Abstract
Context. Alpinia oxyphylla Miquel (Zingiberaceae) is a traditional Chinese herbal medicine widely used for the treatment of intestinal disorders, urosis and diuresis. However, information about antioxidant and cytotoxic properties of its fruits remains to be elucidated.
Objective: The ethanol crude extract (CE) and its fractions [petroleum ether fraction (PF), ethyl acetate fraction (EF), n-butanol fraction (BF) and water fraction (WF) extracted by petroleum ether, ethyl acetate, n-butanol and water, respectively] of A. oxyphylla fruits were investigated for their antioxidant activity and cytotoxicity.
Materials and methods: The total phenolic content (TPC) and antioxidant activity of the extracts were determined by Folin–Ciocalteu reagent, 1,1-diphenyl-2-picrylhydrazyl (DPPH•), Trolox equivalent antioxidant capacity and reducing power assay. Cytotoxicity of the extracts (0–200 μg/mL) was tested on six human cancer cell lines (breast cancer cell line, cervix carcinoma cell line, lung adenocarcinoma cell line, liver carcinoma cell line, gastric cancer cell line and colon cancer cell line) using the sulforhodamine B assay.
Results: The TPC of extracts varied from 8.2 to 20.3 mg gallic acid equivalents/g dry weight. DPPH radical scavenging effect of extracts decreased in the order of EF > BF > CE > PF > WF, with IC50 values ranging from 74.7 to 680.8 μg/mL. 2,2-azo-bis(3-Ethylbenzothiazoline-6-sulfoic acid) diammonium salt scavenging activity ranged from 0.118 to 0.236 mmol Trolox equivalence/mg extract. The extracts exhibited concentration-dependent reducing power, and EF showed the highest reducing ability. A satisfactory correlation (R2 > 0.826) between TPC and antioxidant activity was observed. In addition, EF, PF and CE exhibited potent anticancer effects on six cancer cell lines with IC50 values ranging from 40.1 to 166.3 μg/mL.
Discussion and conclusion: The ethanol extract of A. oxyphylla fruit, especially the EF, was found to possess potent antioxidant and anticancer activities, and thus a great potential for the application in food and drug products.
Introduction
Reactive oxygen species (ROS) are highly reactive free radicals, which can be generated in numerous physiological and biochemical processes in the human body during excessive metabolism (Finkel & Holbrook, Citation2000). It is commonly recognized that ROS cause extensive oxidative damage to cellular and extracellular macromolecules such as proteins, lipids and nucleic acids (Jones et al., Citation2002; Schafer & Buettner, Citation2001), leading to a variety of chronic health problems including cancer (Ames et al., Citation1995), heart disease (Diaz et al., Citation1997) and neuronal degeneration such as Alzheimer’s (Christen, Citation2000). It is generally assumed that frequent consumption of plant-derived antioxidants from different kinds of natural resource such as vegetables, fruit, tea and herbs may contribute to reduce oxidative damage and exert a beneficial effect on human health (Bedda et al., Citation2003; Halliwell et al., Citation1995). Recently, interest has increased considerably in finding naturally occurring antioxidants from natural sources such as foods and traditional herbal medicines for the prevention and treatment of complex diseases such as Alzheimer’s disease and cancer (Bjelakovic et al., Citation2004; Noguchi & Niki, Citation2000; Singal et al., Citation1998). In this regard, traditional Chinese medicinal plants are good sources of natural antioxidants such as terpenoids, anthocyanins, flavonoids and other phenolics compounds.
Alpinia oxyphylla Miquel (Zingiberaceae), a plant named Yi-Zhi in Chinese traditional medicine, is widely distributed in South China. The fruits of this plant have been used as a folk medicine for the treatment of intestinal disorders, urosis, diuresis, ulceration and dementia in traditional Chinese medicine (TCM) and were coded in Chinese Pharmacopoeia as an aromatic stomachic (Chinese Pharmacopoeia Commission, Citation2010). Previous phytochemical and biological investigations of this plant have reported the isolation of some sesquiterpenoids, and some of which showed inhibitory effects on nitric oxide production in lipopolysaccharide-activated mouse peritoneal macrophages and release of β-hexosaminidase from RBL-2H3 cells (Luo et al., Citation2012; Morikawa et al., Citation2002; Muraoka et al., Citation2001). Moreover, A. oxyphylla has also been reported to possess antianaphylactic (Kim et al., Citation2000; Shin et al., Citation2001), anti-inflammatory (Chun et al., Citation2001), antitumor (Lee et al., Citation1998), calcium-antagonist (Shoji et al., Citation1984), neuroprotective (Guan et al., Citation2006a,Citationb; Yu et al., Citation2003; Zhang et al., Citation2012a) and insecticidal (Miyazawa et al., Citation2000, Citation2001) activities.
