Abstract
Context: Exposure to high levels of nitrites for a prolonged time have adverse health effects on several organs especially the liver due to oxidative properties. Meanwhile, cod liver oil has been reported to ameliorate organ dysfunction in animal models that involve oxidative stress.
Objective: Examine the impact of dietary cod liver oil on sodium nitrite-induced liver damage.
Materials and methods: Thirty-two adult male Sprague-Dawely rats were daily treated with sodium nitrite (80 mg/kg) in presence or absence of cod liver oil (5 ml/kg). Morphological changes were assessed in liver sections. Oxidative stress and antioxidant markers were measured in serum and liver homogenates. Liver samples were used for measurements of MCP-1, DNA fragmentation and mitochondrial function.
Results: The hepatoprotective effect of cod liver oil was proved by significant reduction of elevated liver enzymes and normal appearance of hepatocytes. Cod liver oil significantly reduced hepatic malondialdehyde, hydrogen peroxide and superoxide anion (224.3 ± 18.9 nmol/g, 59.3 ± 5.1 and 62.5 ± 5.1 µmol/g, respectively) compared with sodium nitrite (332.5 ± 25.5 nmol/g, 83.1 ± 8.1 and 93.9 ± 6.5 µmol/g, respectively). Cod liver oil restored hepatic cytochrome c oxidase activity after 38% reduction by sodium nitrite. Furthermore, cod liver oil significantly reduced hepatic MCP-1 (79.8 pg/mg) and DNA fragmentation (13.8%) compared with sodium nitrite (168.7 pg/mg and 41.3%, respectively).
Discussion and conclusion: Cod liver oil ameliorates sodium nitrite induced hepatic impairment through several mechanisms including attenuation of oxidative stress, blocking MCP-1, reactivation of mitochondrial function and reduction of DNA fragmentation.
Introduction
Food additives are considered major problems in food safety. All food additives, whether actually in use or being proposed for use, should be subjected to appropriate toxicological testing and evaluation. One important food additive is sodium nitrite. It is an inorganic salt with E number E250, code for chemicals that can be used as food additives within the European Union. Sodium nitrite is well known for its role in inhibiting the growth of Clostridium botulinum spores in refrigerated meats by the inhibition of iron-sulfur clusters essential to energy metabolism (Milkowski et al., Citation2010). In addition, sodium nitrite is responsible for the desirable red color of meat by formation of nitrosylating agents that subsequently react with myoglobin to produce the red color (Sindelar et al., Citation2012). It has been reported that as little as 2 to 14 parts per million is needed to induce the desirable red color change. However, in order to extend the lifespan of this color change, significantly higher levels are needed (Sullivan et al., Citation2012). Moreover, sodium nitrite is able to effectively delay the development of oxidative rancidity by reacting with heme proteins and metal ions and chelating free radicals terminating the cycle of lipid oxidation that leads to rancidity (Sullivan et al., Citation2012). In the European Union, it may be used only as a mixture with salt containing at most 0.6% sodium nitrite.
While sodium nitrite will prevent the growth of bacteria, it can be toxic in high amounts for animals, including humans. Sodium nitrite’s LD50 in rats is 180 mg/kg and its human LD50 is 71 mg/kg, meaning a 65 kg person would likely have to consume at least 4.615 g to result in death (Shuval et al., Citation1972). Exposure to nitrites at levels above health-based risk has been reported to possess adverse health effects especially on infants and children. The cytotoxicity and detrimental effect of nitrite could be attributed to its oxidative properties. In addition, sodium nitrite is generally accepted as a weak carcinogen. Exposure to higher levels of nitrites has been associated with increased incidence of cancer in adults, and possibly increased incidence of brain tumors, leukemia, and nasopharyngeal tumors in children (Klurfeld, Citation2001; Kozisek, Citation2007; Pogoda et al., Citation2001; Ward et al., Citation2000). A principal concern about sodium nitrite is the formation of carcinogenic nitrosamines in charred or overcooked meat. Such carcinogenic nitrosamines can be formed from the reaction of nitrite with secondary amines under acidic conditions, such as what occurs in the human stomach, as well as during the curing process used to preserve meats (Paik et al., Citation2001).
Recent trends in controlling and treating diseases tend to favor natural compounds, as the human diet is essential in protecting the body against the development of diseases. One important natural product is cod liver oil, a nutritional supplement derived from the liver of cod fish. Cod fish is a common name for the genus Gadus of demersal fishes, belonging to the family Gadidae. As with most fish oils, cod liver oil has high levels of the omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA), as well as vitamin A, not less than 850 USP units/g, and vitamin D, not less than 85 USP units/g (Trofimiuk et al., Citation2011). In previous studies, cod liver oil supplementation has been suggested to reduce cardio-metabolic risk factors (Abeywardena et al., Citation2011), have anticancer effects (Dyck et al., Citation2011), ameliorate cognitive impairment induced by chronic stress (Trofimiuk et al., Citation2011), and treat rickets and osteomalacia as a dietary supplement (Khare et al., Citation2008; Wjst, Citation2009).
