± 13.7 h. PD may be the best choice for protection of neuron against mitochondrial injury in severe shock (Wang et al., Citation2013).
Anti-tumor activity
PD has the favorable cytotoxic effects on many human tumor cell lines such as human cervical carcinoma HeLa cells, hepatoma cell line SMMC-7721 cells, epidermal carcinoma A-431 cells and nasopharyngeal carcinoma CNE cells. PD can cause mitochondrial disruption, trigger endoplasmic reticulum (ER) stress and down-regulate Akt phosphorylation in CNE cells, while knock-down of CCAAT/enhancer-binding protein homologous protein dramatically abrogates the inactivation of Akt. Furthermore, PD-induced reactive oxygen species (ROS) are an early event that triggers ER stress mitochondrial apoptotic pathways in CNE cells (Liu et al., Citation2011). Additionally, PD has higher binding affinity to the target G-quadruplex in the proximal VEGF promoter that reduces VEGF expression in PAN-1 cancer cells and NIH3T3 cells (Balasubramanian & Neidle, Citation2009; Li & Yuan, Citation2010; Sun et al., Citation2005). PD inhibits the formation of capillary-like tube networks (angiogenesis) of human umbilical vein endothelial cells (HUVECs) and suppresses DNA synthesis in Lewis lung carcinoma cells (Kimura & Okuda, Citation2000).
Through hydrophobic stacking and hydrogen bond, PD can interact with neurotensin (NT). The polyphenol–protein complexes seem to affect NT metabolism (Richard et al., Citation2005) and diminish the NT-induced metabolic activation of colon carcinoma cells (Briviba et al., Citation2001). PD significantly inhibits COX-1 activity, with the half maximal inhibitory concentration (IC50) value of 10.6 μM in mouse mammary organ culture (Waffo-Téquo et al., Citation2001).
Anti-oxidative activity
PD has significant anti-oxidant properties due to its molecular structure of conjugated double bond, which is closely associated with its many pharmacological effects including protecting against I/R injury, improving learning and memory, lowering lipid and extending lifespan. PD is more resistant to enzymatic oxidation than resveratrol owing to the change of molecule structure (Fabris et al., Citation2008). In fact, the anti-oxidative activity of trans-polydatin is stronger than cis-PD and trans-resveratrol (Mikulski & Molski, Citation2010).
Free radical scavenging effect is one of PD anti-oxidative properties. Both NADH-PMS-NBT system produced oxygen free radicals () and EDTANa2-Fe(II)-H2O2 system produced hydroxyl radicals (·OH) can be reduced by PD in vitro (Tian & Yang, Citation2001), which also scavenges ·OH produced by H2O2 to protect HUVECs (ECV304) in a dose-dependent manner (Su et al., Citation2010). Additionally, PD shows a notable anti-oxidant capacity in fish oil-in-water emulsions (Medina et al., Citation2010). PD enables the extension of mean lifespan of transgenic strain CL2166 due to its protective effects against oxidative stress (Wen et al., Citation2012), shows a slower but prolonged protective action against lipid peroxidation in comparison with BHT (2,6-di-tert-butyl-4-methylphenol) and α-tocopherol (vitamin E) and is more efficacious than resveratrol. The susceptible hydroxyl group of PD is located in the lipid region of the bilayer close to the double bonds of polyunsaturated fatty acids, making it particularly suitable for the prevention and control of the lipid peroxidation of the membranes (Fabris et al., Citation2008).
PD displays no cytotoxicity in HaCaT cells at low concentrations (<100 μg/ml), but reduces HaCaT cell death induced by UVB irradiation. The production of ROS, one of important parameters for an anti-oxidative activity, is apparently increased by UVB irradiation but significantly decreased by PD, which also evidently inhibits COX-2 expression boosted by UVB irradiation in HaCaT cells and the epidermis of BALB/c-nu mice. The mechanism is involved in PD suppressing UVB-induced activation of p38, JNK and ERK1/2 in the cells (He et al., Citation2012).
