Abstract
Context. Ficus religiosa L. (Moraceae) is widely planted in the tropics. Its chemical constituents include tannin, saponin gluanol acetate, β-sitosterol, leucoanthocyanidin and leucoanthocyanin which are used for the treatment of pain, inflammation, impotence, menstrual disturbances, uterine tonic and urine related problems.
Objective: To determine the possible nephroprotective and curative effects of F. religiosa latex methanol extract against cisplatin induced acute renal failure.
Materials and methods: Methanol extract was obtained by maceration process. Rats were divided in five groups. Group 1 was administered acacia (2% w/v) of 5 ml/kg throughout the experiment; group 2 was treated with single dose of cisplatin (5 mg/kg i.p.) on the 1st day; group 3 (200 mg/kg p.o.) of extract control for the 1st to 10th day, group 4 (200 mg/kg p.o.) of extract from the 1st to 10th day and a single dose of cisplatin (5 mg/kg, i.p.) on 11th day while group 5 received the same dose of cisplatin on day 1 and extract (200 mg/kg p.o.) from the 7th to 16th day.
Results: Phytochemical screening of the extract revealed the presence of glycoside, alkaloids, tannins (phenolic compounds), flavonoids and amino acids. The half maximal inhibitory concentration (IC50) values of the extract were 31.75 ± 0.12 and 18.35 ± 0.48 µg/ml, respectively. The cisplatin-treated group 2 showed significant changes; renal functions, biochemical parameters and histopathology were significantly (**p < 0.01) recovered by 200 mg/kg curative and protective groups.
Discussion and conclusion: These findings demonstrated that F. religiosa latex and constituents have excellent nephroprotective and curative activities and thus have great potential as a source for natural health products.
Introduction
Ficus religiosa L. (Moraceae) is widely planted in the tropics (Bailey & Bailey, Citation1976). Its bark contains tannins, saponin gluanol acetate, β-sitosterol, leucopelargonidin-3-o-β-d-glucopyranoside, leucopelargonidin-3-o-α-l-rhamnopyranoside, lupeol, ceryl behenate, lupeol acetate, α-amyrin acetate, leucoanthocyanidin and leucoanthocyanin (Husain et al., Citation1992). F. religiosa has been extensively used in traditional medicine for a wide range of ailments of the central nervous system, endocrine system, gastrointestinal tract, reproductive system, respiratory system and infectious disorders (Singh et al., Citation2011). An aqueous extract of F. religiosa decreased fasting blood glucose and exaggerated the activity of superoxide dismutase (SOD) in streptozotocin-induced type II diabetic rats (Kirana et al., Citation2009). F. religiosa showed in vitro anthelmintic activity (Zafar et al., Citation2001). An aqueous extract showed high antimicrobial activity against selected pathogens such as Bacillus subtilis and Pseudomonas aeruginosa (Preethi et al., Citation2010).
With this background, the current study investigated whether oral administration of a methanol extract of F. religiosa latex has possible protective and curative effects against cisplatin-induced nephrotoxicity in albino rats.
Materials and methods
Drug and reagents
Gallic acid, quercetin, 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl (DPPH), 2,4,6-tripyridyl-S-triazine (TPTZ) and BHT were from Merck Pvt. Ltd., Mumbai, India; cisplatin was from VHB, Life Sciences Inc., Mumbai, India; urea and creatinine span diagnosis kits and 5,5′-dithiobis-(2-nitrobenzoic acid) were from Merck Pvt. Ltd.; thiobarbituric acid was from Loba Chemicals Pvt. Ltd., Mumbai, India; epinephrine, SOD, catalase (CAT) and adenosine triphosphate (ATP) solution were from Sigma Aldrich Chemicals Pvt. Ltd., Bangalore, India.
Plant material
F. religiosa latex was collected from Pipariya village, Vadodara district of Gujarat, Western India in September 2010. The plant was identified by Dr. Nagar (Professor of Botany), M.S. University Vadodara (Gujarat) and a voucher specimen (DPSV/F/01/2010) was deposited in the Department of Pharmacy, Sumandeep Vidyapeeth Vadodara.