Recent understanding of the molecular events of increased levels of sodium nitirte has focused on oxidative stress in different body organs (Hassan et al., Citation2010). Less attention has been given to nutritional treatment using cod liver oil. In addition, we have previously shown that DHA can obliterate lethal cisplatin-induced nephrotoxicity and renal tissue injury by reducing oxidative stress in rats and mice (El-Mesery et al., Citation2009). Moreover, we found that daily treatment of non-alcoholic fatty liver patients with fish oil, which contains both DHA and EPA, for 6 months, improved lipid profiles and ameliorated oxidative stress (Al-Gayyar et al., Citation2012). In addition, we depend on the fact that risks of adverse events caused by the use of polyunsaturated fatty acids seem negligible (De Ley et al., Citation2007). This study examines the impact of dietary cod liver oil on sodium nitrite-induced liver damage in rats.
Materials and methods
Chemicals
All chemicals used in the current study were purchased from Sigma-Aldrich (St. Louis, MO). Cod liver oil was purchased from Silver Seas Co. (El-Obour, Egypt).
Animals and their treatment outlines
The animal protocol was approved by ethical committee in Faculty of Pharmacy, University of Mansoura (protocol code no. 2012-32). Thirty-two adult male Sprague Dawely rats weighing 120–140 g were used. All animals were maintained under standard conditions of temperature, about 25 °C, with regular 12 h light/12 h dark cycle and allowed free access to food and water. Rats were fed with standard rat chow (160.4 g/kg protein, 36.3 g/kg fat, 41 g/kg fiber and metabolizable energy 12.08 MJ). They were classified into the following groups with 8 rats in each group ():
Control group. Rats received the standard diet without any treatment and served as negative control group throughout the study.
Treated control group. Rats received a daily standard diet and supplemented orally with cod liver oil (5 ml/kg body weight) daily for 12 weeks.
Sodium nitrite group. Rats received standard diet and given orally sodium nitrite (80 mg/kg body weight) daily for 12 weeks to serve as positive control group.
Cod liver oil treated group. Rats received standard diet and supplemented orally with cod liver oil followed by sodium nitrite daily for 12 weeks with similar doses that mentioned above.
Table 1. Body weight of different rat groups (mean ± SE).
The doses and time course of experiments used for sodium nitrite were in the range of those used in other studies (Hassan et al., Citation2010). In addition, the dose was determined after appropriate preliminary experiments.
Animal sacrifice and collection of samples
The animals were sacrificed by decapitation. Rat trunk blood was collected and centrifuged at 3000 rpm for 5 min and serum samples were separated and stored at −80 °C. Rat livers were removed, cleaned with ice-cold saline, weighed and chilled on crushed ice. A piece of the liver was fixed in 10% buffered formalin for subsequent morphologic analysis. Another part was homogenized in a 10-fold volume of ice-cold sodium, potassium phosphate buffer (0.01 M, pH 7.4) containing 1.15% KCl. The homogenates were centrifuged at 600 g at 4 °C for 10 min. The supernatant, referred to as homogenate, was stored at −80 °C until used.
Morphologic analysis of hepatic tissue
The liver was cut, fixed in 10% buffered formalin and embedded in paraffin. Five micrometer thickness sections were cut and stained with Mayer's hematoxylin and eosin (H&E) for examination of cell structure by light microscope. Another set of slides were stained with Mallory trichome, histology stain used on connective tissue to visualize collagen and reticular fibers, to study extracellular components. Hepatic specimens were anonymously coded and examined in a masked manner. The morphologic changes were photographed using digital camera-aided computer system (Nikon Digital Camera, Tokyo, Japan).
Measuring liver function
Serum alkaline phosphatase (ALP) and alanine aminotransferase (ALT) activities as well as serum total protein, albumin, and bilirubin concentrations were measured by standard methodologies using commercially available kits provided by Biodiagnostic Company (Giza, Egypt).
Assessment of oxidative stress
This was estimated through the following parameters:
Serum and hepatic malondialdhyde (MDA) concentrations were measured by thiobarbituric acid as described previously by our group (Al-Gayyar et al., Citation2007; Shams et al., Citation2011). In brief, after precipitation of serum proteins by trichloroacetic acid, thiobarbituric acid reacts with MDA to form thiobarbituric acid-reactive substance that is measured at 532 nm.
Leucocytic and hepatic hydrogen peroxide concentrations were measured by horseradish peroxidase (HRPO) method after modification as described previously (Al-Gayyar et al., Citation2007, Citation2012). This method depends on the HRPO mediated oxidation of phenol red by hydrogen peroxide released from leucocytes, which resulted in the formation of a compound that could be read at 610 nm.
Serum and hepatic superoxide anion concentrations were measured by the nitroblue tetrazolium (NBT) method (Baehner et al., Citation1976). It depends on the ability of the superoxide anion to reduce NBT to an insoluble formazan that can be measured at 560 nm.
Assessment of antioxidant activity
This was estimated through the following parameters:
Serum and hepatic SOD activities were determined using phenazinemethosulfate (PMS) method (DeChatelet et al., Citation1974); which depends on the ability of SOD to inhibit the PMS-mediated reaction of NBT.
Blood and hepatic catalase activities were determined by addition of known excess of hydrogen peroxide. The unreacted hydrogen peroxide was determined with potassium permanganate using rapid spectrophotometric determination reflecting catalase activity (Cohen et al., Citation1970).