As a part of our ongoing project toward the discovery of biologically active metabolites from TCMs (Lan et al., Citation2010, Citation2012; Zhang et al., Citation2009, Citation2012b,c), the aim of this work was to study the total phenolic content (TPC) and in vitro antioxidant activities of the ethanol extract and its fractions of A. oxyphylla fruits using the Folin–Ciocalteu method and different antioxidant models. In addition, the anticancer effects of these extracts on six human cancer cell lines were also investigated by sulforhodamine B (SRB) assay. Correlation between antioxidant power and anticancer activity of the extracts was also discussed in this work. Results from this study provide a better understanding of the nutritional and health benefits of the fruits of A. oxyphylla.
Materials and methods
Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH•), 2,2-azo-bis(3-ethylbenzothiazoline-6-sulfoic acid) diammonium salt (ABTS), Trolox, gallic acid, Folin–Ciocalteu reagent, ascorbic acid and SRB were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were of analytical grade and obtained from Sinopharm Chemical Reagent Co. (Shanghai, China).
Plant material
The air-dried fruits of A. oxyphylla collected from Guangxi province were purchased from Lei Yun Shang Medicine Corporation (Shanghai, China). A voucher specimen (NO. 11010) was authenticated by Prof. Yongchuan Zhou from East China University of Science and Technology and deposited at the herbarium of Research Center of Analysis and Test, East China University of Science and Technology, China. The dried fruits of A. oxyphylla were finely powdered with an electric mill and were kept at −20 °C in the dark until use.
Preparation of extracts
Powdered sample (100 g) of A. oxyphylla fruits was extracted by refluxing with 1000 mL of 95% ethanol for 4 h. The extract was then filtered and collected. The residual was extracted for another two times using the same procedure. The collected ethanol extracts were then combined, concentrated under reduced pressure and lyophilized until dry to afford the crude extract (CE). The CE was resuspended in distilled water and then sequentially extracted with petroleum ether, ethyl acetate and water-saturated n-butanol, using liquid–liquid partition, to obtain four fractions termed petroleum ether fraction (PF), ethyl acetate fraction (EF), n-butanol fraction (BF) and water fraction (WF), respectively. The CE and its four fractions were stored in dark bottles at 4 °C after solvent evaporation and freeze-drying.
Determination of TPC
The TPC of the ethanol CE and its fractions was measured by the Folin–Ciocalteu method described with some modifications (Nurmi et al., Citation1996). Briefly, an aliquot of 0.5 mL of sample solution (with appropriate dilution to obtain absorbance in the range of the prepared calibration curve) was mixed with 1.0 mL Folin–Ciocalteu reagent (10 times dilution before use) and allowed to react at 30 °C for 5 min in the dark. Then 2.0 mL of saturated Na2CO3 solution was added, and the mixture was allowed to stand for 1 h before the absorbance of the reaction mixture was read at 747 nm. A calibration curve, using gallic acid with a concentration range of 0.01–0.10 mg/mL, was prepared. The TPC of the samples was standardized against gallic acid and expressed as mg gallic acid equivalent (GAE) per gram of sample on a dry weight basis.
DPPH• assay
The ability of the prepared extracts to scavenge DPPH• radicals was determined by the method described by Brandwilliams et al. (Citation1995) with slight modifications. Briefly, one milliliter methanol solution of each extract in different concentrations was added to 9 mL methanol solution of DPPH• (0.1 mM). The solution was mixed well and then left at room temperature for 15 min in the dark. The absorbance of the resulting solution was read at 517 nm. Ascorbic acid was employed as a reference, and the radical scavenging activity was calculated as a percentage of DPPH• discoloration using the equation:
where Asample is the absorbance of the solution when the extract/reference has been added at a particular level, and Acontrol is the absorbance of the DPPH• solution without extract added. All analyzes were run in triplicate. IC50 values calculated denote the concentration of a sample required to decrease the absorbance at 517 nm by 50%.