Sample preparation
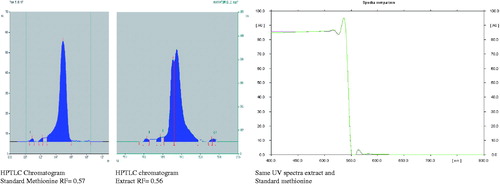
F. religiosa latex was extracted by a maceration process (48 h) in methyl alcohol after defatted with petroleum ether at 72 h at room temperature. The extract was dried with a rotatory evaporator under reduced pressure. The yield of latex was 18.56% (w/w). Phytochemical identification and standardization of F. religiosa latex was performed by thin layer chromatography (TLC) method and high performance TLC (HPTLC) (CAMAG, Basel, Switzerland, Linomet 5 and Scanner 3, Win Cat Software), mobile phase: butanol:formic acid:water (7.5 ml:1.5 ml:1.0 ml). HPTLC analysis was performed by the use of various standard amino acid markers like glutamine, glycine, cysteine, methionine, lysine, arginine tyrosine, leucine, etc. and extract in which many compounds were observed on the extract track. One spot retention factor value 0.56 of extract was similar to standard methionine marker. The methionine content of F. religiosa latex extract standardized was found to be 0.648 ± 0.0425% of standard methionine.
In vitro antioxidant activity
Determination of DPPH scavenging activity
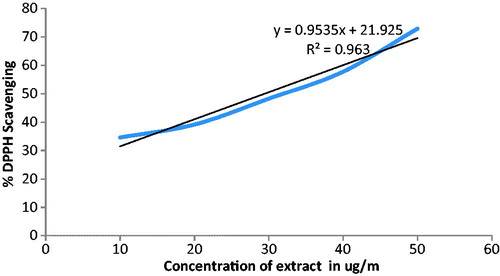
DPPH radical scavenging activity of the methanol extract of F. religiosa latex was determined as described by Blois (1958). An aliquot of 0.5 ml of sample solution in methanol was mixed with 2.5 ml of 0.5 mM methanol solution of DPPH. The mixture was shaken vigorously and incubated for 37 min in the dark at room temperature. The absorbance was measured at 517 nm using a UV-vis spectrophotometer. Ascorbic acid was used as a positive control. DPPH free radical scavenging ability (%) was calculated by using the formula:
Determination of phosphor-molybdenum scavenging activity
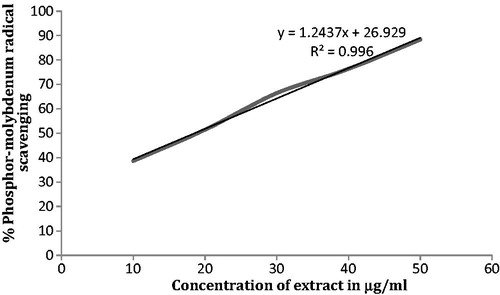
The antioxidant activity of the extract was determined by the phosphor-molybdenum method as described by Prieto et al. (Citation1999). The extract (0.3 ml) was mixed with 3 ml of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The reaction mixture was incubated at 95 °C, for 90 min and cooled to room temperature. Finally, absorbance was measured at 695 nm using a spectrophotometer (Merck Thermo Spectronic, Model NO. UV-1, double beam, Jawa Barat, Indonesia) against blank. Methanol (0.3 ml) in place of extract was used as the blank. Ascorbic acid was used as a positive control. Phosphor-molybdenum free radical scavenging ability (%) was calculated by using the formula:
Animals
Wistar adult male rats 12–13 week old (180–210 g) obtained from Sun Pharma Advanced Research Company (Gujarat, India) were allowed to access water and food ad libitum, and maintained under constant temperature (25 ± 1 °C), humidity (65 ± 10%) and a 12-h light/dark cycle. The experiment was carried out in accordance to the guidelines mentioned in the Committee for the Purpose of Control and Supervision of Experiments on Animals, and Institutional Animal Ethics Committee approved the experiment protocols (SVU/PH/IAEC/26.03.2010/02).
Acute toxicity study
Each group (n = 3) of Wistar rats fasted overnight prior to the experiment. Each group of rats was given increasing doses of 5 mg/kg body weight (bw), and one dose at 50 mg/kg bw, one dose at 100 mg/kg bw, one dose at 200 mg/kg bw, one dose at 400 mg/kg bw, one dose at 1000 mg/kg bw and one dose at 2000 mg/kg bw. The animals were observed continuously for 2 h and then every 2 h up to 24 and 72 h for gross behavior changes (Annie et al., Citation2005). It was lethal at a dose of 2000 mg. The extract was administered at one-tenth of the lethal dose for in vivo experiments which was 200 mg/kg bw for safe treatment (OCED 423).