Blood and hepatic reduced glutathione concentrations were determined by 5,5′-dithiobis (2-nitrobenzoic acid; DTNB) method (Beutler et al., Citation1963) as modified by our group (Al-Gayyar et al., Citation2007). It depends on the fact that virtually all of the non-protein sulfhydryl of red cells is in the form of reduced glutathione. The disulfide compound, DTNB, is readily reduced by sulfhydryl compounds, forming a highly colored yellow anion that can be measured at 412 nm.
Assessment of hepatic fibrosis
Hepatic fibrosis was assessed through determination of hepatic monocyte chemotactic protein (MCP)-1 concentration using a commercially available ELISA kit (eBioscience Inc, San Diego, CA).
Determination of hepatic mitochondrial function
Hepatic mitochnodiral function was measured via determination of hepatic cytochrome c oxidase using a commercially available kit (Sigma-Aldrich). It is based on observation of the decrease in absorbance at 550 nm of ferrocytochrome c caused by its oxidation to ferricytochrome c by cytochrome c oxidase.
DNA fragmentation assay
The DNA fragmentation assay was conducted using the procedure of Wu et al. (Citation2005). Briefly, liver tissue (50 mg) was homogenized in 10 volumes of 5 mM Tris–HCl pH 8, containing 20 mM EDTA and 0.2% Triton X-100. An aliquot of each sample (1 mL) was centrifuged at 27 000 × g for 20 min to separate the intact chromatin (pellet, B) from the fragmented DNA (supernatant, T). Pellet and supernatant fractions were assayed for DNA content using a freshly prepared diphenylamine solution and the optical density was read at 620 nm. Results are expressed as percent fragmented DNA using the following formula: Percent fragmented DNA = T × 100/(T + B).
Data analysis
The mean values ± standard error was used for quantitative variables. For comparison between two groups Student's t-test was used. Statistical computations were done on a personal computer using the computer software SPSS version 13 (Chicago, IL). Statistical significance was predefined as p≤ 0.05.
Results
Effect of cod liver oil on liver function
Oral sodium nitrite in rats produces hepatotoxicity (Hassan et al., Citation2010). As shown in , sodium nitrite caused significant increases in serum ALT and ALP activities and bilirubin concentration as compared with the control group (p < 0.05). In addition, sodium nitrite caused significant decreases in serum concentrations of total protein and albumin as compared with the control rats (p < 0.05). Daily treatment with 5 ml/kg body weight cod liver oil for 12 weeks significantly reduced serum concentration of bilirubin and activities of ALT and ALP as well as significantly elevated the serum concentrations of total protein and albumin in sodium nitrite group and did not affect the control group.
Table 2. Effect of cod liver oil on liver function (mean ± SE).
Effect of cod liver oil on sodium nitrite-induced morphological damage
The hepatoprotective effect of cod liver oil was examined in liver sections stained with H&E or Mallory trichome, histological stain used for distinguishing cellular from extracellular components. Liver sections of rats treated with sodium nitrite that were stained with H&E showed disrupted endothelial lining of central vein and many hepatocytes appear vacuolated (). In parallel, liver sections of sodium nitrite group that stained with Mallory trichome showed increased drainage of collagen fibers surrounding the central vein (). However, rats simultaneously received daily cod liver oil (5 ml/kg body weight, po) for 12 weeks showed nearly normal appearance of heypatocytes in the sodium nitrite group and did not affect the control group.
Figure 1. Effect of cod liver oil on sodium nitrite-induced hepatocyte damage. Liver sections from different rat groups were stained with H/E and examined under a microscope (400 × magnification). (A) Sections from the control group showing hpatocytes radiating from the central vein (asterisk). Some hepatocytes appear binucleated (arrows). Blood sinusoids appear between the hepatocytes (arrow head). (B) Treated control group sections showing a portal tract containing a branch of portal vein (arrow) and a branch of hepatic artery (arrow head). (C) Sodium nitrite cussed disrupted endothelial lining of central vein (arrow head). Many hepatocytes appear with vacuolated cytoplasm (arrows). An area with disorganized liver cell is seen (asterisk). Cellular infilterate is present between hepatocytes (thick arrows). (D) Sections from rats treated with daily cod liver oil showing nearly normal appearance of hepatic lobule. Some hepatocytes appear with dark nuclei (arrow heads).
