Abstract
Context: Fruits of Ternstroemia sylvatica Schltdl. and Cham. (Theaceae) are used in Mexican traditional medicine to alleviate anxiety, sleep disorders and seizures; however, the active principles have not been identified.
Objective: To identify the neuroactive principles of T. sylvatica fruits using neuropharmacological tests on mice.
Materials and methods: The methanol and aqueous extracts of pericarp or seeds of T. sylvatica fruits were intraperitoneally administered (1–562 mg/kg, single doses) to mice. The exploratory cylinder, hole board, open field, Rota-rod and sodium pentobarbital-induced hypnosis tests were used to evaluate the CNS depressant effect after 30 min single administration of extracts. From aqueous seeds extract, triterpene glycoside 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol was isolated an active compound.
Results: Crude extracts of T. sylvatica fruits, separated from seed and pericarp, showed sedative effect in mice. The aqueous (ED50 = 4.9 ± 0.8 mg/kg) seed extracts is the most active among them. This extract also decrease locomotor activity and disrupt motor coordination of mice. This extract was also the most toxic extract (LD50 = 5.0 ± 1.4 mg/kg; i.p.). The triterpene glycoside 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol was identified in this extract as one of the active sedative compounds (ED50 = 0.12 ± 0.01 mg/kg) also with toxic effect (LD50 = 1.11 ± 0.23 mg/kg).
Conclusion: The results suggest that T. sylvatica fruits has toxic activity rather than CNS depressant activity in mice and that this effect might be related to the presence of 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol, one of the active principles of T. sylvatica fruits with sedative and toxic effect.
Introduction
Ternstroemia is the largest genus in the Theaceae family with an estimated 130 species worldwide. It has been reported that 12 Ternstroemia species occur in México (Boom, Citation1989). Fruits of several species from Ternstroemia are commonly known in México as “Flor de Tila”, and they are used in Mexican traditional medicine to alleviate anxiety, sleep disorders and seizures (Aguilar-Santamaría & Tortoriello, Citation1996; Molina et al., Citation1999; Tortoriello & Romero, Citation1992). Ternstroemia pringlei (Rose) Standl. (synonym Ternstroemia lineata DC) and Ternstroemia sylvatica Schltdl. and Cham. are the two major Ternstroemia species used as decoctions in Mexican traditional medicine to treat insomnia and fear (Molina et al., Citation1999). The T. sylvatica fruits contain 40% of seeds and 60% of pericarp. Traditionally, the decoctions of these plants are prepared from their whole ground fruits. Pharmacological studies have shown that fruits of T. pringlei (Bartholomew & McVaugh, Citation1997; Kobuski, Citation1942) produce sedative and anticonvulsive effects in rats (Aguilar-Santamaría & Tortoriello, Citation1996), and interact in a complex form with CNS depressant drugs (Balderas et al., Citation2008). Fruits of T. sylvatica have sedative effects more than anxiolytic effects in rats (Aguilar-Santamaría & Tortoriello, Citation1996; Molina et al., Citation1999; Tortoriello & Romero, Citation1992). Recently, the isolation of jacaranone as a sedative substance from T. pringlei was reported (Lozada-Lechuga et al., Citation2010). Phytochemical studies of Ternstroemia species have reported the isolation of oleanane- and ursane-type triterpenoids, triterpenoid glycosides, triterpenoid saponins, carotenoids, monoterpenoids, tannins and other aromatic compounds (Balderas et al., Citation2008; Ikuta et al., Citation2003; Jo et al., Citation2005; Kikuchi & Yamaguchi, Citation1974; Shin et al., Citation2003; Tori et al., Citation2005). However, there are no additional reports concerning neuropharmacological effects of T. sylvatica fruits. Therefore, this study was undertaken to evaluate the neuropharmacological effects of T. sylvatica fruits using several experimental models to identify the active principle(s) of this medicinal plant. However, the experimentation led to the identification of a toxic effect greater than the neuropharmacological effect of this plant and to the identification of a new glycoside triterpenoid as one of the toxic compounds from T. sylvatica.
