Abstract
Context: The aqueous extracts of Cetraria islandica (L.) Ach. (Parmeliaceae) is traditionally used in many countries against a number of conditions, including inflammatory conditions.
Objective: The present study aimed to assess, for the first time, the effectiveness of C. islandica in cultured primary blood cells of Type 1 diabetes subjects.
Materials and methods: Diabetic and control blood samples were treated with or without aqueous lichen extract (5 and 10 μg mL−1) for 48 h. The activity of antioxidant enzymes in erythrocytes and also malondialdehyde levels in plasma were determined to evaluate the oxidative status. DNA damages were analyzed by SCE, MN and comet assays in cultured human lymphocytes. Additionally, proliferation index (PI) was evaluated in peripheral blood lymphocytes.
Results: There were significant increases in observed total DNA damage (comet assay) (240.2%) and SCE (168.8%), but not in MN frequencies of cultures with diabetes as compared (p > 0.05) to controls. Whereas, the significant reductions of total DNA damage (69.2 and 65.3%) and SCE frequencies (17.7 and 12.3%) were determined when the 5 and 10 mg mL−1 lichen extract was added to the cell culture medium, respectively. However, lichen extract did not completely inhibit the induction of SCEs in lymphocytes of patients with diabetes. C. islandica extract was also useful on PI rates.
Discussion: In conclusion, the antioxidant role of C. islandica in alleviating diabetes-induced genomic instability and for increasing cell viability was firstly indicated in the present study.
Introduction
Lichens are ecologically obligate symbiotic associations between a fungus (mycobiont) and at least one photosynthetic organism (photobionts; an alga, a cyanobacterium or both) (Widmer et al., Citation2012). We documented that they are effective in the treatment of tuberculosis (Vartia, Citation1973), hemorrhoids and dysentery (Dülger et al., Citation1998) and induce apoptosis in colon (Bezivin et al., Citation2003; Ren et al., Citation2009) and prostate cancers (Mitrović et al., Citation2011; Russo et al., Citation2006). The lichens also have antioxidant, antimicrobial and anticancer properties (Cansaran, Citation2010; Cansaran et al., Citation2007; Halici et al. Citation2005; Odabasoglu et al., Citation2006; Kosanic & Rankovic, Citation2011; Zambare & Christopher, Citation2012). Unique lichen flora has attracted many researchers on a systematic basis (Aslan et al., Citation2002). It is pointed out that lichens may be easily accessible sources of natural drugs that could be used as a possible food supplement or in the pharmaceutical industry (Archer et al., Citation2008).
Type 1 diabetes (T1D) is a chronic condition with a rising incidence worldwide in developed as well as in developing countries (Malerbi et al., Citation2012). Because of the early age of onset and longer diabetes duration, children and adolescents are at risk for developing diabetes-related complications at a younger age. As these youth age, this profoundly affects their productivity, quality of life, and life expectancy and increases health care costs (Imperatore et al., Citation2012). Over the past two decades our increased understanding of the pathogenesis of this disease has led to the development of new treatments. Oxidative stress has been implicated in the T1D pathogenesis (Bhatti et al., Citation2011; Lao-ong et al., Citation2012). In recent years, there has been a growing interest in the antioxidant defense system as a regulator of disease development. This opens up an important strategy for therapy of diabetes and may provide a promising avenue for future approaches to lichens. In response to pharmacological activation or oxidative stress, we studied well-known lichen species Cetraria islandica Ach. (1830) (Parmeliaceae) in relation to the survival of the blood cells in subjects with T1D. In many countries, C. islandica is used medicinally, e.g., for colds, bronchitis, and asthma (Safonova et al., Citation1999; Senchilo & Senchilo, Citation2004) and is studied for antioxidant properties with in vitro conditions (Kotan et al., Citation2011; Odabasoglu et al., Citation2006). The medicine is usually taken in the form of a tea (decoction or infusion) of the dry lichen. C. islandica extract demonstrates hepatoprotective and also immune-stimulating effects in blood tissue (Cernescu et al., Citation2011a; Freysdottir et al., Citation2008). Our study investigated the efficacy of C. islandica against diabetes-induced DNA damage with in vitro conditions. Firstly, some oxidative parameters including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) and malondialdehyde (MDA) which are used to monitor the development and extent of damage due to oxidative stress in human blood were investigated. Secondly, genotoxicity using single cell gel electrophoresis (SCGE or comet), sister-chromatid exchange (SCE), micronucleus (MN) assays, which are rapid and sensitive methods for measuring the genetic damage, were evaluated. In addition, the proliferation index (PI) rate was assessed to provide information on cell viability. Ultimately, the data may be used to devise strategies to reduce the risk of T1D in humans. To our knowledge, these results provide for the first time cellular information of C. islandica on blood of humans with T1D.