Trolox equivalent antioxidant capacity assay
Determination of the ABTS•+ radical scavenging effect of the extracts was performed according to the method described by Re et al. (1999) with some modifications. The radical cation ABTS•+ was generated by persulfate oxidation of ABTS. A mixture of ABTS (7.0 mM) and potassium persulfate (2.45 mM) was allowed to stand overnight at room temperature in the dark to produce a dark green solution containing ABTS•+ radical cations. The initial absorbance at 734 nm was read. A working solution was diluted with ethanol to an absorbance of about 0.700 ± 0.02 at 734 nm and equilibrated at 30 °C. Different concentrations of the methanol solution of each extract (0.05–0.8 mg/mL) were prepared, and 0.5 mL of the extract was mixed with 4.5 mL of ABTS•+ working solution, and the decrease of absorbance was measured at 734 nm after 10 min at 37 °C in the dark. A Trolox calibration curve was constructed by measuring the reduction in absorbance of the ABTS•+ solution in the presence of different concentrations of Trolox (0–200 μM). The ABTS•+ radical scavenging activity of the extracts was measured by comparing the ratios of the gradients of the concentration plot of the extracts with that of Trolox over a linear concentration range. Results were expressed as mmol Trolox equivalent antioxidant capacity (TEAC) per mg dried extract. All determinations were performed in triplicate. Ascorbic acid was used as a reference compound.
Reducing power test
The reducing power of the extracts was determined according to the method of Oyaizu (Citation1986). Different concentrations (0.05–0.8 mg/mL) of each extract in methanol (2.5 mL) was mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of potassium ferricyanide [K3Fe(CN)6] (1%, w/v). After incubation at 50 °C for 20 min, 2.5 mL of trichloroacetic acid (TCA; 10%, w/v) was added to the mixture before centrifugation at 3000 rpm for 10 min. Then 2.5 mL of the supernatant was mixed with 2.5 mL ddH2O and 0.5 mL of aqueous FeCl3 (0.1%, w/v). The absorbance was measured 10 min later at 700 nm against a blank. Increased absorbance of the reaction mixture indicated increased reducing power. Ascorbic acid was used as a reference compounds. All tests and analyses were run in triplicate.
Cell lines and culture
Human breast cancer cell line (MCF-7), human cervix carcinoma cell line (HeLa), human liver carcinoma cell line (HepG2), human gastric cancer cell line (MNK-45), human lung adenocarcinoma cell line (A549) and human colon cancer cell line (SW480) were obtained from the Committee of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). These cell lines were grown and maintained in a high glucose concentration (4.5 g/L) Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, and 1% penicillin–streptomycin (100 IU–100 μg/mL) in a humidified incubator at 37 °C and in 5% CO2 atmosphere.
Cytotoxicity assay
The effects of the extracts on inhibition of cancer cell growth were measured by the SRB assay, which was performed as the procedure described previously (Monks et al., Citation1991). Briefly, after being harvested from culture flasks, the cells (1 × 104) were seeded in each well of a 96-well plate containing 100 μL fresh growth medium per well and permitted to adhere for 24 h. Then they were treated with 200 μL of different concentrations (0–200 μg/mL) of the extracts in each well, respectively. After 48 h of treatment, the cells were fixed with 10% TCA and stained with 100 μL of SRB solution in 1% acetic acid for 15 min. Unbound dye was removed by washing with 1% acetic acid. The bound dye was extracted with 10 mM Tris-HCl for determination of optical density at 492 nm in a 96-well microtiter plate reader. Cytotoxicity was expressed as the concentration of the extracts inhibiting cell growth by 50% (IC50). All tests and analyses were run in triplicate.
Statistical analysis
The statistical analyzes were performed using the Statistical Package for Social Science (SPSS Inc., Chicago, IL). All data were expressed as means ± SD for at least three replications. The 50% inhibitory concentration (IC50) was calculated according to concentration–effect regression line. Correlation analysis was carried out to determine the relationship between the antioxidant activity and the TPC.