Inhibition of thrombus formation
PD shows an antagonistic action on thrombosis, which significantly reduces the fibrinogen content and platelet adhesive rate in acute blood-stasis model rats (Wang et al., Citation2004), inhibits platelet aggregation and decreases the production of thromboxane B2 (TXB2) in platelet-rich plasma induced by arachidonic add or Adenosine diphosphate (Shan et al., Citation1990). The thrombin-induced platelet neutrophil adhesion and platelet aggregation in nutmeg phorbol-activated neutrophilic granulocyte suspension are markedly inhibited by PD. In addition, PD is also capable of lowering TXB2 content and elevating 6-keto-PGF1α level in plasma (Chen et al., Citation2006a,b).
Inhibition of melanogenesis
PD inhibits tyrosinase activity and melanin production in melanocytes better than arbutin, which is well known to inhibit melanin formation. Furthermore, the mRNA and protein expression of tyrosinase, tyrosinase-related proteins 1, 2 and microphthalmia-associated transcription factor is significantly suppressed in melanocytes (Jeong et al., Citation2010). However, PD displays only weak effects on tyrosinases from mushroom and murine melanoma B-16 in vitro with IC50 value more than 100 μM (Kim et al., Citation2002). Presumably different species of cells are responsible for these two diametrically opposed results.
Anti-microbial activity
P. cuspidatum ethyl acetate fraction, including PD, resveratrol, anthraglycoside B and emodin, displays a effective anti-bacterial activity, which inhibits dental caries-related factors of Streptococcus mutans and Streptococcus sobrinus and significantly reduces glycolytic acid production at a low level (Ban et al., Citation2010). PD also has weak inhibitory effects on the acidogenicity of S. mutans UA 159 and subsequent dental caries formation (Kwon et al., Citation2010).
Immunoregulatory effects
The therapeutic effects of PD on passive cutaneous anaphylaxis are carried out by decreasing antigen-stimulated mast cell degranulation. The possible mechanism is that PD suppresses high-affinity IgE receptors-mediated Ca2+ mobilization by inhibiting Ca2+ entry through Ca2+ release-activated Ca2+ channel, which are the major contributors to PD-induced mast cell stabilization (Yuan et al., Citation2012).
Pharmacokinetic studies
Pharmacokinetic studies are often necessary to the clinical use of drugs effectively and safely. The absorption, distribution and metabolism of PD are closely combined with its bioactivity. To date, however, few analytical methods have been used for determination of trans-stilbene glycoside in biological samples when its pharmacokinetics profiles are studied (Lv et al., Citation2011; Ren et al., Citation2010).
The absorption of some phenols from the diet is enhanced by conjugation with glucose. It seems that PD can be more efficiently conjugated with glucose (Hollman et al., Citation1995; Hollman, 1997). PD can be absorbed in two different transport systems: passive diffusion and active transport via sodium-dependent glucose transporter (SGLT1) mainly existing in the stomach and intestines (Henry et al., Citation2005). PD, unlike resveratrol that penetrates cell membrane passively, enters the cell via an active mechanism using glucose carriers. PD is absorbed at 4 °C, the initial rate of trans-PD incorporation is about 1.6-times lower than that estimated at 37 °C, while the content in cells is not much lower (He et al., Citation2007), suggesting PD active transport by SGLT1. Therefore, PD solution is essential for its uptake in the intestines. Although the accumulation rate and residual amount of resveratrol are higher than PD in Caco-2 cells, the half life time of PD is nearly 4 h, and Cmax is higher than resveratrol at the same dosage. In addition, AUC(0–∞) and t1/2 of PD are also increased in a dose-dependent manner (Zhou et al., Citation2009). Transepithelial transport of PD clearly occurs with the favorable apparent permeability coefficient (Papp) about 10 × 10−6 cm/s for the apical to basolateral flux, suggesting virtually complete absorption of PD in humans (Yee, 1997).