Materials and methods
Drugs and reagents
Tween 80 was purchased from Sigma Co. (St. Louis, MO), diazepam (Valium™) was purchased from Roche S.A. (México). The extracts were dissolved in saline solution (0.9%) and the drugs were dissolved in 0.5% Tween 80 in saline solution. All solutions were freshly prepared each time and administered intraperitoneal or orally in a volume of 0.1 mL/10 g body weight in single administration. Control animals received the same volume of vehicle (0.5% Tween 80 in saline solution or saline solution only).
Experimental animals
All experiments were performed on adult male ICR mice (25–34 g; Centro UNAM-Harlan, Harlan México, S. A. de C. V.). The experimental groups consisted of six animals. The animals were maintained at constant room temperature (22 ± 2 °C) and subjected to a 12 h light/dark cycle with free access to food and water.
To optimize the number of laboratory animals used, exploratory cylinder and hole board tests and open field and sodium pentobarbital-induced hypnosis tests were performed consecutively.
Procedures involving animals and their care were conducted in conformity with the Mexican Official Norm for Animal Care and Handling (NOM-062-ZOO-1999) adopted in our laboratory, and in compliance with international rules on care and use of laboratory animals. Furthermore, clearance for conducting the studies was obtained from the Ethics Committee for the Use of Animals in Pharmacological and Toxicological Testing (CICUAL/020/11, 2011), Facultad de Química, UNAM. Behavioral experiments were carried out between 10:00 and 14:00 h.
Plant material
The fruits of T. sylvatica Schltdl. and Cham. were collected at Pahuatlán, Puebla, Mexico, in June 2007. The authenticity of the plant materials was certified by M.E. Flores-Villafranco, botanist of the FES Iztacala UNAM Herbarium. Samples were deposited in this herbarium with the voucher number IZTA42169. The fruits of T. sylvatica have an average weight of 543 ± 123 mg (n = 10); 40% of them correspond to seeds (221 ± 81 mg). Therefore, we decided to test the pericarp and seeds separately.
Preparation of extracts
The seeds and pericarp from dried fruits of T. sylvatica were manually separated. After grinding using a manual miller, 374 g of pericarp and 56.2 g of seeds were extracted separately at room temperature with hexane (3 × 2 L, 24 h each), then with dichloromethane (3 × 2 L, 24 h each) and, finally, with methanol (3 × 2 L, 24 h each); evaporation of the solvents in vacuum gave 1.44 g of hexane (0.39% yield), 2.01 g of dichloromethane (0.54% yield) and 50.17 g of methanol (13.41% yield) extracts as syrupy residues from pericarp, and 17.59 g of hexane (31.30% yield), 5.51 g of dichloromethane (9.81% yield) and 3.80 g of methanol (6.76% yield) extracts from seeds. Aqueous extracts were prepared with 200 g of dried and powdered seeds or 200 g of pericarp by boiling them in 1.8 L of distilled water for 10 min. Afterwards, the extracts were filtered by gravity and concentrated through air current at room temperature (22 ± 2 °C) obtaining 27.9 g (13.9% yield) of a breakable reddish solid for pericarp and 28.4 g (14.2% yield) of a pale beige solid for seeds.