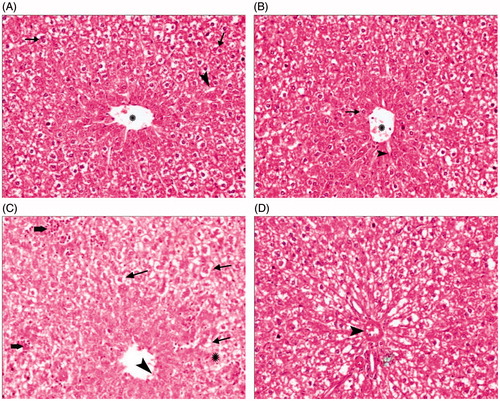
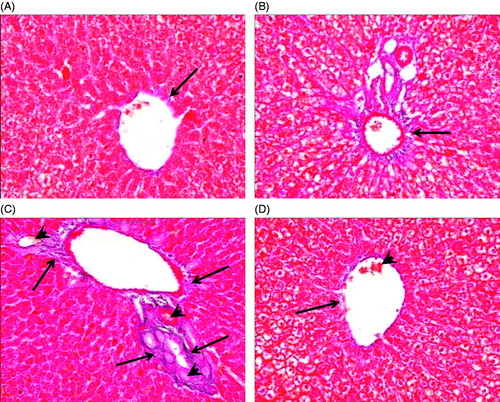
Figure 2. Effect of cod liver oil on sodium nitrite-induced drainage of connective tissue. Liver sections from different rat groups were stained with Mallory trichome and examined under a microscope (400 × magnification). (A) Sections from control group showing minimal amount of collagen fibers around the central vein (arrow). (B) Sections from treated control group showing minimal amount of collagen fibers in the portal tract (arrow). (C) Administration of sodium nitrite increased amount of collagen fibers surrounding a central vein (arrows). Delicate collagen fibers appear in the sinusoidal wall (arrow head). (D) Sections from rats treated with cod liver oil showing minimal collagen fibers surrounding a central vein (arrow) with an area exhibiting some increased fibers (arrow head).
Effect of cod liver oil on oxidative stress
Hassan et al. (Citation2010) reported previously that sodium nitrite exerts oxidative stress and retrograde the endogenous antioxidant system. However, we found significant increases in the serum and hepatic levels of malondialdehyde (MDA), hydrogen peroxide and superoxide anion as compared with the control group (p < 0.05). Treatment with cod liver oil (5 ml/kg body weight, po) daily for 12 weeks significantly reduced both the serum and hepatic concentrations of MDA, hydrogen peroxide and superoxide anion in sodium nitrite group but did not affect the control group ().
Table 3. Effect of cod liver oil on oxidative stress (mean ± SE).
Effect of cod liver oil on antioxidant activity
We examined the effect of administering sodium nitrite and cod liver oil on the antioxidant activity in experimental rats. We found significant reduced blood and hepatic concentration of glutathione and the activities of SOD and catalase in sodium nitrite group as compared with the control group (p < 0.05). Daily treatment with 5 ml/kg body weight cod liver oil for 12 weeks significantly increased glutathione concentration and the activities of SOD and catalase in sodium nitrite group and did not affect the control group ().
Table 4. Effect of cod liver oil on antioxidant activity (mean ± SE).
Effect of cod liver oil on hepatic fibrosis
We examined the effect of sodium nitrite and cod liver oil on monocyte chemotactic protein (MCP)-1 as a marker of liver fibrosis. We found significant (p < 0.05) elevation in hepatic concentration of MCP-1 in sodium nitrite group (168.7 ± 12.6 pg/mg protein) as compared with the control group (39.6 ± 3.9 pg/mg protien). Daily treatment with 5 ml/kg body weight cod liver oil for 12 weeks significantly reduced MCP-1 concentration in the sodium nitrite (79.8 ± 6.4 pg/mg protein) group but still significantly higher than the control group (). MCP-1 showed significant positive correlations with MDA (r = 0.83), hydrogen peroxide (r = 0.82) and superoxide anion (r = 0.91), as well as, significant negative correlations with catalase (r = −0.75), SOD (r =− 0.73) and glutathione (r =− 0.65; ).
Figure 3. Effect of cod liver oil on hepatic fibrosis. Statistical analysis showing significant increase in hepatic concentration of monocyte chemotactic protein (MCP)-1 in sodium nitrite group as compared with the control group (p < 0.05). Treatment with cod liver oil significantly reduced MCP-1 concentration in sodium nitrite group but still significantly higher than the control group. *: significant difference as compared with the rest of the groups at p < 0.05. #: significant difference as compared with the control groups at p < 0.05.
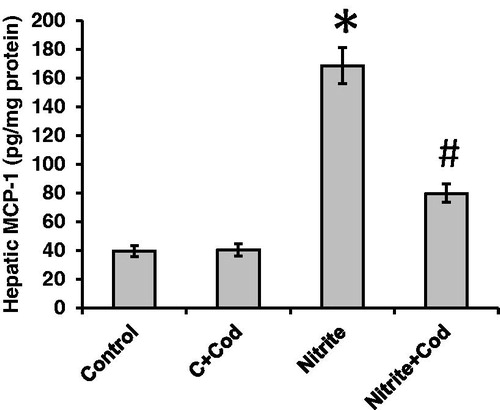
Table 5. Correlation between different measured parameters.