Results and discussion
Total phenolic content
Phenolic compounds in plants are powerful free radical-scavengers that can inhibit lipid peroxidation by neutralizing peroxyl radicals generated during the oxidation of lipids (Shahidi et al., Citation1992). The TPC of the ethanol CE and its four fractions from A. oxyphylla fruit was assayed by the Folin–Ciocalteu method using gallic acid as standard (y = 0.0061x + 0.0905, R2 = 0.9982). The data presented in indicated that the TPC of different extracts was in the order of EF > BF > CE > PF > WF. The highest TPC of 20.3 mg GAE/g was obtained in EF, whereas the lowest TPC of 8.2 mg GAE/g was achieved in WF. It is worthily mentioned that the TPC of CE was lower than that of EF and BF, but higher than that of PF and WF, which may result from enrichment of the phenolic components during the solvent–solvent partitioning processes.
Table 1. The total phenolic content and radical scavenging activities of the ethanol crude extract and its four fractions from Alpinia oxyphylla fruits using DPPH• and TEAC assay.
DPPH radical scavenging activity
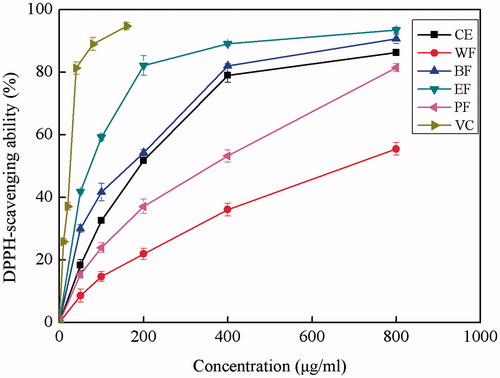
DPPH radical scavenging assay is one of the most common methods to evaluate the radical scavenging activity of antioxidants because of its quickness, reliability and reproducibility. This method depends on the reduction of the purple DPPH• by accepting an electron or hydrogen radical to become a stable diamagnetic molecule with discoloration. The degree of discoloration indicates the free radical scavenging potentials of the antioxidant compounds or extracts in terms of hydrogen-donating ability (Alasalvar et al., Citation2009; Shahidi et al., Citation2007).
DPPH free radical scavenging activities of the ethanol CE and its four fractions are shown in . For each sample, five concentrations (50–800 μg/mL) were tested. All tested extracts showed a promising DPPH• scavenging effect in a concentration-dependent manner. EF exhibited considerably higher DPPH radical scavenging activity than other fractions, and the lowest DPPH• scavenging rate was found in WF. The free radical scavenging activities of extracts decreased in the order of EF > BF > CE > PF > WF. This trend was similar to that observed in the TPC. The IC50 values were also calculated to further evaluate the antioxidant activity, as shown in . The lower the IC50 value is, the greater the free radical scavenging activity is. The highest DPPH radical scavenging effect was obtained in EF with the lowest IC50 of 74.7 μg/mL, followed by BF (173.7 μg/mL), CE (188.9 μg/mL), PF (354.9 μg/mL) and WF (680.8 μg/mL). However, when compared with the extracts, ascorbic acid showed higher radical scavenging ability with IC50 of 51.7 μg/mL.