The different tissue concentrations reach maximum values at 10 min postdose, which are 1.53 ± 0.13, 5.22 ± 0.46, 4.59 ± 0.59 and 6.41 ± 0.77 μg/g in heart, liver, lung and kidney, respectively, after intravenous administration of 20 mg/kg PD (Gao et al., Citation2006). But after oral administration of 50 mg/kg PD to male rats, the maximum concentrations in heart, liver, spleen, lung, kidney, stomach, small intestine, brain and testis are 0.50 ± 0.26, 4.47 ± 2.51, 28.03 ± 13.81, 10.42 ± 3.86, 2.58 ± 1.19, 168.79 ± 77.45, 108.66 ± 29.79, 6.07 ± 2.85 and 5.30 ± 2.40 μg/g, respectively (Lv et al., Citation2006).
PD is deglycosylated in trans-resveratrol through two possible pathways: the first is a cleavage by cytosolic-β-glucosidase, after passing the brush-border membrane by SGLT1. The second is deglycosylation on the luminal side of the epithelium by the membrane-bound enzyme lactase-phlorizin hydrolase, followed by passive diffusion of the released aglycone, which is further metabolized inside the cells into two glucuronoconjugates (Henry-Vitrac et al., Citation2006). Resveratrol, dihydroresveratrol monosulfate, PD-monosulfate and PD-monoglucuronide are detected in rat urine after oral administration of PD, which can be transformed into resveratrol, dihydropiceid and dihydroresveratrol after incubation with gut microbiota (Wang et al., Citation2011). Resveratrol, glucuronidated resveratrol and glucuronidated trans-PD, the first-pass metabolites of PD in rat small intestine and liver, are detected in plasma after perfusion of trans-PD in situ rat small intestine and oral administration of PD (Zhang et al., Citation2008b; Zhou et al., Citation2009). After oral administration, 98.4% of PD is metabolized in the intestines and liver, and glucuronidated resveratrol is the main metabolite, reaching 84% (Zhou et al., Citation2009). In addition, the small intestine extracts are more active than the liver extracts (Zhou et al., Citation2007).
Conclusion
P. cuspidatum, a traditional Chinese medicine, has long been used as analgesic, anti-pyretic, diuretic and expectorant in clinical practice. PD, a monocrystalline compound isolated from P. cuspidatum, shows many pharmacological effects confirmed by numerous investigations, including cardiovascular protection, neuroprotection, anti-inflammation, immunoregulation, anti-oxidation, anti-tumour and liver and lung protective effects.
However, further studies are required for PD development. Although various bioactivities of PD are confirmed in laboratory animals, organs or cells, few molecular mechanisms of action are known, and the definitive target proteins bound by PD still remain undetermined, which will make against further clinical applications of PD. When a drug is used in clinical practice, its safety is especially important. Unfortunately, there are also few toxicological evaluations reported on PD.
The documents summarized above strongly support the point of view that PD has favorable therapeutic properties, indicating its potential as an effective material. This review presents and assesses the pharmacological and pharmacokinetic studies of PD in the past 22 years. It may be important for interested readers to easily identify and research further into PD.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This work was supported by the National Natural Science Foundation of China (No. 81173462) and the Open Research Fund of State Key Laboratory Breeding Base of Systematic Research, Development and Utilization of Chinese Medicine Resources.