Effect of cod liver oil on hepatic cytochrome c oxidase activity
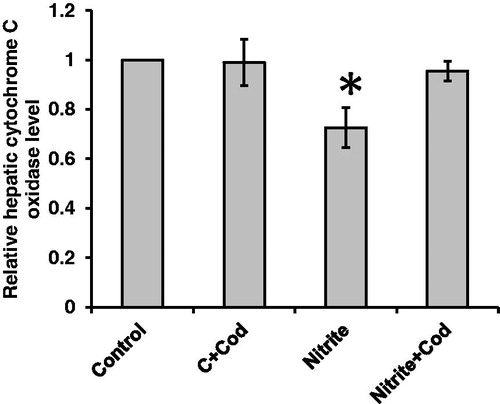
As shown in , we found 38% reduction in hepatic cytochrome c oxidase in rats that received sodium nitrite as compared with the control rats. Treatment with cod liver oil (5 ml/kg body weight, po) daily for 12 weeks restores cytochrome c oxidase activity in sodium nitrite group but did not affect the control group. In addition, as shown in , cytochrome c oxidase showed significant positive correlations with catalase (r = 0.48), SOD (r = 0.47) and glutathione (r = 0.34) and significant negative correlations with MDA (r =− 0.49), hydrogen peroxide (r =− 0.4), superoxide anion (r =− 0.79) and MCP-1 (r =− 0.67).
Figure 4. Effect of cod liver oil on hepatic cytochrome c oxidase activity. Statistical analysis showing about 38% reduction in hepatic cytochrome c oxidase in rats received sodium nitrite as compared with the control group. Treatment with cod liver oil restores cytochrome c oxidase activity in sodium nitrite group but did not affect the control rats. *: significant difference as compared with the rest of the groups at p < 0.05.
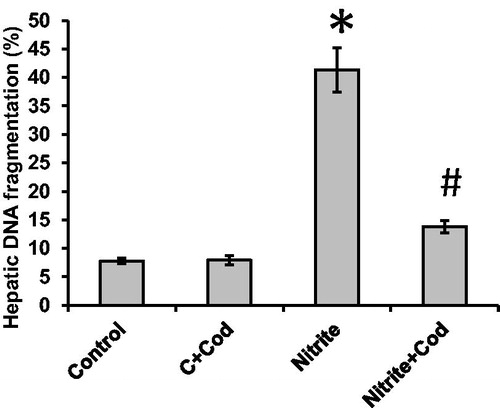
Effect of cod liver oil on hepatic DNA fragmentation
As shown in , sodium nitrite caused a significant (p < 0.05) increase in the percent of hepatic DNA fragmentation (41.3 ± 3.9%) as compared with the control group (7.8 ± 0.5%). Treatment with 5 ml/kg cod liver oil daily for 12 weeks significantly reduced the percent of DNA fragmentation in sodium nitrite group (13.8 ± 1.1%) but did not affect the control group. However, the level of DNA fragmentation in rats simultaneously treated with sodium nitrite and cod liver oil is still significantly higher than those of the control group (p < 0.05). DNA fragmentation showed significant positive correlations with MDA (r = 0.79), hydrogen peroxide (r = 0.85), superoxide anion (r = 0.87) and MCP (r = 0.91), as well as, significant negative correlations with catalase (r =− 0.68), SOD (r =− 0.7), glutathione (r =− 0.61) and cytochrome c oxidase (r =− 0.67; ).
Figure 5. Effect of cod liver oil on hepatic DNA fragmentation. Statistical analysis showing significant increase in hepatic DNA fragmentation in sodium nitrite group as compared with the control group (p < 0.05). Treatment with cod liver oil significantly reduced the percent of DNA fragmentation in the sodium nitrite group but did not affect the control group. *: significant difference as compared with the rest of the groups at p < 0.05. #: significant difference as compared with the control groups at p < 0.05.
Discussion
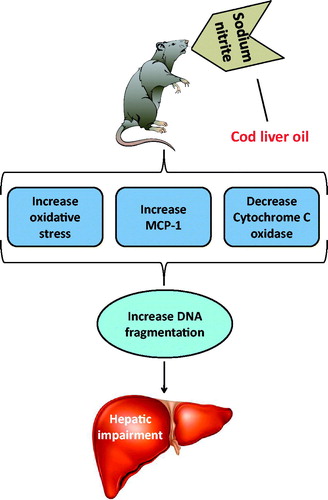
The main findings of the current study are that cod liver oil ameliorates the impairment of hepatic function in rats ingesting sodium nitrite via multiple mechanisms including: (1) reducing sodium nitrite-induced oxidative stress, as indicated by reducing MDA, hydrogen peroxide and superoxide anion levels in serum and hepatic tissues; (2) blocking sodium nitrite-induced inhibition of antioxidant activity as set out by restoring the concentration of reduced glutathione and the activity of superoxide dismutase and catalase in serum and hepatic tissues of rats; (3) blocking sodium nitrite-induced elevation of hepatic fibrosis markers as MCP-1; (4) inhibiting sodium nitrite-induced deactivation of mitochondrial function as indicated by restoring cytochrome c oxidase activity; and (5) reducing sodium nitrite-induced increase in the percent of DNA degradation in the hepatic tissues of rats. The mechanism of action is summarized in . To the best of our knowledge, our study demonstrates for the first time hepatic-protection role of cod liver oil in sodium nitrite-induced hepatic impairment in rats.
Figure 6. Schematic representation of the mechanism of hepatoprotective action of cod liver oil against sodium nitrite-induced liver damage.