Materials and methods
Lichen extract and dose adjustment
C. islandica was collected from the Giresun, Erzurum and Artvin province in Turkey, during summer of 2009. The samples were identified using various flora books and papers (Aslan, Citation2000; Aslan et al., Citation2002). The identified sample was air-dried and stored in the herbarium of Kazım Karabekir Education Faculty. For water extraction of lichen, 20 g sample was mixed with 400 mL distillated and boiling water using magnetic stirrer for 15 min. Then the extract was filtered over Whatmann No. 1 paper.
Experimental design and protocol
The study described effects of C. islandica in blood cultures of patients with T1D. Thus, this study is divided into two sections. Section 1: The assessment of antioxidant role of C. islandica on T1D-induced oxidative stress and Section 2: Study of effectiveness of C. islandica on T1D-induced DNA damage.
Blood samples were obtained by vein puncture from 25 children (age 10–18 years) diagnosed with T1D (disease duration <18 months) and also age and sex matched control subjects. Human peripheral blood lymphocyte cultures were set up according to a slight modification of the protocol described by Evans and O’Riordan (Citation1975). The heparinized blood (0.5 mL) was cultured in 5 mL of culture medium (Chromosome Medium B, Biochroms, Berlin) with 5 mg mL−1 of phytohemagglutinin (Biochrome®, Berlin, Germany). C. islandica extract (5 and 10 μg mL−1) was added to the cultures just before incubation for biochemical and cytogenetic analysis. The experiments were performed on different groups as follows: T1D alone, C. islandica alone and T1D plus C. islandica. The dosages of the lichen extract were determined by preliminary dose-response work in our laboratory and also the literature data (Kotan et al., Citation2011). The study was performed in accordance with the Declaration of Helsinki and with the approval of the local ethics committee. In the patients with T1D, after supplementation of C. islandica, the blood was incubated for 2 h at 37 °C to adjust body conditions, except for testing comet, SCE, MN and PI (see below). Each individual whole blood culture without disease or lichen extract was studied as a control group (n = 8).
Erythrocytes
Erythrocytes were obtained from heparinized blood samples by centrifugation (2500 g, for 20 min) at 4 °C. The red cells were then washed three times with 5 volumes of phosphate buffered saline (PBS; 150 mmol L−1 NaCl, 1.9 mmol L−1 NaH2PO4, 8.1 mmol L−1 Na2HPO4, pH 7.4), and with a ratio of 1:1 divided in appropriate aliquots and stored at −80 °C until further analysis.
Biochemical methods
SOD activity
SOD activity was determined by the method of Misra and Fridovich (Citation1972), which is based on the ability of superoxide dismutase to inhibit the process of epinephrine self-oxidation in alkaline medium. In the reaction of colored adrenochrome formation, the superoxide anion-radical is formed as an intermediate product. Erythrocyte SOD activity was measured by monitoring the increase in the absorbance at 480 nm.
CAT activity
CAT was determined in erythrocytes by the method of Aebi (Citation1984). To 3 mL H2O2 (54 mM H2O2 in 50 mM phosphate buffer, pH 7.0), 5 μL of a catalase solution was added and the decrease in H2O2 was measured spectrophotometrically (Beckman DU 500, USA) at 240 nm for 60 s at 25 °C. In the erythrocyte preparations, hemolysates were centrifuged (9000 g) and estimation of activity was made with 1% hemolysates. One unit of catalase activity was defined as the activity required for degrade 1 μmol hydrogen peroxide in 60 s.
GSH-Px activity
GSH-Px activity of erythrocytes was measured using hydrogen peroxide as substrate (Carlberg & Mannervik, Citation1972). Potassium azide was added to inhibit catalase. Potassium ferricyanide was added to inhibit the pseudo-peroxidase activity of hemoglobin. Conversion of NADPH was monitored continuously in a spectrophotometer at 340 nm for 3 min at 25 °C.