References
- Balasubramanian S, Neidle S. (2009). G-Quadruplex nucleic acids as therapeutic targets. Curr Opin Chem Biol 13:345–53
- Ban SH, Kwona YR, Pandit S, et al. (2010). Effects of a bio-assay guided fraction from Polygonum cuspidatum root on the viability, acid production and glucosyltranferase of mutans streptococci. Fitoterapia 81:30–4
- Bian HN, Sun CW, Chen HD, et al. (2012). Effects of polydatin on the biological features of cultured fibroblasts. Chin J Tissue Eng Res 16:6111–15
- Briviba K, Abrahamse SL, Pool-Zobel BL, et al. (2001). Neurotensin- and EGF-induced metabolic activation of colon carcinoma cells is diminished by dietary flavonoid cyanidin but not by its glycosides. Nutr Cancer 41:172–9
- Chen L, Lan Z, Lin Q, et al. (2013). Polydatin ameliorates renal injury by attenuating oxidative stress-related inflammatory responses in fructose-induced urate nephropathic mice. Food Chem Toxicol 52:28–35
- Chen P, Hu XL, Lei WY, et al. (2006a). Effects of polydatin on thrombosis and interactions of platelets to neutrophils. J Yunnan Univ (Natural Sci) 28:364–8
- Chen P, Yang LC, Hu XL, et al. (2006b). Investigation of polydatin on antithrombotic effects and plasma levels of TXB2 and 6-keto-PGF1α. Nat Prod Res Dev 18:398–400, 404
- Cheng Y, Zhang HT, Sun L, et al. (2006c). Involvement of cell adhesion molecules in polydatin protection of brain tissues from ischemia–reperfusion injury. Brain Res 1110:193–200
- Deng J, Liu W, Wang Y, et al. (2012). Polydatin modulates Ca2+ handling, excitation–contraction coupling and β-adrenergic signaling in rat ventricular myocytes. J Mole Cell Card 53:646–56
- Deng YH, Alex D, Huang HQ, et al. (2011). Inhibition of TNF-α-mediated endothelial cell-monocyte cell adhesion and adhesion molecules expression by the resveratrol derivative, trans-3,5,4′-trimethoxystilbene. Phytother Res 25:451–7
- Du J, Sun LN, Xing WW, et al. (2009). Lipid-lowering effects of polydatin from Polygonum cuspidatum in hyperlipidemic hamsters. Phytomedicine 16:652–8
- Fabris S, Momo F, Ravagnan G, et al. (2008). Antioxidant properties of resveratrol and piceid on lipid peroxidation in micelles and monolamellar liposomes. Biophys Chem 135:76–83
- Fei J, Xie K, Tao FS. (2009). Effects of polydatin on expressions of NF-κB and MPO during renal ischemia–reperfusion injury. J Clin Anesthesiol 25:511–13
- Gao JP, Chen CX, Gu WL, et al. (2010). Effects of polydatin on attenuating ventricular remodeling in isoproterenol-induced mouse and pressure-overload rat models. Fitoterapia 81:953–60
- Gao S, Fan G, Hong Z, et al. (2006). HPLC determination of polydatin in rat biological matrices: Application to pharmacokinetic studies. J Pharmaceut Biomed 41:240–5
- He H, Zhao Y, Chen X, et al. (2007). Quantitative determination of trans-polydatin, a natural strong antioxidative compound, in rat plasma and cellular environment of a human colon adenocarcinoma cell line for pharmacokinetic studies. J Chromatogr B 855:145–51
- He YD, Liu YT, Lin QX, et al. (2012). Polydatin suppresses ultraviolet B-induced cyclooxygenase-2 expression in vitro and in vivo via reduced production of reactive oxygen species. Br J Dermatol 167:941–4
- Henry C, Vitrac X, Decendit A, et al. (2005). Cellular uptake and efflux of trans-piceid and its aglycone trans-resveratrol on the apical membrane of human intestinal Caco-2 cells. J Agric Food Chem 53:798–803