Sodium nitrite is an important antimicrobial additive to food products for as long as 5000 years. Nitrite in meat greatly delays the development of botulinum toxin, develops cured meat flavor and color, retards the development of rancidity during storage, inhibits the development of warmed-over flavor, and preserves flavors of spice and smoke (Binkerd et al., Citation1975; Stokes et al., Citation2009). Despite the enormous effort over the past few decades to limit or even restrict dietary nitrite consumption due to the potential to form carcinogenic N-nitrosamines, to date there are no conclusive data to indicate that dietary sources of nitrite may be unsafe. Since the early 1980s, there have been numerous reports on the association of N-nitrosamines and human cancers (Craddock, Citation1983). The negative connotations of nitrite have led the government to regulate and restrict levels in food and drinking water, particularly in cured and processed meats. However, we found significant decreases in serum total protein and albumin and significant increases in serum activities of both ALT and ALP in rats treated with sodium nitrite. In parallel, sodium nitrite showed disrupted endothelial lining of central hepatic vein and drainage of collagen fibers surrounding a central vein. These results demonstrated hepatic impairment effects of sodium nitrite in rats, which is consistent with a previous study (Hassan et al., Citation2010).
The relationship between diet and health has been recognized throughout recorded history. Disease prevention through healthy preparation of foods and eating habits has been discussed in religious and civil writings for thousands of years. Therefore, we aimed to investigate the role of cod liver oil in a sodium nitrite rat model. Of note, daily treatment with cod liver oil for 12 weeks ameliorates the altered hepatocyte structure as well as reduced the elevated liver enzymes in the sodium nitrite group and did not affect the control group. Cod liver oil, widely used as a dietary supplement, is obtained from the liver of cod fish and is different from fish oil. The main difference is that cod liver oil is rich in vitamin A and vitamin D besides the essential omega-3 fatty acids, especially eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA; Trofimiuk et al., Citation2011). In previous studies, cod liver oil supplementation has been suggested to reduce cardio-metabolic risk factors (Abeywardena et al., Citation2011), have anticancer effects (Dyck et al., Citation2011), and ameliorate cognitive impairment induced by chronic stress (Trofimiuk et al., Citation2011). To our knowledge, no study has yet investigated the potential beneficial effects of cod liver oil in preventing sodium nitrite-induced hepatic impairment.
Next we aimed to figure out the mechanism of hepatoprotective effect of cod liver oil. Inside the body, the formed reactive nitrogen species produced by the exposure to nitrite is considered one of the most important causes of body tissues destruction like triggering lipid peroxidation, DNA lesions, enzyme inactivation and damage of different organs. The high oxidative stress indicator, MDA, could be attributed to the oxidative cytotoxicity of nitrite (Patsoukis et al., Citation2007). It has been reported previously that sodium nitrite and other food additives may react with amines of food in the stomach and produce nitrosamines and free radicals. Such products may increase lipid peroxidation, which can be harmful to different organs including liver (Choi et al., Citation2002; Hassan et al., Citation2010). Consistent with observations from these previous studies, we found significant increases in oxidative stress markers (MDA, hydrogen peroxide and superoxide anion) and significant decreases in antioxidant activity (SOD, catalase and reduced glutathione) in both serum and hepatic homogenates. All these effects were blocked by cod liver oil. Of note, it has been reported previously that cod liver oil reduced oxidative stress and augmented the antioxidant activity in other animal models that involve oxidative stress like streptozotocin-induced diabetes in rats (Hunkar et al., Citation2002) and daunomycin-induced nephropathy in mice (Ohtake et al., Citation2002). Moreover, dietary supplementation with cod liver oil inactivates circulating neutrophils and plasma lipoperoxides (Guarnieri et al., Citation1991). These antioxidant effects of cod liver oil can be attributed to its omega-3 fatty acids and vitamin A contents. Many previous studies showed strong antioxidant activity of omega-3 fatty acids in different models (Al-Gayyar et al., Citation2012; Diggle, Citation2002; El-Mesery et al., Citation2009; Mesa et al., Citation2004). In addition, cod liver oil is a good source of vitamin A supplementation. The antioxidant activity of vitamin A and related carotenoids is conferred by the hydrophobic chain of polyene units that can quench singlet oxygen, neutralize thiyl radicals, and combine with and stabilize peroxyl radicals. The majority of research performed to date has examined the antioxidant effects of vitamin A and carotenoids, as the observation by Das (Citation1989) suggests that vitamin A supplementation can exert protective effects against neurodegenerative and cardiovascular diseases, keeping in mind that the oxidative stress play a major role in the pathogenesis of such conditions (Lee et al., Citation2009). However, many previous studies reported that intake of large doses of vitamin A can cause nausea, vomiting, headache, and dry scaly skin. More severe health problems can arise from storing excess vitamin A in the body, including liver damage, osteoporosis, and nervous system disorders. For these reasons, the total vitamin A intake is recommended as less than 3000 µg per day from retinol. On the other hand, supplementation of moderate amounts of cod liver oil appears to be relatively safe (Huang et al., Citation2011).