Cisplatin-induced renal injury
Five groups of rats (n = 6) were used, in which group 1 was administered acacia (2% w/v) of 5 ml/kg throughout the experiment for 16 days. The group 2 (modal control) was administered normal saline (5 ml/kg) and treated with a single dose of cisplatin (5 mg/kg i.p.) on the first day. Group 3 (extract control) was administered 200 mg/kg p.o. of extract from the 1st to 10th day. Group 4 (protective) was administered extract (200 mg/kg p.o.) of F. religiosa latex from the 1st to the 10th day and administered a single dose of cisplatin (5 mg/kg, i.p.) on the 11th day, and group 5 (curative) received the same dose of cisplatin on day 1 and administered extract (200 mg/kg p.o.) from the 7th to the 16th day (Annie et al., Citation2005).
On the 6th day in model control, 10th day in extract control and 16th day in control, protective and curative, blood was withdrawn from the retro-orbital sinus of rats for biochemical estimation for serum urea and creatinine levels and the kidneys were dissected out for estimation of antioxidant enzymes and histopathological work.
Biochemical assays
The collected blood was centrifuged using a table top centrifuge (REMI) at 3000 rpm to get serum. Serum urea and creatinine were estimated using a diagnostic kit from Span Diagnostic, Kolkata, India, on a chemical analyzer (microlab 3000) for assessment of renal toxicity. Later, kidneys were removed, homogenized and centrifuged at 10 000 rpm at 0 °C for 20 min. The supernatant was used for the estimation of different antioxidant enzyme levels by calorimetric methods using a spectrophotometer (Merck thermo spectronic, Model NO. UV-1, double beam); glutathione reductase (GSH) was estimated by the method of Sedlak and Lindsay (Citation1968); lipid peroxidation by the thiobarbuturic acid-reactive substances method of Uchiyama and Mihara (Citation1978). SOD was estimated by the method Mishra and Fridovich (Citation1972), CAT by a colorimetric assay (Sinha, Citation1972) and the sediment of the centrifuge was used for estimation of the Na+K+ATPase by Bontin (Citation1970) methods, Ca2+ATPase by Hjerken and Pan (Citation1983) method and Mg2+ATPase by Ohinishi et al. (Citation1982) method.
Histopathological examination
The Wistar rats were sacrificed on the day of blood withdrawal. Kidneys were isolated, processed and embedded in paraffin wax. The sections were stained in hematoxylin and eosin and permanently mounted for viewing and reporting (Chandrasekaran et al., Citation1978).
Statistical analysis
Results were analyzed by one way analysis of variance followed by Dunnett’s test, and p < 0.05 was considered as significant.
Results and discussion
F. religiosa latex has revealed the presence of glycosides, alkaloids, methionine (), tannins (phenolic compound) and flavonoids. In vitro evaluation of F. religiosa for its antioxidant potential revealed DPPH and phosphor-molybdenum scavenging effects observed by significant decreases in the concentration of DPPH and phosphor-molybdenum radical due to the scavenging potential of the extract ( and ). IC50 values for DPPH and phosphor-molybdenum were 31.75 ± 0.12 and 18.35 ± 0.48 µg/ml, respectively.
Figure 3. Percent phosphor-molybdenum radical scavenging activity of the methanol extract of Ficus religiosa L. latex.
Orally administered doses ranging from 10–2000 mg/kg of extract did not produce any significant changes in the autonomic or behavior responses.
The cisplatin-treated group 2 (model control) showed a significant increase in urine volume, serum urea and creatinine levels (), lipid peroxidation () and decreases in bw (), GSH, SOD, CAT (), Na+/K+ ATPase, Ca++ ATPase and Mg++ATPase of kidney () on the sixth day as compared to the group 1 (control). They were significantly (p < 0.01) recovered in both protective and curative regimens with a treatment dose at 200 mg/kg of latex extract. However, the protective group was more effective than the curative group.
Figure 4. Effect of methanol extract of Ficus religiosa latex the on change in body weight of various groups. Each group represents mean ± SD of six animals as compared to the model control. **p < 0.01, *p < 0.05 as compared to the model control (2) with 1, 3, 4, 5 group.