Isolation of 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol
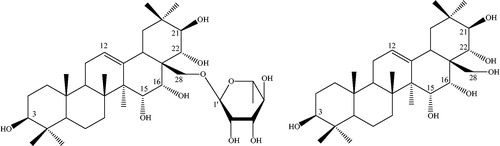
The active aqueous seeds extract of T. sylvatica (20 g) was partitioned with n-BuOH (2 L). Both aqueous and n-butanol fractions were concentrated under reduced pressure (40 °C at 72 mbar for water and 40 °C at 25 mbar for n-BuOH) to obtaining 9.3 g and 9.0 g of aqueous and n-BuOH fractions, respectively. A sample of the n-butanol soluble fraction (1.0 g) was chromatographed using a Sephadex LH-20 column (400 mm × 40 mm i.d.) and eluted with MeOH to give 20 fractions of 50 mL each. Fractions 3–5 (200 mg) were pooled and subjected to an additional medium pressure RP-C18 silica gel column chromatography (450 mm × 25 mm i.d.) eluted with MeOH:H2O (9:1, 8:2, 7:3 and 5:5, each 30 mL) to afford 78.60 mg of white powder. A part of this powder (50 mg) was applied to a medium pressure C18 column (450 mm × 25 mm i.d.) and eluted with MeOH to afford 30 mg of white amorphous powder. A posterior purification by preparative RP-TLC eluted with H2O:MeOH:CH3CN (2:1:1) yielded a pure white amorphous powder (20 mg) with a melting point (m.p.) 288–290 °C. This compound was identified as 28-O-[β-l-6- rhamnopyranosyl]-R1-barrigenol (1, ) by mass spectrometry and nuclear magnetic resonance (). A portion of this powder (15 mg) was refluxed with 2 N HCl for 3 h. After neutralization with Na2CO3 (10% w/v in water), the reaction mixture was extracted with CH2Cl2 (5 mL × 3). The aqueous phase was dried under vacuum and then treated with 40 μL of Sigma-Sil-A reagent at 60 °C by 10 min and analyzed by GC/MS for sugars. The organic phase was washed with H2O (2 mL × 2) and evaporated to yield a pale yellow solid (7 mg). From this, reaction mixture was separated by preparative silica gel TLC (eluting with CH2Cl2:MeOH 9:1) a white solid with a m.p. >300 °C. The MS and proton NMR (400 MHz, Pyridine-d5) data corresponded to data previously reported (Fu et al., Citation2005) for R1-barrigenol (1a).
Table 1. 1H NMR and 13C NMR spectral data for compounds 1 and 1a.
Pharmacological studies
Sedative activity in the exploratory cylinder test
The apparatus consisted of a glass cylinder (30 cm in height, 11 cm in diameter, with wall of 3 mm). The cylinder was placed on a filter paper in a room with constant lighting and isolated from external noise. An individual naïve mouse was put on the filter paper-covered floor of the glass cylinder; the number of rears performed over a 5 min period was recorded. The inner side of the apparatus and floor were cleaned with ethanol solution (10% v/v) and the filter paper was changed between each animal test session. The extracts, compounds or drugs were administered 30 min before testing in different doses. During observation, the experimenter stood next to the apparatus always at the same place. The observations were made without prior knowledge of the experimental conditions applied to the animal. Reduced exploratory rearing showed by naïve mice after placement in an unfamiliar environment reveals a sedative effect (Hiller & Zetler, Citation1996; Oliva et al., Citation2004; Rolland et al., Citation1991; Ugalde et al., Citation2005).
Open field test
The locomotor behavior was assessed on open field test. The open field was made with an acrylic cage of 20 cm in height. The floor of the open field, 26 cm in length and 18 cm in width, was divided into 12 squares (4 cm × 4 cm) with 2 cm strips. Thirty minutes after the administration of extracts or compounds, each mouse was placed individually at the center of the apparatus and observed for 2 min to record the locomotion as number of segments crossed with the four paws (Walsh & Cummins, Citation1976).
Hole board test
Thirty minutes after the administration of extracts or compounds, each mouse was placed into the hole board apparatus (Ugo Basile 6650 Hole Board). The number of holes explored was recorded automatically during 3 min. This test was used to evaluate emotionality, anxiety and/or response to stress in animals. A decrease in the number of head-dips reveals the anxiolytic behavior (Boissier & Simon, Citation1962).
Sodium pentobarbital-induced hypnosis.