- Henry-Vitrac C, Desmoulière A, Girard D, et al. (2006). Transport, deglycosylation, and metabolism of trans-piceid by small intestinal epithelial cells. Eur J Nutr 45:376–82
- Hollman PC. (1997). Bioavailability of flavonoids. Eur J Clin Nutr 51:S66–9
- Hollman PC, de Vries JH, van Leeuwen SD, et al. (1995). Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr 62:1276–82
- Huang ZS, Wang ZW, Liu MP, et al. (1999). Protective effects of polydatin against CCl4-induced injury to primarily cultured rat hepatocytes. World J Gastroenterol 5:41–4
- Jeong ET, Jin MH, Kim MS, et al. (2010). Inhibition of melanogenesis by piceid isolated from Polygonum cuspidatum. Arch Pharm Res 33:1331–8
- Ji H, Zhang X, Du Y, et al. (2012). Polydatin modulates inflammation by decreasing NF-κB activation and oxidative stress by increasing Gli1, Ptch1, SOD1 expression and ameliorates blood-brain barrier permeability for its neuroprotective effect in pMCAO rat brain. Brain Res Bulletin 87:50–9
- Jin XF, Xu ZJ, Wang WT, et al. (2009). The regulative effects of polydatin on toll-like receptor 4 signal transduction pathway in lung ischemia/reperfusion injury in rabbits. Chin J Appl Physial 25:41–4
- Kennedy S, Bergqvist A, Chapron C, et al. (2005). ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod 20:2698–704
- Kim YM, Yun J, Lee CK, et al. (2002). Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J Biol Chem 227:16340–4
- Kimura Y, Okuda H. (2000). Effects of naturally occurring stilbene glucosides from medicinal plants and wine, on tumour growth and lung metastasis in Lewis lung carcinoma-bearing mice. J Pharm Pharmacol 52:1287–95
- Kwon YR, Son KJ, Pandit S, et al. (2010). Bioactivity-guided separation of anti-acidogenic substances against Streptococcus mutans UA 159 from Polygonum cuspidatum. Oral Diseases 16:204–9
- Lanzilli G, Cottarelli A, Nicotera G, et al. (2012). Anti-inflammatory effect of resveratrol and polydatin by in vitro IL-17 modulation. Inflammation 35:240–8
- Li H, Yuan G. (2010). Electrospray ionization mass spectrometry probing of formation and recognition of the G-quadruplex in the proximal promoter of the human vascular endothelial growth factor gene. Rapid Commun Mass Sp 24:2030–4
- Li RP, Wang ZZ, Sun MX, et al. (2012). Polydatin protects learning and memory impairments in a rat model of vascular dementia. Phytomedicine 19:677–81
- Liu H, Zhang G, Bie X, et al. (2010). Effect of polydatin on dynamic changes of excitatory amino acids in cerebrospinal fluid of cerebral hemorrhage rats. China J Chin Mate Med 35:3038–42
- Liu H, Zhao S, Zhang Y, et al. (2011). Reactive oxygen species-mediated endoplasmic reticulum stress and mitochondrial dysfunction contribute to polydatin-induced apoptosis in human nasopharyngeal carcinoma CNE cells. J Cell Biochem 112:3695–703
- Luo SF, Yu CL, Zhang PW. (1990). Influences of 3,4′,5-trihydroxystibene 3-beta-mono-d-glucoside on beat rate and injury of cultured newborn rat myocardial cells. Acta Pharmacol Sin 11:147–50
- Lv C, Zhang L, Wang Q, et al. (2006). Determination of piceid in rat plasma and tissues by high-performance liquid chromatographic method with UV detection. Biomed Chromatogr 20:1260–6
- Lv G, Lou Z, Chen S, et al. (2011). Pharmacokinetics and tissue distribution of 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside from traditional Chinese medicine Polygonum multiflorum following oral administration to rats. J Ethnopharmacol 137:449–56