MDA level
The content of MDA was measured in plasma preparations by the thiobarbutiric acid (TBA) method which was modified from methods of Satoh (Citation1978) and Yagi (Citation1984). Peroxidation was determined as the production of MDA that, in combination with TBA, forms a pink chromogen compound whose absorbance at 532 nm was measured.
Cytogenetic analysis
SCE assay and proliferation index
With the aim of providing a better visualization of SCEs, 5-bromo-2-deoxyuridine (Sigma®, St. Louis, MO, final concentration 20 mM) was added after culture initiation. The cultures were incubated in complete darkness for 72 h at 37 °C. Exactly 70 h and 30 min after beginning of the incubations, colcemid (Sigma®) was added to the cultures to achieve a final concentration of 0.5 mg L−1. After hypotonic treatment (0.075 M KCl) followed by three repetitive cycles of fixation in methanol/acetic acid solution (3.1, v/v), centrifugation and resuspension. The cell suspension was dropped on to chilled and grease-free microscopic slides, air-dried, aged and then differentially stained for inspection of the SCE rate according to the fluorescence plus Giemsa (FPG) procedure (Perry & Wolff, Citation1974). For each treatment condition, 25 well-spread second division metaphases were scored and the values obtained were calculated as SCEs per cell.
In addition to SCEs, cells were analyzed for the relative frequency of first-division metaphases (M1; identifiable by uniform staining of both sister chromatids), second-division metaphases (M2; identifiable by differential staining of the sister chromatids), and third- and subsequent division metaphases (M3; identifiable by nonuniform pattern of staining). PI is the average number of replications completed by metaphase cells and is calculated as follows:
MN assay
The MN test was performed by adding cytochalasin B (Sigma®; final concentration of 6 µg mL−1) after 44 h of culture. At the end of the 72 h incubation period, the lymphocytes were fixed with ice-cold methanol acetic acid (1:1). The fixed cells were put directly on slides using a cytospin, and stained with May Grünwald-Giemsa. All slides were coded before scoring. The criteria for scoring micronuclei were as described by Fenech (Citation1993). At least 2000 binucleated lymphocytes were examined per concentration (two cultures per concentration) for the presence of one, two or more micronuclei.
Comet assay
The comet assay also known as single cell gel electrophoresis (SCGE) was performed and scored according to Singh et al. (Citation1988), Banu et al. (Citation2001) and Prabhavathy Das et al. (Citation2006). The cultures were set up by incubating lymphocytes for 72 h with C. islandica in diabetic groups. The control cultures were set up by incubating lymphocytes with the phosphate buffered saline (PBS) (at a final concentration of 1%). Ten milliliters of the 100 mL aliquots of the lymphocytes treated as above along with untreated samples were mixed with 120 mL of 0.5% low melting agarose and layered on the surface on glass slides previously coated with 140 mL of 1% normal melting agarose. After the application of cover slips, the slides were allowed to gel at 4 °C for 20 min. After carefully removing the cover slips, a second layer of 0.5% low-melting agarose was pipetted on to the slides and allowed to gel for a further 20 min at 4 °C. The slides were immersed in freshly prepared coldly lysing solution and refrigerated overnight followed by alkali treatment, electrophoresis and neutralization. The dried slides were then stained using silver nitrate solution after appropriate fixing. The whole procedure was carried out in dim light to minimize artifactual. DNA damage analysis was performed at a magnification of 100× using a light microscope after coding the slides. A total of 100 cells were screened per slide. A total damage score for each slide was derived by multiplying the number of cells assigned to each grade of damage by the numeric value of the grade and summing over all grades (giving a maximum possible score of 400, corresponding to 100 cells at grade 4).
Statistics
The statistical analysis of experimental values was performed by one-way analysis of variance (ANOVA) and Duncan’s test using the SPSS 13.0 software the level of 0.05 was regarded as indicative of statistical significance for all tests
Results
The effects of C. islandica on biochemical parameters in blood cultures of diabetic patients are present in . As compared with the controls, the activity of SOD, CAT and also GSH-Px enzymes were markedly decreased in erythrocytes of patients with T1D while MDA increased (p < 0.05). In the alone group, the C. islandica extract significantly increased the level of antioxidant enzymes at both dosage (5 and 10 μg mL−1) but the effect of extract on the parameters was not dose related. As presented in , lichen extracts did not affect the MDA level measured in the human blood plasma. Noteworthy, C. islandica brought the antioxidant activity of erythrocytes closer to the control levels in blood cultures with T1D. The increase of antioxidant capacity was significant statistically in diabetic groups (p < 0.05) and MDA level returned to the control levels. However, the effect of extract on the MDA was not dose related (data not shown) ().