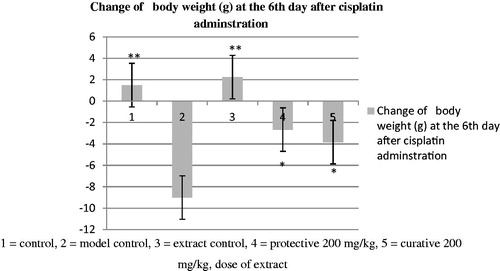
Table 1. Effect of methanol extract of Ficus religiosa latex on the excretion urine volume, urea and creatinine in blood serum on the sixth day after cisplatin administration.
Table 2. Effect of methanol extract of Ficus religiosa latex on lipid peroxidation and antioxidant enzymes of kidney on the sixth day after cisplatin administration.
Table 3. Effect of the methanol extract of Ficus religiosa latex on the Na+/K+ ATPase, Mg++ATPase and Ca++ ATPase in kidney tissue on the sixth day after cisplatin administration.
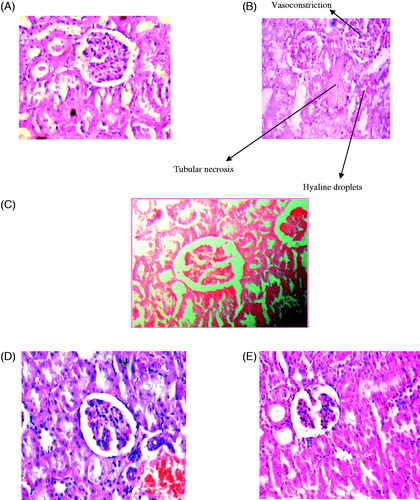
Histopathological sections of the kidneys showed marked vasoconstriction, hyaline droplets, proinflammatory and tubular necrosis observed cisplatin-treated group II, Plates 1(B) (), and in the protective and curative regimens, treated with extract (200 mg/kg bw, p.o.) which were reduced hyaline droplets, tubular dilation and recovery of tubular in Plate D and E, respectively, and extract control regimen, treated with extract (200 mg/kg bw, p.o.) was normal histology as a control group in plate A and C.
Figure 5. Histopathological evidence of cisplatin-induced proximal tubular toxicity. Representative histopathology stained sections from kidney of plate (A) vehicle-treated (magnification 20×), (B) cisplatin-treated rats (5 mg/kg, six days) (magnification 20×), (C) extract control Ficus religiosa treated with 200 mg/kg rats (magnification 20×), (D) protective Ficus religiosa treated with 200 mg/kg rats (magnification 20×), and (E) curative F. religiosa treated with 200 mg/kg rats (magnification 20×).
In the present study, cisplatin-induced renal impairment was evidenced by an increase in serum urea and creatinine and acute tubular necrosis. These changes persisted up to the sixth day following administration of a single dose of 5 mg/kg cisplatin. The methanol extract of F. religiosa latex normalized serum urea and creatinine levels and lipid peroxidation, GSH, SOD, CAT, Na+/K+ ATPase, Ca++ ATPase and Mg++ATPase of kidney.
According to previous findings, we confirmed that the single dose of cisplatin (5 mg/kg, i.p.) causes a significant increase in two serum markers of the kidney function, viz, serum urea and serum creatinine (Francescato et al., Citation2001). The present study revealed the significant decrease in the levels of urea and creatinine in blood serum after treatment with extract that have nephroprotective and curative effects. A relationship between free radical oxidative stress and nephrotoxicity has been well demonstrated in many experimental models (Devipriya & Shyamaladevim, Citation1999). Evidence points out that cisplatin induces nephrotoxicity partly via free radical oxidative stress.
In vitro studies on F. religiosa latex were used to evaluate antioxidant potential because it was revealed that DPPH and phosphor-molybdenum free radical scavenging effects with lower IC50 values due to rich sources of flavonoids, polyphenol and methionine. Deegan et al. (Citation1994) also reported nephrotoxicity, cytotoxicity and renal handling of a cisplatin–methionine complex in male Wistar rats. The present phytochemical screening data demonstrated that methionine antagonized cisplatin nephrotoxicity.