- Medina I, Alcántara D, González MJ, et al. (2010). Antioxidant activity of resveratrol in several fish lipid matrices: Effect of acylation and glucosylation. J Agric Food Chem 58:9778–86
- Miao Q, Shi XP, Ye MX, et al. (2012). Polydatin attenuates hypoxic pulmonary hypertension and reverses remodeling through protein kinase C mechanisms. Int J Mol Sci 13:7776–87
- Miao Q, Wang S, Miao S, et al. (2011). Cardioprotective effect of polydatin against ischemia/reperfusion injury: Roles of protein kinase C and mito KATP activation. Phytomedicine 19:8–12
- Mikulski D, Molski M. (2010). Quantitative structure-antioxidant activity relationship of trans-resveratrol oligomers, trans-4,4′-dihydroxystilbene dimer, trans-resveratrol-3-O-glucuronide, glucosides: Trans-piceid, cis-piceid, trans-astringin and trans-resveratrol-4′-O-β-d-glucopyranoside. Eur J Med Chem 45:2366–80
- Mo ZX, Shao HX, Zheng YS. (1999). Protective effects of polydatin on hepatocyte injury induced by pyrogallic acid in mice. Pharm Clin Chin Mater Med 15:6–8
- Pastore S, Lulli D, Fidanza P, et al. (2012). Plant polyphenols regulate chemokine expression and tissue repair in human keratinocytes through interaction with cytoplasmic and nuclear components of epidermal growth factor receptor system. Antioxid Redox Sign 16:314–28
- Potapovich AI, Lulli D, Fidanza P, et al. (2011). Plant polyphenols differentially modulate inflammatory responses of human keratinocytes by interfering with activation of transcription factors NF-κB and AhR and EGFR-ERK pathway. Toxicol Appl Pharm 255:138–49
- Qin J, Chen YZ, Zhou QX, et al. (2004). Effect of polydatin on endothelial function in aorta vascular strips of healthy rabbits treated with ADMA. Chin Tradit Herbal Drugs 35:535–8
- Ravagnan G, De Filippis A, Cartenì M, et al. (2013). Polydatin, a natural precursor of resveratrol, induces β-defensin production and reduces inflammatory response. Inflammation 36:26–34
- Ren X, Ouyang H, Wang G, et al. (2010). Study on excretion of stilbene glycoside (THSG) and its beta-cyclodextrin inclusion. China J Chin Mater Med 35:2620–3
- Reqev-Shoshani G, Shoseyov O, Bilkis I, et al. (2003). Glycosylation of resveratrol protects it from enzymic oxidation. Biochem J 374:157–63
- Richard T, Vitrac X, Merillon JM, et al. (2005). Role of peptide primary sequence in polyphenol-protein recognition: An example with neurotensin. Biochim Biophys Acta 1726:238–43
- Rivière C, Papastamoulis Y, Fortin PY, et al. (2010). New stilbene dimers against amyloid fibril formation. Bioorg Med Chem Lett 20:3441–3
- Rivière C, Richard T, Quentin L, et al. (2007). Inhibitory activity of stilbenes on Alzheimer’s β-amyloid fibrils in vitro. Bioorg Med Chem Lett 15:1160–7
- Rivière C, Delaunay JC, Immel F, et al. (2009). The polyphenol piceid destabilizes preformed amyloid fibrils and oligomers in vitro: Hypothesis on possible molecular mechanisms. Neurochem Res 34:1120–8
- Shan CW, Yang SQ, He HD, et al. (1990). Influence of 3,4′,5-trihydroxystibene-3-β-mono-d-glucoskle on rabbits' platelet aggregation and thromboxane B2 production in vitro. Acta Pharmacol Sin 11:527–30
- Sheng C, Yu YH, Zhao KS, et al. (2011). Hypotensive resuscitation combined with polydatin improve microcirculation and survival in a rabbit model of uncontrolled hemorrhagic shock in pregnancy. J Surg Res 168:103–10
- Shi YW, Wang CP, Liu L, et al. (2012). Antihyperuricemic and nephroprotective effects of resveratrol and its analogues in hyperuricemic mice. Mol Nutr Food Res 56:1433–44