Table 1. The effects of C. islandica extract on biochemical parameters in healthy or diabetic human blood cultures.
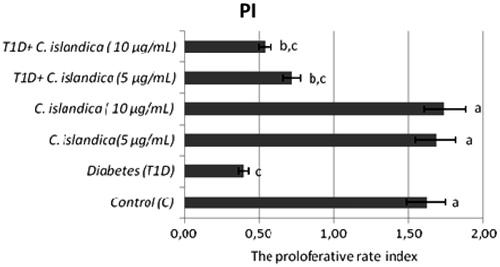
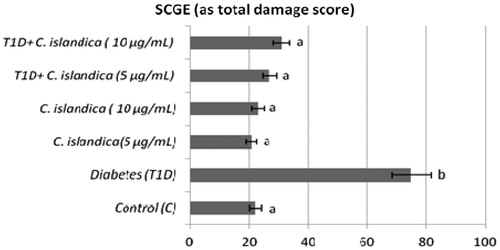
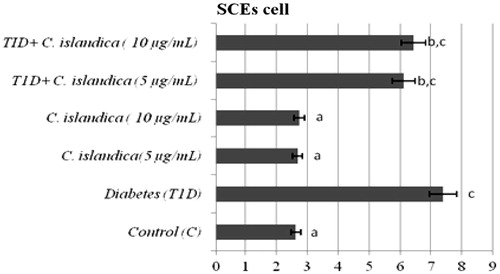
The frequency of MN, SCEs, PI and the rate of primary DNA damage (SCGE) in human lymphocytes were given in , respectively. In T1D + C. islandica groups, separately and together, it was determined that the values of MN were near to control groups (p > 0.05) (). Diabetes caused to generate a significant SCE rate in human lymphocytes as compared with controls (p < 0.05). We also compared SCE frequency in controls with and without C. islandica and did not find any significant difference in the frequency of SCE. On the contrary, C. islandica at both doses reduced the SCE frequency, but the reduction of the SCEs by lichen extract was sought in the blood with T1D (p > 0.05) (). Regarding PI values, our results showed that treatments with C. islandica alone did not alter PI values compared to the control. Whereas, a statistically important decrease in the rate of PI was observed in patients with DM. However, the treatments with C. islandica (at both concentrations) increased the rate of PI as compared to the group with T1D alone (). In the present study, comet assay as DNA damage analysis was performed and quantified with regard to diabetes and C. islandica administration (). It was shown that the level of primary DNA damage significantly increased with T1D (p > 0.05), whereas there were no differences between controls and C. islandica groups. At the concentrations of C. islandica, it was clearly being seen that the rate of DNA damage was significantly decreased against T1D (p < 0.05). Thus, C. islandica was established to display beneficial effects on DNA, by returning these values close to those of the control group after T1D disease. However, the lichen extract did not show any evidence of dose-related effect (data not shown).
Figure 1. The frequencies of MN in cultured human lymphocytes from diabetic patients and control groups. Values are means ± standard deviation; n = 8. Different letters within each bar present a statistical difference by Duncan multiple range tests at p < 0.05.
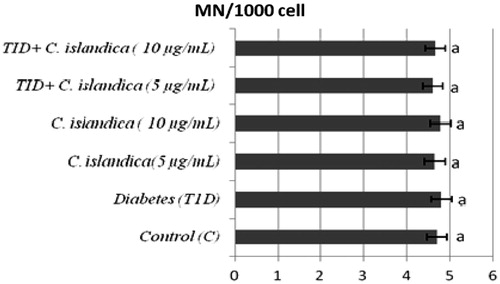
Figure 2. The frequencies of SCEs in cultured human lymphocytes from diabetic patients and control groups. Abbreviations are as defined in .