Monocyte chemoattractant protein (MCP)-1 is a chemokine capable of recruiting monocytes/macrophages into sites of inflammation as well as stimulating the respiratory burst required for macrophage activation (Mitchell et al., Citation2009). Several independent studies highlighted the importance of MCP-1 for monocyte recruitment during experimental hepatic fibrosis (Seki et al., Citation2009; Mitchell et al., Citation2009). However, we found a significant increase in hepatic MCP-1 in the sodium nitrite group. Baeck et al. (Citation2012) reported that pharmacological inhibition of MCP-1 efficiently inhibited monocyte chemotaxis in vitro and in vivo reducing liver tissue injuries and the development of steatosis. However, treatment with cod liver oil significantly reduced hepatic MCP-1 in the sodium nitrite group. To the best of our knowledge, our study is the first to investigate the effect of both sodium nitrite and cod liver oil on hepatic MCP-1 in vivo.
Moreover, effect of sodium nitrite on mitochondrial activity, as indicated by cytochrome c oxidase, was assessed in the present study. Mitochondrial cytochrome c oxidase is the final electron acceptor in the mitochondrial electron transport chain and is required for aerobic ATP production through catalyzes of electron transfer from cytochrome c to molecular oxygen (Horn et al., Citation2008). We found a significant decrease in the cytochrome c oxidase activity in rats that received oral sodium nitrite. It was proposed previously that this down-regulation of cytochrome c oxidase activity may be linked to reactive oxygen species and apoptosis (Papadopoulou et al., Citation1996; You et al., Citation2002). Of note, results of our study elucidated significant elevation in oxidative stress that was accompanied by significant reduction in cytochrome c oxidase in rats received sodium nitrite. However, we found for the first time the significance of using cod liver oil in restoring hepatic cytochrome c oxidase activity in rats.
The morphological features induced by high sodium nitrite completely reflected the classic apoptotic features. This was further confirmed by DNA fragmentation. In addition, lipid peroxidation induced by the free radicals of sodium nitrite combine with DNA to form adducts and accelerates DNA fragmentation (Khan et al., Citation2012). However, we found that sodium nitrite exhibited a remarkable increase in hepatic DNA fragmentation percent. On treating sodium nitrite stressed hepatocytes with cod liver oil, DNA fragmentation was significantly decreased. Hence, cod liver oil was found to be effective in ameliorating oxidative stress, mitochondria deactivation and DNA fragmentation caused by sodium nitrite.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Abeywardena MY, Patten GS. (2011). Role of omega3 long-chain polyunsaturated fatty acids in reducing cardio-metabolic risk factors. Endocr Metab Immune Disord Drug Targets 11:232–46
- Al-Gayyar MM, Eissa LA, Rabie AM, El-Gayar AM. (2007). Measurements of oxidative stress status and antioxidant activity in chronic leukaemia patients. J Pharm Pharmacol 59:409–17
- Al-Gayyar MM, Shams ME, Barakat EA. (2012). Fish oil improves lipid metabolism and ameliorates inflammation in patients with metabolic syndrome: Impact of nonalcoholic fatty liver disease. Pharm Biol 50:297–303
- Baeck C, Wehr A, Karlmark KR, et al. (2012). Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 61:416–26
- Baehner RL, Boxer LA, Davis J. (1976). The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood 48:309–13
- Beutler E, Duron O, Kelly BM. (1963). Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–8
- Binkerd EF, Kolari OE. (1975). The history and use of nitrate and nitrite in the curing of meat. Food Cosmet Toxicol 13:655–61
- Choi SY, Chung MJ, Sung NJ. (2002). Volatile N-nitrosamine inhibition after intake Korean green tea and Maesil (Prunus mume Sieb. et Zacc.) extracts with an amine-rich diet in subjects ingesting nitrate. Food Chem Toxicol 40:949–57
- Cohen G, Dembiec D, Marcus J. (1970). Measurement of catalase activity in tissue extracts. Anal Biochem 34:30–8
- Craddock VM. (1983). Nitrosamines and human cancer: Proof of an association? Nature 306:638
- Das NP. (1989). Effects of vitamin A and its analogs on nonenzymatic lipid peroxidation in rat brain mitochondria. J Neurochem 52:585–8
- DeChatelet LR, McCall CE, McPhail LC, Johnston RB Jr. (1974). Superoxide dismutase activity in leukocytes. J Clin Invest 53:1197–201
- De Ley M, de Vos R, Hommes DW, Stokkers P. (2007). Fish oil for induction of remission in ulcerative colitis. Cochrane Database Syst Rev (4): CD005986
- Diggle CP. (2002). In vitro studies on the relationship between polyunsaturated fatty acids and cancer: Tumour or tissue specific effects? Prog Lipid Res 41:240–53
- Dyck MC, Ma DW, Meckling KA. (2011). The anticancer effects of vitamin D and omega-3 PUFAs in combination via cod-liver oil: One plus one may equal more than two. Med Hypotheses 77:326–32