- Shu SY, Ling ZY, Wang XY, et al. (2004). Effect of polydatin on phospholipase A2 in lung tissues in rats with endotoxic shock. Chin J Traumatol 7:239–43
- Shu SY, Ling ZY, Ye M, et al. (2011). Polydatin up-regulates Clara cell secretory protein to suppress phospholipase A2 of lung induced by LPS in vivo and in vitro. BMC Cell Biol 12:31
- Su D, Zhang BL, Huan ML, et al. (2010). Comparison of resveratrol and polydatin on antioxidative activities. Chin Pharma 13:471–3
- Sun D, Guo K, Rusche JJ, et al. (2005). Facilitation of a structural transition in the polypurine/polypyrimidine tract within the proximal promoter region of the human VEGF gene by the presence of potassium and G-quadruplex-interactive agents. Nucleic Acids Res 33:6070–80
- Sun J, Qu XY, He HM, et al. (2012). Effects of polydatin on expression of BDNF in cortex of neonatal rats with hypoxic-ischemic brain damage. Chin J Public Health 28:783–5
- Tian JW, Yang JX. (2001). In vitro antioxidative effect of polydatin. Chin Trad Herb Drugs 32:918–20
- Waffo-Téquo P, Hawthorne ME, Cuendet M, et al. (2001). Potential cancer-chemopreventive activities of wine stilbenoids and flavans extracted from grape (Vitis vinifera) cell cultures. Nutr Cancer 40:173–9
- Wang D, Zhang Z, Ju J, et al. (2011). Investigation of piceid metabolites in rat by liquid chromatography tandem mass spectrometry. J Chromatogr B 879:69–74
- Wang FY, Xu ZJ, Zhang XL, et al. (2008). Protective effects of polydatin against lung ischemia/reperfusion injury and the initial exploration for its mechanism. Chin J Appl physiol 24:62–5
- Wang M, Li ZC, Wang JB. (2001). Protective effect of polydatin on hypoxia-induced injury in rat pulmonary microvascular endothelial cells. Northwest Pharmaceut J 26:185–9
- Wang X, Song R, Bian HN, et al. (2012). Polydatin, a natural polyphenol, protects arterial smooth muscle cells against mitochondrial dysfunction and lysosomal destabilization following hemorrhagic shock. Am J Physiol Regul Integr Comp Physiol 302:R805–14
- Wang X, Song R, Chen Y, et al. (2013). Polydatin – A new mitochondria protector for acute severe hemorrhagic shock treatment. Expert Opin Investig Drugs 22:169–79
- Wang Y, Xue J, Sun XD, et al. (2004). Study on decreasing effects of polydatin on blood viscosity in the rat model of acute blood stasis. China Pharm 15:275–7
- Wen H, Shi W, Qin J. (2012). Multiparameter evaluation of the longevity in C. elegans under stress using an integrated microfluidic device. Biomed Microdevices 14:721–8
- Wu MJ, Gong X, Jiang R, et al. (2012). Polydatin protects against lipopolysaccharide-induced fulminant hepatic failure in d-galactosamine-sensitized mice. Int J Immunopathol Pharmacol 25:923–34
- Xie X, Peng J, Huang K, et al. (2012). Polydatin ameliorates experimental diabetes-induced fibronectin through inhibiting the activation of NF-κB signaling pathway in rat glomerular mesangial cells. Mole Cell Endocrinol 362:183–93
- Xing WW, Wu JZ, Jia M, Du J, Zhang H, Qin LP. (2009). Effects of polydatin from Polygonum cuspidatum on lipid profile in hyperlipidemic rabbits. Biomed Pharmacother 63:457–62
- Xu B, Lin HB, Zhou H, et al. (2010). Protective effect of polydatin on a PC12 cell model of oxygen-glucose deprivation. J South Med Univ 30:1041–3
- Xu CY, Li S, Hao W, et al. (2011). Effect of polydatin on learning and memory and expression of NR2B in the prefrontal cortex of rats with chronic alcoholism. Chin J Appl Physiol 27:213–14, 220, 235