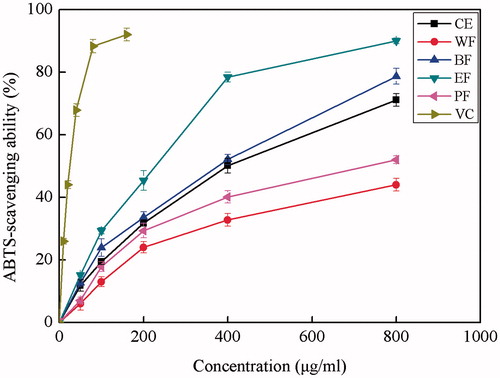
ABTS•+ scavenging activity
The ABTS•+ radical formed from the reaction ABTS-e− → ABTS•+ reacts quickly with electron/hydrogen donors to form colorless ABTS. A decrease of the ABTS•+ concentration is linearly dependent on the antioxidant concentration. The antioxidant abilities of the CE and its fractions determined by TEAC method are shown in . All the samples were found to have the ability to scavenge ABTS•+ at concentrations of 50 μg/mL. A steady increase in the percentage inhibition of the ABTS•+ with concentrations of the extracts was observed, while ascorbic acid showed a sharp increase of scavenging ability with the concentration. The TEAC values were calculated and tabulated in to facilitate the comparison of the scavenging activity of ABTS•+ of different extracts. TEAC values of the ABTS•+ scavenging activity ranged from 0.118 to 0.236 mmol Trolox equivalence per mg extracts with the descending order of EF > BF > CE > PF > WF. The rank order was the same as that of the DPPH assay. The highest ABTS•+ scavenging activity was found for EF with the TEAC value of 0.236 mmol TE/mg extract, suggesting that active radical scavenging compounds were selectively concentrated in this fraction.
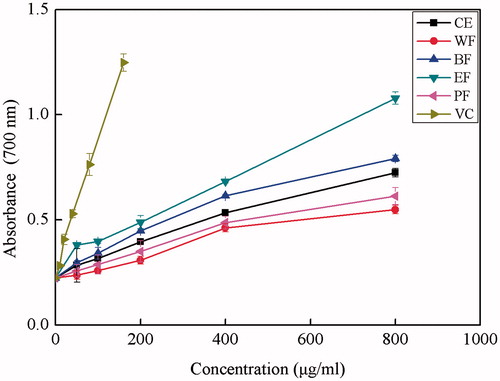
Reducing power
In reducing power assay, potential antioxidants reduce the Fe3+/ferricyanide complex to its ferrous form which can then be monitored spectrophotometrically at 700 nm. Increased absorbance of the reaction mixture indicates increased reducing power. The antioxidant activities of natural components may have a reciprocal correlation with their reducing powers. shows the dose-response curves for the reducing power of the CE and its four fractions. The reducing power values were positively correlated with the concentrations of the extracts and ascorbic acid in the range of 0–800 μg/mL. The corresponding correlation coefficients were 0.9871, 0.9602, 0.9832, 0.9706, 0.9478 and 0.9952 for EF, BF, CE, PF, WF and ascorbic acid, respectively. The highest reducing power among the extracts was found in EF, followed by BF, CE, PF and WF. At a concentration of 0.8 mg/mL, the reducing power of EF was 1.079, whereas at the same concentration, the value of BF, CE, PF and WF were only 0.792, 0.724, 0.613 and 0.549, respectively. These observations were similar to those for DPPH• and ABTS•+ radical scavenging activities. Although all the tested extracts showed much lower reducing power than ascorbic acid, it was evident that the extracts, especially EF, have ability to reduce Fe (III) to Fe (II), which might be due to the presence of phenolic compounds in these extracts.
Relationship between antioxidant activity and the TPC
Polyphenols have been reported to be responsible for the antioxidant activities of plant extracts. As shown in , the correlations of the TPC against the antioxidant activity based on the DPPH• assay, TEAC assay and reducing power test were satisfactory (R2 > 0.826). A strong linear correlation appeared between the TPC and TEAC value with an excellent correlation coefficient (R2 = 0.936). The results indicated that polyphenols in extracts may largely contribute to their antioxidant properties. These findings were in good accordance with previous studies, which showed that extracts with higher TPC possessed stronger antioxidant activity (Abdel-Hameed, Citation2009; Cai et al., Citation2004). As a result, the various phenolic compounds isolated from the EF of A. oxyphylla fruits such as protocatechuic acid, yakuchinone A, yakuchinone B and tectochrysin (Di et al., Citation2011) may play a major role in its highest antioxidant activity among extracts. Furthermore, excellent correlation coefficient (R2 > 0.817) between two kinds of IC50 and TEAC values was also obtained, indicating that the antioxidant methods used in this study were strongly correlated. Therefore, antioxidants present in different extracts cannot only be capable of effectively scavenging DPPH• and ABTS•+ but also have potent ability to reduce ferric ions.
Table 2. Correlation coefficients (R2) for relationship between assays.