Sodium pentobarbital at a dose of 42 mg/kg was intraperitoneally administered 33 min after the intraperitoneal administration of extracts. Each mouse was observed for the loss of righting reflex (hypnosis) and duration of sleep. The time between loss and recovery of the righting reflex was recorded as sleeping time (González-Trujano et al., Citation1998).
Effect on motor coordination on rota-rod test.
Mice which remained on the rod (Rota-rod Treadmills for mice, constant speed model 7600, Ugo Basile; 4 cm diameter, 16 rpm) for at least 3 min were selected and allocated to groups of six animals each. Immediately after administration extracts, diazepam or vehicle by intraperitoneal route, mice were placed on the rod. The time each animal remained on the rod (“time-on-rod”) was recorded every 10 min for a total recording of 120 min. Effect on motor coordination was considered when mice remained on the rod for less than 2 min (González-Trujano et al., Citation1998).
Acute toxicity study (LD50)
The acute toxicity of extracts was estimated by intraperitoneal administration. Mice were kept under observation for the following 14 d after single administration of extracts (1–316 mg/kg single doses) at constant room temperature (22 ± 2 °C) and submitted to a 12 h light/dark cycle with free access to food and water. Their weights were registered daily, and at the end of the study a macroscopic tissue evaluation was done. The acute toxicity of 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol was estimated by a single intraperitoneal administration (0.1–10.0 mg/kg) and observation for the following 14 d in the same conditions described above.
Statistical analysis
Results are expressed as mean ± S.E.M. of six repetitions. Statistical differences between experimental and control groups were performed by one-way analysis of variance (ANOVA), followed by Dunnett’s test. A value of p < 0.05 was considered to be significant.
Results
The methanol and aqueous extracts of T. sylvatica seeds showed potent sedative effect in exploratory cylinder test after intraperitoneal administration in mice (). The aqueous seeds extract showed a sedative effect (ED50 = 4.9 ± 0.8 mg/kg) like diazepam (ED50 = 3.04 ± 1.14 mg/kg), which was used as reference a sedative drug (). However, single or repeated intragastric administration of aqueous extracts did not show sedative effect (). In addition, the sodium pentobarbital-induced hypnosis time was prolonged by the intraperitoneal administration of methanol or aqueous extracts of T. sylvatica seeds (). However, higher doses of methanol (32 and 100 mg/kg) or aqueous (56 and 100 mg/kg) extracts of T. sylvatica seeds induced death of the animals during sodium pentobarbital-induced hypnosis.
Figure 2. Effect of methanol (A) and aqueous (B) extracts of Ternstroemia sylvatica on latency and duration of pentobarbital-induced hypnosis in mice. Bars represent mean ± S.E.M. n = 6. *p < 0.05 significantly different from vehicle; ANOVA followed by Dunnett’s test.
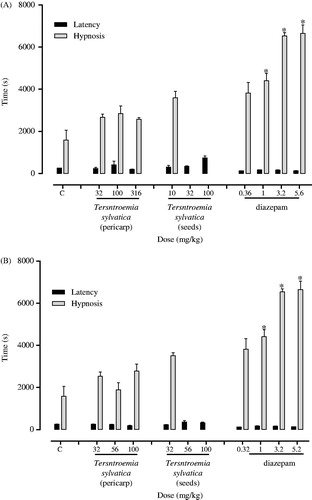
Table 2. Sedative effect in the exploratory cylinder test of Ternstroemia sylvatica extracts after intraperitoneal administration in mice.
Table 3. Sedative effect of Ternstroemia sylvatica aqueous extracts in the exploratory cylinder test by acute and repeated intragastric administration.