- Xu CY, Li S, Hao W, et al. (2012). Effect of polydatin on expression of cdk5 in the prefrontal cortex of rats with chronic alcoholism. Chin J Appl Physiol 28:158–9, 188
- Xue X, Jin CH, Li L, et al. (2008). Influence of polydatin on myocardial function and ultrastructure of LPS infected rats. Chin Practical Med 3:3–4
- Yao J, Wang JY, Liu L, et al. (2011). Polydatin ameliorates DSS-induced colitis in mice through inhibition of nuclear factor-kappaB activation. Planta Med 77:421–7
- Yee S. (1997). In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man-fact or myth. Pharm Res 14:763–6
- Yuan M, Li J, Lv J, et al. (2012). Polydatin (PD) inhibits IgE-mediated passive cutaneous anaphylaxis in mice by stabilizing mast cells through modulating Ca2+ mobilization. Toxicol Appl Pharmacol 264:462–9
- Zhang DQ, Sun LS, Xu JP. (2006). Effect of piceid on the dog with an acute myocardial infarction model. Herald Med 25:510–13
- Zhang H, Yu CH, Jiang YP, et al. (2012a). Protective effects of polydatin from Polygonum cuspidatum against carbon tetrachloride-induced liver injury in mice. Plos One 7:e46574
- Zhang J, Tan Y, Yao F, et al. (2012b). Polydatin alleviates non-alcoholic fatty liver disease in rats by inhibiting the expression of TNF-α and SREBP-1c. Mol Med Report 6:815–20
- Zhang L, Lv ZP. (2010). Effect of polydatin on nonalcoholic fatty liver disease and the TNF-α level in serum. LiShizhen Med Mater Med Res 21:1007–8
- Zhang LP, Ma HJ, Bu HM, et al. (2009). Polydatin attenuates ischemia/reperfusion-induced apoptosis in myocardium of the rat. Acta Physiol Sin 61:367–72
- Zhang LP, Wei Y, Song SL, et al. (2011a). Effect of polydatin on action potential in ventricular papillary muscle of rat and the underlying ionic mechanism. Acta Physiol Sin 63:48–54
- Zhang LP, Yang CY, Wang YP, et al. (2008a). Protective effect of polydatin against ischemia/reperfusion injury in rat heart. Acta Physiol Sin 60:161–8
- Zhang W, Li Q, Zhu M, et al. (2008b). Direct determination of polydatin and its metabolite in rat excrement samples by high-performance liquid chromatography. Chem Pharm Bull 56:1592–5
- Zhang Y, Chen LY, Liang B. (2011b). Effects of polydatin on bleomycin-induced pulmonary fibrosis in rats. China J Chin Materia Med 24:3528–34
- Zhao J, Li HY, Wang ZH, et al. (2010). Effect of polydatin on ultrastructure of cardiac myocytes in rats with adriamycininduced myocardial damage. Acta Acad Med CPAPF 19:629–30, 634
- Zhao KS, Jin C, Huang X, et al. (2003). The mechanism of polydatin in shock treatment. Clin Hemorheol Microcirc 29:211–17
- Zhao Q, Huang HX, Jin CH. (2004). The regulation and its mechanism of polydatin on the β-adrenoreceptor in cardiac myocytes stimulated by lipopolysaccharide. Chin Pharm Bull 20:769–72
- Zhou MJ, Chen XY, Zhong DF. (2007). Metabolism of trans-resveratrol-3-O-glucoside in vitro in rat tissues. Acta Pharm Sin 42:520–4
- Zhou S, Yang R, Teng Z, et al. (2009). Dose-dependent absorption and metabolism of trans-polydatin in rats. J Agric Food Chem 57:4572–9
- Zhu LX, Jin ZY. (2005). Effects of piceid on blood lipid metabolism, nitric oxide and nitricoxide synthase in experimental hyperlipidemia rats. Pharm Clin Chin Materia Med 21:16–18
- Zhu LX, Jin ZY. (2006). Effect of polydatin on metabolism of blood lipid of hyperlipidemia rats and its antioxidation. Chin Trad Patent Med 28:260–1