Cytotoxic activity
The growth inhibitory effects of CE and its four fractions (PF, EF, BF and WF) on six human cancer cell lines (A549, HepG2, SW480, HeLa, MCF-7 and MNK-45 cells) were tested with the SRB assay. Cells were treated with various concentrations (0–200 μg/mL) of the extracts for 48 h. BF and WF did not exhibit significant effect on cells viability at the indicated concentrations. However, CE, PF and EF with the same doses inhibited cell proliferation in a significant concentration-dependent manner. The IC50 values of the extracts and 5-fluorouracil (5-FU; a positive control anticancer drug) on six cancer cell lines are shown in . Among those cancer cells tested, HepG2 was the most sensitive cancer cell to the extracts with IC50 values of 79.1, 40.1 and 40.9 μg/mL for CE, PF and EF, respectively. The most resistant cancer cell to the extracts-induced growth inhibition was found to be SW480 with IC50 values of 166.3, 86.6 and 95.4 μg/mL for CE, PF and EF, respectively. As shown in , the IC50 values of EF and PF are much lower than those of CE, indicating that the active anticancer compounds were mainly concentrated in the fractions of ethyl acetate and petroleum ether. Furthermore, EF was found to have the strongest growth inhibition effect on A549 and HeLa cells with the IC50 values of 69.4 and 61.4 μg/mL, respectively, while PF had the most potent ability to inhibit MNK-45 and SW480 cells with the IC50 values of 57.1 and 86.6 μg/mL, respectively. The growth inhibition ability of EF and PF on HepG2 and MCF-7 cells were nearly the same with similar IC50 values. Interestingly, the antiproliferation effect of EF and PF on HepG2 cells was comparable to that of 5-FU (IC50: 43.8 μg/mL), whereas other five cancer cells was found to be more sensitive to 5-FU than the extracts of A. oxyphylla fruits, as shown in .
Table 3. Cytotoxicity (IC50) of the ethanol crude extract and its four fractions from Alpinia oxyphylla fruits and 5-fluorouracil (5-FU) on six human cancer cell lines.
Correlation between antioxidant power and anticancer activity of the extracts
It is worthy noted that the rank order of extracts against tested cancer cells can be summarized as EF and PF > CE ≫ BF and WF, which is not in agreement with that of antioxidant activities and TPC. The fraction of EF not only exhibited excellent antioxidant activity but also possessed a potent effect on inhibiting cancer cell growth, suggesting that the active phenolic components serving as the natural antioxidants in EF including yakuchinone A and yakuchinone B, which have been reported to possess antiproliferative activity on mouse skin tumor and HL-60 cells (Surh, Citation1999), might be also responsible for the chemoprotective effects against cancer cells. PF showed significant anticancer capacity but relatively weak antioxidant activity, indicating that the main active compounds serving as anticancer agents in PF may not belong to polyphenols and deserve further study. In our previous study, the essential oil of A. oxyphylla fruits was found to have strong anticancer activities against human cancer cells, which might be attributed to the presence of abundant sesquiterpene hydrocarbons and oxygenated sesquiterpenes such as α-humulene, β-caryophyllene, caryophyllene oxide and nootkatone in it (unpublished data). Therefore, the potent anticancer activity of PF might be associated to such nonphenolic compounds. The interesting results were also found in this study concerning the fraction of BF, which showed potent antioxidant activity but weak anticancer ability in the tested concentration range. CE exhibited moderate antioxidant and anticancer capacities among extracts, while weak corresponding activities were observed for WF. The results suggested that the compounds serving as antioxidant and/or anticancer agents could be effectively concentrated in some subfractions. Therefore, the differences of correlation between antioxidant activity and anticancer effect of extracts could be attributed to the diversity of chemical constituents and mechanisms of action among extracts.
Conclusions
The results of this study provided evidence that the ethanol extracts and its four fractions (PF, EF, BF and WF) had strong antioxidant capacities in different models used, and the antioxidant capacity in order: EF > BF > CE > PF > WF, which was the same as the rank of the TPC. Satisfactory correlations between the antioxidant activities and the TPC further suggested that the antioxidant properties were attributed to phenolic compounds in extracts. Furthermore, the fractions of EF and PF also showed potent cytotoxic activity on six human cancer cells. Therefore, A. oxyphylla fruit ethanol extract, especially its EF, deserves further investigation in active compounds responsible for the antioxidant and anticancer properties as it might be used in the field of pharmaceutical products and functional foods for the prevention and treatment of cancers.