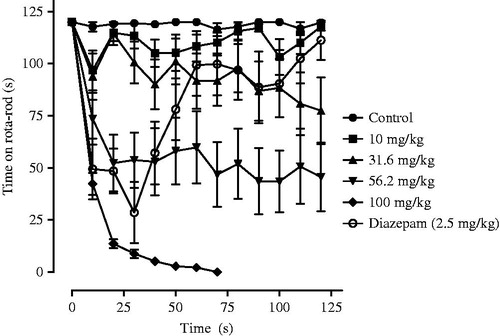
Aqueous extract of T. sylvatica seeds significantly decreased the motor coordination of the animals in a dose-dependent manner (ED50 = 52.21 ± 4.45 mg/kg) without recuperation in the rota-rod test (). However at 100 mg/kg this extract induced the death of all animals 70 min after its administration. Methanol extract did not change the motor coordination of the mice.
Figure 3. Effect of aqueous extract of Ternstroemia sylvatica seeds on motor coordination in the rota-rod test. Symbols represent mean ± S.E.M. n = 6.
From the aqueous seeds extract, it was isolated as a white amorphous powder (Compound 1) with a m.p. 288–290 °C (recrystallized from MeOH). This compound was identified as 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol by mass spectrometry and nuclear magnetic resonance (). This pure compound showed potent sedative activity in the exploratory cylinder test (ED50 = 0.12 ± 0.01 mg/kg, ) and significantly reduced the nose-poking activity in the hole board test (ED50 = 1.02 ± 0.0 mg/kg, ). Whereas the aqueous extract of seeds of T. sylvatica was less active (ED50 =8.81 ± 2.83 mg/kg) than compound 1. Diazepam showed an ED50 = 2.56 ± 1.35 mg/kg in this test.
Figure 4. Effect of 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol in Exploratory Cylinder test (A) and hole board test (B) in mice. Symbols represent mean ± S.E.M. n = 6. *p < 0.05 significantly different from vehicle; ANOVA followed by Dunnett’s test.
![Figure 4. Effect of 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol in Exploratory Cylinder test (A) and hole board test (B) in mice. Symbols represent mean ± S.E.M. n = 6. *p < 0.05 significantly different from vehicle; ANOVA followed by Dunnett’s test.](/cms/asset/377616bd-56ad-42dc-9585-f56da4682388/iphb_a_799706_f0004_b.jpg)
Compound 1 and aqueous extract of seeds from T. sylvatica significantly decreased, in a dose-dependent manner, the locomotor activity (ED50 = 0.35 ± 0.11 and 26.64 ± 2.37 mg/kg, respectively) in the open field test ().
Figure 5. Effect of 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol (A) and aqueous extract of seeds from Ternstroemia sylvatica (B) in open field test in mice. Symbols represent mean ± S.E.M. n = 6. *p < 0.05 significantly different from vehicle; ANOVA followed by Dunnett’s test.
![Figure 5. Effect of 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol (A) and aqueous extract of seeds from Ternstroemia sylvatica (B) in open field test in mice. Symbols represent mean ± S.E.M. n = 6. *p < 0.05 significantly different from vehicle; ANOVA followed by Dunnett’s test.](/cms/asset/510e50e9-8f76-4f38-ad6f-92926e4fa219/iphb_a_799706_f0005_b.jpg)
T. sylvatica seed aqueous extract was the most toxic extract by the intraperitoneal route (LD50 = 5.0 ± 1.4 mg/kg). T. sylvatica aqueous extract produced a weight loss of 15% of the animals during observation period (14 d). Autopsy of mice showed peritoneal extravasations and bleedings in stomach and gut. The intragastric administration of the aqueous extract up to 1000 mg/kg neither induced death nor weight loss of the animals during the observation period (14 d). But repeated intragastric administration of 316 mg/kg of T. sylvatica seeds aqueous extract every 24 h for 5 d induced 50% mortality, while the repeated intragastric administration of 1000 mg/kg of T. sylvatica seeds aqueous extract every 24 h for 5 d induced 83% mortality ().
The 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol showed also high toxicity in mice (LD50 = 1.11 ± 0.23 mg/kg).