- El-Mesery M, Al-Gayyar M, Salem H, et al. (2009). Chemopreventive and renal protective effects for docosahexaenoic acid (DHA): Implications of CRP and lipid peroxides. Cell Div 4:6
- Guarnieri C, Li YZ, Roth E, et al. (1991). Reduced neutrophil superoxide generation and plasma lipoperoxide levels in pigs fed with cod liver oil. Cell Biochem Funct 9:239–43
- Hassan HA, Hafez HS, Zeghebar FE. (2010). Garlic oil as a modulating agent for oxidative stress and neurotoxicity induced by sodium nitrite in male albino rats. Food Chem Toxicol 48:1980–5
- Horn D, Barrientos A. (2008). Mitochondrial copper metabolism and delivery to cytochrome C oxidase. IUBMB Life 60:421–9
- Huang WB, Fan Q, Zhang XL. (2011). Cod liver oil: A potential protective supplement for human glaucoma. Int J Ophthalmol 4:648–51
- Hunkar T, Aktan F, Ceylan A, Karasu C. (2002). Effects of cod liver oil on tissue antioxidant pathways in normal and streptozotocin-diabetic rats. Cell Biochem Funct 20:297–302
- Khan RA, Khan MR, Sahreen S. (2012). CCl4-induced hepatotoxicity: Protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complement Altern Med 12:178
- Khare S, Asad M, Dhamanigi SS, Prasad VS. (2008). Antiulcer activity of cod liver oil in rats. Indian J Pharmacol 40:209–14
- Klurfeld DM. (2001). Maternal cured meat consumption during pregnancy and risk of paediatric brain tumour in offspring: Potentially harmful levels of intake. Public Health Nutr 4:1303–5
- Kozisek F. (2007). Influence of nitrate levels in drinking water on urological malignancies: A community-based cohort study. BJU Int 99:1550–1
- Lee HP, Casadesus G, Zhu X, et al. (2009). all-trans Retinoic acid as a novel therapeutic strategy for Alzheimer's disease. Expert Rev Neurother 9:1615–21
- Mesa MD, Buckley R, Minihane AM, Yaqoob P. (2004). Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on the oxidizability and thrombogenicity of low-density lipoprotein. Atherosclerosis 175:333–43
- Mitchell C, Couton D, Couty JP, et al. (2009). Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol 174:1766–75
- Milkowski A, Garg HK, Coughlin JR, Bryan NS. (2010). Nutritional epidemiology in the context of nitric oxide biology: A risk–benefit evaluation for dietary nitrite and nitrate. Nitric Oxide 22:110–19
- Ohtake T, Kimura M, Takemura H, Hishida A. (2002). Effects of dietary lipids on daunomycin-induced nephropathy in mice: Comparison between cod liver oil and soybean oil. Lipids 37:359–66
- Paik DC, Saborio DV, Oropeza R, Freeman HP. (2001). The epidemiological enigma of gastric cancer rates in the US: Was grandmother's sausage the cause? Int J Epidemiol 30:181–2.
- Papadopoulou LC, Tsiftsoglou AS. (1996). Effects of hemin on apoptosis, suppression of cytochrome C oxidase gene expression, and bone-marrow toxicity induced by doxorubicin (adriamycin). Biochem Pharmacol 52:713–22
- Patsoukis N, Georgiou CD. (2007). Effect of glutathione biosynthesis-related modulators on the thiol redox state enzymes and on sclerotial differentiation of filamentous phytopathogenic fungi. Mycopathologia 163:335–47
- Pogoda JM, Preston-Martin S. (2001). Maternal cured meat consumption during pregnancy and risk of paediatric brain tumour in offspring: Potentially harmful levels of intake. Public Health Nutr 4:183–9
- Seki E, de Minicis S, Inokuchi S, et al. (2009). CCR2 promotes hepatic fibrosis in mice. Hepatology 50:185–97
- Shams ME, Al-Gayyar MM, Barakat EA. (2011). Type 2 diabetes mellitus-induced hyperglycemia in patients with NAFLD and normal LFTs: Relationship to lipid profile, oxidative stress and pro-inflammatory cytokines. Sci Pharm 79:623–34
- Shuval HI, Gruener N. (1972). Epidemiological and toxicological aspects of nitrates and nitrites in the environment. Am J Publ Health 62:1045–52
- Sindelar JJ, Milkowski AL. (2012). Human safety controversies surrounding nitrate and nitrite in the diet. Nitric Oxide 26:259–66
- Stokes KY, Dugas TR, Tang Y, et al. (2009). Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am J Physiol Heart Circ Physiol 296:H1281–8
- Sullivan GA, Jackson-Davis AL, Niebuhr SE, et al. (2012). Inhibition of Listeria monocytogenes using natural antimicrobials in no-nitrate-or-nitrite-added ham. J Food Prot 75:1071–6
- Trofimiuk E, Braszko JJ. (2011). Long-term administration of cod liver oil ameliorates cognitive impairment induced by chronic stress in rats. Lipids 46:417–23
- Ward MH, Pan WH, Cheng YJ, et al. (2000). Dietary exposure to nitrite and nitrosamines and risk of nasopharyngeal carcinoma in Taiwan. Int J Cancer 86:603–9
- Wjst M. (2009). Introduction of oral vitamin D supplementation and the rise of the allergy pandemic. Allergy Asthma Clin Immunol 5:8
- Wu B, Ootani A, Iwakiri R, et al. (2005). T cell deficiency leads to liver carcinogenesis in azoxymethane-treated rats. Exp Biol Med 231:91–8
- You KR, Wen J, Lee ST, Kim DG. (2002). Cytochrome c oxidase subunit III: A molecular marker for N-(4-hydroxyphenyl)retinamise-induced oxidative stress in hepatoma cells. J Biol Chem 277:3870–7