Discussion
This study provides the first evidence that C. islandica has a protective role against T1D-induced oxidative stress and genomic instability in human blood. For T1D is an important observation that this disease increases oxidative stress in tissues (Perez-Gutierrez & Damian-Guzman, Citation2012; Tan et al., Citation2007). Our results are in agreement with previous studies in which T1D increases plasma level of MDA, an important biological marker of lipid peroxidation (Bulum & Duvnjak, Citation2011; Yousefi et al., Citation2011). And it is suggested that the positive relationship between MDA and T1D is significant because disease leads to increased production of ROS and it may also involve lipid peroxidation (Országhová et al., Citation2009). Under these conditions, diabetes may induce vascular dysfunction and hypertension (Naka et al., Citation2012). On the other hand, lichens has been used for centuries in traditional medicine and, especially over the last decade, many works dealing with lichens have been published, but it still is a matter of investigation by researchers of different properties (Valente et al., Citation2011). Those are the reasons why we decided to investigate the role of C. islandica in both the effects on oxidative stress of T1D and its effect on DNA damages in blood cells. The human body possesses a range of antioxidant components, which are distributed among biological fluids, tissues and cell compartments (Simões-Ambrosio et al., Citation2010). Both enzymatic and non-enzymatic antioxidants play an important role in inhibiting and scavenging free radicals, thus providing protection to humans against infectious and degenerative diseases (Nader et al., Citation2010). The oxidative stress develops when the levels of antioxidants are lowered (Tapiero et al., Citation2004). We have considered whether the effectiveness of C. islandica in response to T1D is associated with the induction of antioxidant activity. Our findings revealed that there were statistically significant differences in MDA level between the T1D group and T1D + C. islandica group. In the current study, it was also demonstrated that lichen extract administration in T1D group significantly decreased the level of MDA without depending on the dose. From previous reports, C. islandica extract determined adaptogenic-antistress effects, confirmed by its actions on oxidative stress parameters as MDA in laboratory animals (Cernescu et al., Citation2011b). Recent studies suggested a strong antioxidant effect of lichen extract that could ameliorate oxidative stress (Kosanic & Rankovic, Citation2011). The effects of antioxidants are pronounced in erythrocytes because these cells have increased amounts of hemoglobin and iron ions and consequently they are under higher oxidative stress (Vitturi et al., Citation2013). The results obtained in vitro suggest that the membrane fragility increases under oxidative stress conditions for the patient RBCs and the protection effect of antioxidants is due to their antioxidant properties (Moreira et al., Citation2011).
The protective effect of C. islandica can be explained by both the direct scavenging of free radicals produced by the indirect effect and the activation of oxidative repair enzymes. Gülçin et al. (Citation2002) have reported that C. islandica has strong superoxide radical scavenging activity on peroxidation of linoleic acid. Thus, authors have underlined the occurrence of alterations in antioxidants upon the administration of C. islandica. It is known that the phenolic compounds of lichen may contribute directly to antioxidative action (Duh et al., Citation1999). And these compounds have inhibitory effects on mutagenesis and carcinogenesis in humans, when up to 1.0 g daily ingested from a diet rich in fruits and vegetables (Tanaka et al., Citation1988). In the present study, C. islandica had a pronounced effect on the T1D. In our study, some important key enzymes, such as erythrocyte SOD, CAT and GSH-Px can be involved in the inhibitory effect of ROS generation, which shall be further investigated. In previous studies erythrocyte SOD and GSH-Px activities were measured. The administration of C. islandica concluded to increase activities of these enzymes and thereby to result in the alleviation of oxidative stress (Kotan et al., Citation2011).
As known, antioxidant capacity comes from non-enzymes like glutathione (GSH), as well as enzymes such as SOD, CAT and GSH-Px (Murri et al., Citation2010). T1D included vascular abnormalities, associated with the reduction of SOD, CAT and GSH-Px activities (Yang et al., Citation2013). SOD is the primary step of the defense mechanism in the antioxidant system against oxidative stress. It catalyzes the dismutation of superoxide radicals () into molecular oxygen (O2) and H2O2 (Kakarla et al., Citation2005). CAT activity is decreased in erythrocytes during T1D and its activity is inhibited by the superoxide radical (Shailey & Basir, Citation2012). The suppression of these enzyme activities has been linked to induction of oxidative stress (Stoppa et al., Citation2006). In addition, the observed decreases in GSH-related enzyme activities indicate that diabetes may induce oxidative stress in human blood by altering GSH metabolism (Yavari & Azizova, Citation2012). GSH-Px can protect DNA and lipids of the cell against the peroxidation products (Han, Citation2011; Kostic et al., Citation2007). The observed decline in the activity of GSH-Px in the blood of DM patients may be ascribed to the reduction in the level of the GSH and an increase in the level of peroxides (Al-Shebly & Mansour, Citation2012). GSH is one of the most abundant intracellular antioxidants in animal cells. Since it is considered to be one of the most abundant tripeptides in human organism and its action against oxidative radicals is well known. Thus, the balance of GSH-Px enzyme system may also be essential to remove superoxide anion and peroxides generated in erythrocytes (Allen & Bradley, Citation2011).