Identification of 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol
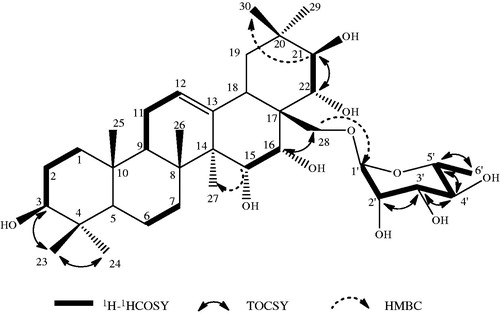
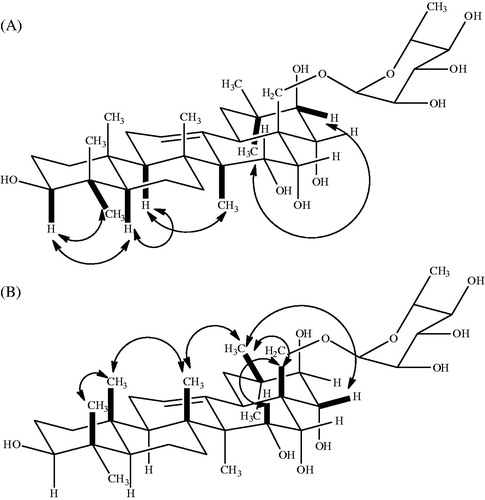
In the negative ion FAB-MS of compound 1, quasi-molecular ion peak was observed at m/z 651 (M − H)− and high-resolution negative ion FAB-MS revealed the molecular formula to be C36H60O10. Another significant fragment ion peak was observed at m/z 505 [(M − H)−146]−, which revealed the elimination of one 6-desoxyhexosyl moiety. Full assignment of the 1H and 13C signals of 1 were accomplished through analysis of the 1H-1H COSY, TOCSY, HSQC, HMBC () and NOESY () spectra. The 1H NMR spectrum of 1 showed signals for seven angular methyl groups as singlets [1.88 (H-23), 1.08 (H-24), 1.01 (H-25), 1.25 (H-26), 1.14 (H-27), 1.37 (H-29), 1.41(H-30)], one olefinic proton at δH 5.55 (1H, t, J = 4 Hz, H-12), five oxygen-bearing methyne protons at 3.06 (1H, t, J = 12 Hz, H-3), 4.46 (1H, m, H-15), 4.98 (1H, m, H-16), 4.85 (1H, d, J = 12 Hz, H-21) and 4.63 (1H, d, J = 12 Hz, H-22), and one primary alcoholic function at 4.51 (2H, m, H-28). In the NOESY experiment, cross-peaks between δH 4.46 (H-15) and δH 4.98 (H-16), and between δH 4.98 (H-16) and δH 4.51 (H-28), allowed the location of two secondary alcoholic functions at C-15 and C-16. The hydroxyl group at C-15 was confirmed by a correlation between δC 67.51 (C-15) and δH 1.14 (s) (H-27) in the HMBC experiment. In this spectrum, it was also observed the correlation between C-21 at δC 78.45 with the two methyl group signals at δH 1.37 (s, H-29) and 1.41 (s, H-30). A correlation in the HMBC experiment between H-28 at δH 4.51 and the anomeric signal at δC 102.8 suggested that a sugar was attached to C-28. The sugar was identified as l-rhamnose with the help of 1H-1H COSY and TOCSY spectra. The characteristic double signal for C-6′of l-rhamnose appeared at δH 1.64 (J = 8 Hz, ).
The relative configurations of C-3, C-15, C-16, C-21 and C-22 of the R1-barrigenol unit were determined by the multiplicity and the coupling constants of carbinol protons, supported by the connectivity observed in the NOESY spectrum: H-3, H-5 and H-24; H-15, H-16, H-26 and H-28; H-22 and H-30; and H-21 and H-29. On the basis of the above results, the structure of 1 was elucidated as 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol, a new glycoside triterpene. Acid hydrolysis of 1 with 2 N HCl yielded 1a as an aglycone, together with l-rhamnose identified by GC/MS analysis of their trimethylsilyl derivative (Kirmizigül et al., Citation1995). The structure of the aglycone of 1 was thus recognized to be the triterpene R1 barrigenol and it was in agreement with the literature data (Fu et al., Citation2005).