Actually, cisplatin-contributed various mechanisms of renal dysfunction, such as cellular toxicity, vasoconstriction in the renal microvasculature and proinflammatory effects, by producing free radicals oxidative stress which participated in the decline of antioxidant enzyme levels of the kidney and led to renal failure (Portilla et al., Citation2007). Hence, the possible mechanism of nephroprotection and the curative effect of F. religiosa latex could be due to its good antioxidant potential and amino acid content. These factors might contribute to free radical scavenging and antagonism nephrotoxicity which were produced by cisplatin in renal failure.
Conclusion
Finally, on the basis of these investigations, we may partially conclude that the methanol extract of F. religiosa latex with antioxidant properties could be a potent nephroprotective and curative agent for the next generation.
Declaration of interest
The present study was performed with financial support from Sumandeep Vidyapeeth University.
This investigation was supported with the technical assistance of Dr. D. N. Srivastava and Dr. A. K. Seth. Thanks to all for deep motivation and technical assistance.
References
- Annie S, Rajagopal PL, Malini S. (2005). Effect of Cassia auriculata L., root of extract on cisplatin and gentamicin-induced renal injury. Phytomedicine 12:555–60
- Bailey LH, Bailey EZ. (1976). Hortus. 3rd ed. New York: MacMillan General Reference
- Blois MS. (1958). Antioxidant determinations by the use of stable free radical. Nature 181:1199–200
- Bontin SL. (1970). Presence of enzyme system of mammalian tissue. Wiley Inter Sci 197:257–363
- Chandrasekaran SK, Benson H, Urquhart J. (1978). Methods to achieve controlled drug delivery: The biochemical engineering approach. In: Robinson JR, ed. Sustained and Controlled Drug Delivery Systems. New York: Marcel Dekker, 557–93
- Deegan PM, Pratt IS, Ryan MP. (1994). The nephrotoxicity, cytotoxicity and renal handling of a cisplatin methionine. Toxicology 89:1–14
- Devipriya S, Shyamaladevim CS. (1999). Protective effect of quercetin in cisplatin induced cell injury in the rat kidney. Indian J Pharmacol 31:422–6
- Francescato HDC, Costa RS, Camargo SMR, et al. (2001). Effect of dietary oral selenium on cisplatin induced nephrotoxicity in rats. Pharmacol Res 43:77–82
- Hjerken S, Pan H. (1983). Purification and characterization of two forms of the low affinity calcium ion ATPase from erythrocyte membrane. Biochem Biophys Acta 728:281–5
- Husain A, Virmani OP, Popli SP, et al. (1992). Dictionary of Indian Medicinal Plants. Lucknow, India: CIMAP, 546
- Kirana H, Agarwal SS, Srinivasan BP. (2009). Aqueous extract of Ficus religiosa Linn reduce oxidative stress in experimentally induced type 2 diabetic rats. IJEB 47:822–6
- Mishra HP, Fridovich I. (1972). The role superoxide anion in the auto-oxidation of epinephrine and a simple assay of SOD. J Bio Chem 247:3170–5
- Ohinishi T, Suzuki Y, Ozawa K. (1982). Comparative study of plasma membrane magnesium ion ATPase activities in normal regenerating and malignant cell. Biochem Biophys Acta 684:67–74
- Portilla D, Arany I, Kaushal GP, et al. (2007). Cellular mechanism of nephrotoxicity. Clin Nephrotoxicol 1:155–70
- Preethi R, Devanathan VV, Loganathan M. (2010). Antimicrobial and antioxidant efficacy of some medicinal plants against food borne pathogens. Adv Biol Res 4:122–5
- Prieto P, Pineda M, Aguilar M. (1999). Spectrophotometric quantization of antioxidant capacity through the formation of a phosphor-molybdenum complex: Specific application to the determination of vitamin E. Anal Biochem 269:337–41
- Sedlak M, Lindsay R. (1968). Estimation of total, protein-bound and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
- Singh D, Singh B, Goel RK. (2011). Traditional uses, phytochemistry and pharmacology of Ficus religiosa: A review. JEP 12:565–83
- Sinha AK. (1972). Colorimetric assay of catalase. Anal Biochem 47:389–94
- Uchiyama M, Mihara M. (1978). Determination of malonaldeyde precursor in tissues by thiobarbuturic acid test. Anal Biochem 86:271–8
- Zafar Iqbal, Qazi Khalid Nadeem, Khan MN, et al. (2001). In vitro anthelmintic activity of Allium sativum, Zingiber officinale, Curcurbita mexicana and Ficus religiosa. IJAB 3:454–7