Declaration of interest
The authors report no conflicts of interest. The authors are alone responsible for the content and writing of this article.
Acknowledgements
This work was supported by a grant from the Science and Technology Commission of Shanghai Municipality (STCSM; No. 08DZ1974000 and No. 10DZ2220500).
References
- Abdel-Hameed ESS. (2009). Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem 114:1271–7
- Alasalvar C, Karamac M, Kosinska A, et al. (2009). Antioxidant activity of hazelnut skin phenolics. J Agric Food Chem 57:4645–50
- Ames BN, Gold LS, Willett WC. (1995). The cause and prevention of cancer. Proc Natl Acad Sci USA 92:5258–65
- Bedda S, Laurent A, Conti F, et al. (2003). Mangafodipir prevents liver injury induced by acetaminophen in the mouse. J Hepatol 39:765–72
- Bjelakovic G, Nikolova D, Simonetti RG, et al. (2004). Antioxidant supplements for prevention of gastrointestinal cancers: A systematic review and meta-analysis. Lancet 364:1219–28
- Brandwilliams W, Cuvelier ME, Berset C. (1995). Use of a free-radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28:25–30
- Cai YZ, Luo Q, Sun M, et al. (2004). Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74:2157–84
- Chinese Pharmacopoeia Commission. (2010). Pharmacopoeia of the People’s Republic of China. Vol. 1. Beijing, China: China Medical Science and Technology Press, 274
- Christen Y. (2000). Oxidative stress and Alzheimer disease. Am J Clin Nutr 71:621S–9S
- Chun KS, Park KK, Lee J, et al. (2001). Inhibition of mouse skin tumor promotion by anti-inflammatory diarylheptanoids derived from Alpinia oxyphylla Miquel (Zingiberaceae). Oncol Res 13:37–45
- Di L,Wang ZY, Wang Z, et al. (2011). Chemical constituents in Alpinia oxyphylla seed. J Plant Resour Environ 20:94–6
- Diaz MN, Frei B, Vita JA, et al. (1997). Antioxidants and atherosclerotic heart disease. N Engl J Med 337:408–16
- Finkel T, Holbrook NJ. (2000). Oxidants, oxidative stress and the biology of ageing. Nuture 408:239–47
- Guan S, Bao YM, Jiang B, et al. (2006a). Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur J Pharmacol 538:73–9
- Guan S, Jiang B, Bao YM, et al. (2006b). Protocatechuic acid suppresses MPP+-induced mitochondrial dysfunction and apoptotic cell death in PC12 cells. Food Chem Toxicol 44:1659–66
- Halliwell B, Murcia MA, Chirico S, et al. (1995). Free radicals and antioxidants in food and in vivo: What they do and how they work? Crit Rev Food Sci Nutr 35:7–20
- Jones DP, Mody VC, Carlson JL, et al. (2002). Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med 33:1290–300
- Kim SH, Choi YK, Jeong HJ, et al. (2000). Suppression of immunoglobulin E-mediated anaphylactic reaction by Alpinia oxyphylla in rats. Immunopharmacol Immunotoxicol 22:267–77
- Lan MB, Guo J, Zhao HL, et al. (2012). Optimization of the extraction of the Magnolia officinalis polysaccharides using response surface methodology. Asian J Chem 24:2290–4
- Lan MB, Zhang YH, Zheng Y, et al. (2010). Antioxidant and immunomodulatory activities of polysaccharides from moxa (Artemisia argyi) leaf. Food Sci Biotechnol 19:1463–9
- Lee E, Park KK, Lee JM, et al. (1998). Suppression of mouse skin tumor promotion and induction of apoptosis in HL-60 cells by Alpinia oxyphylla Miquel (Zingiberaceae). Carcinogenesis 19:1377–81
- Luo JG, Lv XQ, Wang XB, et al. (2012). Sesquiterpenoids from the fruits of Alpinia oxyphylla and inhibition of nitric oxide production in lipopolysaccaride-activated macrophages. Phytochem Lett 5:134–8