Discussion
According to obtained results on the neuropharmacological evaluation of T. sylvatica fruits, it is not possible to consider that this plant possesses a convincing CNS depressant effect. Despite the aqueous and methanol extracts showed sedative effect in two very well-known sedative models, and these results apparently are in agreement with a previous work (Molina et al., Citation1999). The aqueous extract obtained from T. sylvatica seeds was the most active extract, but it was also the most toxic extract for mice. The neurotoxicity was also evidenced for this extract because it decreased the loco-motor activity, disturbed the motor coordination and decreased the exploratory behavior with signs of discomfort for the animals. These results suggest that sedative effect for this plant might be caused more by a toxic effect than by an effect on the CNS. The fruits of T. sylvatica are used to prepare the decoctions used as sedative traditional remedies. These fruits are constituted by 40% of seeds and 60% of pericarp. We were interested in knowning if seeds or pericarp showed the same activity; therefore, we studied separately seeds and pericarp, finding that seeds are more active than pericarp.
From the aqueous seeds extract, the glycoside triterpenoid 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol was isolated as a new natural product. This substance showed also CNS depressant and toxic effect (LD50 = 1.11 ± 0.23 mg/kg). Therefore, these results suggested that this substance is responsible for the sedative and toxic effects of T. sylvatica seeds. A preliminary HPLC analysis of the extracts of T. sylvatica showed that this glycoside triterpenoid is present in methanol and aqueous extracts of T. sylvatica seeds but it was not detected in the extracts of pericarp. Therefore, the activity of this plant may be related to the presence of this glycoside.
Previous pharmacological work did not report any toxic effect for this species, probably since the evaluation was performed in an acute single doses regimen and the experiments were performed in a time less than 3 h (Balderas et al., Citation2008). On the other hand, the repeated oral administration for 5 d of aqueous extract was less active and less toxic than the single intraperitoneal administration. These results suggest this compound is poorly absorbed from the gastrointestinal tract.
On the other hand, jacaranone was identified as the sedative principle in T. pringlei (Lozada-Lechuga et al., Citation2010). However, Xu and coworkers reported neurotoxic effects for this natural quinone that lead to death of mice in 30–90 min, with a LD50 of 150–200 mg/kg (Xu et al., Citation2003). Here, we are reporting the triterpene glycoside 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol as a new natural product and as the second one sedative and toxic principle in Ternstroemia genus. Toxic effect seems to be the major effect of fruits of these medicinal plants, and the neuropharmacological effects observed here and those previously reported might be signs of this toxic effect. These findings highlight the need to consider not only the pharmacological effect to support the traditional use of the medicinal plants but also to take in count their toxicity.
In conclusion, we demonstrated that the aqueous extract of T. sylvatica seeds, which represent 40% of the fruits, has toxic activity rather than CNS depressant activity in mice and that this effect might be related to the presence of 28-O-[β-l-6-rhamnopyranosyl]-R1-barrigenol, a new triterpenoid glycoside.
Declaration of interest
There are no conflicts interest related with the present investigation.
Acknowledgements
This work was supported by a grant from DGAPA IN210112 and CONACYT 82613. José Luis Balderas acknowledges fellowship from CONACYT (116413).
References
- Aguilar-Santamaría L, Tortoriello J. (1996). Anticonvulsant and sedative effects of crude extracts of Ternstroemia pringlei and Ruta chalapensis. Phytother Res 10:531–3
- Balderas J, Reza V, Ugalde M, et al. (2008). Pharmacodynamic interaction of the sedative effects of Ternstroemia pringlei (Rose) Standl. with six central nervous system depressant drugs in mice. J Ethnopharmacol 119:47–52
- Bartholomew B, McVaugh R. (1997). Identification and typification of Ternstroemia lineata de Candolle (Theaceae). Novon 7:14–16
- Boissier J, Simon P. (1962). La reaction d'exploration chez la souris. Therapie 17:1225–32
- Boom B. (1989). New species of Ternstroemia (Theaceae) from Guayana Highland. Brittonia 41:136–42
- Fu G, Wang Y, Gao S, et al. (2005). Five new cytotoxic triterpenoid saponins from the roots of Symplocos chinensis. Planta Med 71:666–72
- González-Trujano M, Navarrete A, Reyes B, Hong E. (1998). Some pharmacological effects of the ethanol extract of leaves of Annona diversifolia on the central nervous system in mice. Phytother Res 12:600–2
- Hiller K, Zetler G. (1996). Neuropharmacological studies on ethanol extracts of Valeriana officinalis L.: Behavioral and anticonvulsant properties. Phytother Res 10:145–51
- Ikuta A, Tomiyasu H, Morita Y, Yoshimura K. (2003). Ursane- and oleanane-type triterpenes from Ternstroemia gymnantheracallus tissues. J Nat Prod 66:1051–4
- Jo Y, Suh J, Shin M, et al. (2005). Jacaranone and related compounds from the fresh fruits of Ternstroemia japonica and their antioxidative activity. Arch Pharm Res 28:885–8
- Kikuchi K, Yamaguchi M. (1974). The structure of ternstroemiaxantina, a new aldehydic C40-carotenoid. B Chem Soc Jpn 47:885–7
- Kirmizigül S, Hüseyin H, Rose M. (1995). Triterpenoid glycosides from Cephalaria transsylvanica. Phytochemistry 39:1171–4
- Kobuski C. (1942). Studies in the Theaceae – XIII. Notes on the Mexican and Central American species of Ternstroemia. J Arnold Arboretum 23:464–78
- Lozada-Lechuga J, Villareal M, Fliniaux M, et al. (2010). Isolation of jacaranone, a sedative constituent extracted from the flowers of the Mexican tree Ternstroemia pringlei. J Ethnopharmacol 127:551–4
- Molina M, Contreras C, Téllez-Alcántara P, Rodríguez F. (1999). Sedative action of Ternstroemia sylvatica in the male rat. Phytomedicine 6:115–18
- Oliva I, González-Trujano M, Arrieta J, et al. (2004). Neuropharmacological profile of hydroalcoholic extract of Valeriana edulis ssp. procera roots in mice. Phytother Res 18:290–6
- Rolland A, Fleurentin J, Lanhers MC, et al. (1991). Behavioral effects of the American traditional plant Eschscholzia californica: Sedative and anxiolytic properties. Planta Med 57:212–16
- Shin M, Wang W, Nam K, et al. (2003). Triterpenoid saponins from the fruits of Ternstroemia japonica. J Nat Prod 66:1351–5
- Tori M, Fukuyama H, Nakashima K, Sono M. (2005). Degraded terpenoids and aromatic compounds from Ternstroemia gymnanthera. Lett Org Chem 2:262–4
- Tortoriello J, Romero O. (1992). Plants used by Mexican with presumable sedative properties: An ethnobotanical approach. Arch Med Res 23:111–16
- Ugalde M, Reza V, González-Trujano ME, et al. (2005). Isobolographic analysis of the sedative interaction between six central nervous system depressant drugs and Valeriana edulis hydroalcoholic extract in mice. J Pharm Pharmacol 57:631–9
- Walsh R, Cummins R. (1976). The open-field test: A critical review. Psychol Bull 83:482–504
- Xu H, Zhang N, Casida JE. (2003). Insecticides in Chinese medicinal plants: Survey leading to Jacaranone, a leurotoxicant and glutathione-reactive quinol. J Agric Food Chem 51:2544–7