- Miyazawa M, Nakamura Y, Ishikawa Y. (2000). Insecticidal sesquiterpene from Alpinia oxyphylla against Drosophila melanogaster. J Agric Food Chem 48:3639–41
- Miyazawa M, Nakamura Y, Ishikawa Y. (2001). Insecticidal diarylheptanoid from Alpinia oxyphylla against larvae of Drosophila melanogaster. Nat Prod Lett 15:75–9
- Monks A, Scudiero D, Skehan P, et al. (1991). Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83:757–66
- Morikawa T, Matsuda H, Toguchida I, et al. (2002). Absolute stereostructures of three new sesquiterpenes from the fruit of Alpinia oxyphylla with inhibitory effects on nitric oxide production and degranulation in RBL-2H3 cells. J Nat Prod 65:1468–74
- Muraoka O, Fujimoto M, Tanabe G, et al. (2001). Absolute stereostructures of novel norcadinane- and trinoreudesmane-type sesquiterpenes with nitric oxide production inhibitory activity from Alpinia oxyphylla. Bioorg Med Chem Lett 11:2217–20
- Noguchi N, Niki E. (2000). Phenolic antioxidants: A rationale for design and evaluation of novel antioxidant drug for atherosclerosis. Free Radic Biol Med 28:1538–46
- Nurmi K, Ossipov V, Haukioja E, et al. (1996). Variation of total phenolic content and individual low-molecular-weight phenolics in foliage of mountain birch trees (Betula pubescens ssp. tortuosa). J Chem Ecol 22:2023–40
- Oyaizu M. (1986). Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 44:307–15
- Re R, Pellegrini N, Proteggente A, et al. (1999). Antioxidant activity applying an improved ABTS radical cation decoloration assay. Free Radic Biol Med 26:1231–7
- Schafer FQ, Buettner GR. (2001). Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30:1191–212
- Shahidi F, Alasalvar C, Liyana-Pathirana CM. (2007). Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L.) and hazelnut byproducts. J Agric Food Chem 55:1212–20
- Shahidi F, Janitha PK, Wanasundra PD. (1992). Phenolic antioxidants. Crit Rev Food Sci Nutr 32:67–103
- Shin TY, Won JH, Kim HM, et al. (2001). Effect of Alpinia oxyphylla fruit extract on compound 48/80-induced anaphylactic reactions. Am J Chin Med 29:293–302
- Shoji N, Umeyama A, Asakawa Y, et al. (1984). Structural determination of nootkatol, a new sesquiterpene isolated from Alpinia oxyphylla Miquel possessing calcium-antagonist activity. J Pharm Sci 73:843–4
- Singal PK, Khaper N, Palace V, et al. (1998). The role of oxidative stress in the genesis of heart disease. Cardiovasc Res 40:426–32
- Surh YJ. (1999). Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res-Fundam Mol Mech Mutagen 428:305–27
- Yu XY, Ceballos YQW, Zhao H, et al. (2003). Neuroprotective effect of Alpinia oxyphylla extract against glutamate-induced apoptosis in cultured mouse cortical neurons. Neurosci Res Commun 33:105–33
- Zhang ZJ, Cheang LCV, Wang MW, et al. (2012a). Ethanolic extract of Fructus Alpinia oxyphylla protects against 6-hydroxydopamine-induced damage of PC12 cells in vitro and dopaminergic neurons in zebrafish. Cell Mol Neurobiol 32:27–40
- Zhang CY, Lan MB, Dai YJ. (2009). The hemagglutination activities of the extractions from 20 kinds of antimicrobial Chinese herbs. Lishizhen Med Mater Med Res 20:2972–3
- Zhang CY, Wu WH, Wang J, et al. (2012b). Antioxidant properties of polysaccharide from the brown seaweed Sargassum graminifolium (Turn.), and its effects on calcium oxalate crystallization. Mar Drugs 10:119–30
- Zhang YH, Xue MQ, Bai YC, et al. (2012c). 3,5-Dicaffeoylquinic acid isolated from Artemisia argyi and its ester derivatives exert anti-Leucyl-tRNA synthetase of Giardia lamblia (GlLeuRS) and potential anti-giardial effects. Fitoterpia 83:1281–5