The enzyme inactivations induced by T1D also corroborate by the genotoxic findings. The results obtained by us indicate a significant increase in the ratios of the SCEs in lymphocytes, which is in accordance with the previous reports (Cinkilic et al., Citation2009; Sheth et al., Citation2006). The SCEs are formed by toxic oxygen metabolites in cultured human leukocytes and other mammalian cells (Madrigal-Santillán et al., Citation2010). T1D is related to LPO and oxidation of DNA in vivo and in vitro (Chistiakov et al., Citation2012). So this disease could contribute to the formation of the genome leading to carcinogenesis (Dobrila-Dintinjana et al., Citation2012). Lipid peroxides enter the nucleus where they react with Fe+2 to generate the alkoxyl radical which attacks DNA (Fraga & Tappel, Citation1988). Also, intracellular calcium levels increase as a result of oxidative damage to cell membranes, calcium then enters the nucleus where it can activate nucleases which cause DNA strand breaks (McConkey et al., Citation1989). DNA damage and defective DNA repair cause SCEs (Dumitrache et al., Citation2011). In this study, the abnormal signs of T1D on blood cells were dramatically and dose-dependently unchanged by C. islandica. Hence the rates of SCEs were not unfortunately fully decreased in lymphocyte cells.
MNs are the results of acentric fragments or lagging chromosomes that fail to incorporate into either of the daughter nuclei during telophase of the mitotic cells (Albertini et al., Citation2000). In the present study, there were no differences in MN frequencies in the lymphocytes of T1D patients when compared with controls. Our current data confirm and expand previous findings by showing that patients with T1D have unchanged levels of MNs (Cinkilic et al., Citation2009).
Notably, previous studies of oxidative damage in DM patients have used tests, including the comet assay, to investigate DNA damage (Ross, Citation2011; Sardas et al., Citation2001). Diabetic patients have increased DNA damage as identified by the comet assay, and this damage may cause an increase in the risk of cancer (Bonassi et al., Citation2007). Our results showed a significant increase in the ratios of the primary DNA damage in lymphocytes in accordance with the previous reports. Investigators reported oxidative DNA damage in the comet assay in both type-1 and type-2 diabetic patients (Palazzo et al., Citation2012; Sardas et al., Citation2001). Moreover, these authors reported that DNA damage as observed in the comet assay was high in individuals with T1D. The comet assay has been proposed to be the most sensitive procedure for detecting DNA fragmentation. Due to its simplicity and sensitivity, the comet assay has rapidly gained acceptance as a genotoxicity assay. Additionally, the comet assay has shown that strand breaks arise from DNA damage generated by oxidative stress. Furthermore, the comet assay has been found to be technically suitable for the routine measurement of DNA damage (Moller et al., Citation2000). Our study revealed that C. islandica extract significantly decreased the ratios of the primary DNA damage when the values were comparable with that of the T1D groups.
Growing evidence suggest that one of causes of increased level of oxidative DNA damage in diabetic patients is reduced antioxidant defense system (Piwowar et al., Citation2007). The biologically fundamental macromolecules such as nucleic acids and proteins in mammalian cells defend themselves with antioxidants (Kedziora-Kornatowska et al., Citation2004). At this point, our results showed that lichen extract exhibited antigenotoxic properties at concentrations ranged at 5 and 10 μg mL−1 concentrations. C. islandica, as mentioned above, supported the antioxidant defense mechanism against T1D. The results obtained in the present study elucidated that DM also induced a reduction in PI in human lymphocytes. These data were similar to the findings from earlier studies (Varga et al., Citation2011). PI has been used for the assessment of the cytostatic action of various therapeutic agents (Baka et al., Citation2009). C. islandica was useful and increased the PI, which was decreased by diabetes.
Conclusion
The biochemical and genotoxic tests dealing with C. islandica are used frequently in the food industry reveal safe results for a food supplement. The present study is the first report describing C. islandica’s ability to protect nuclear DNA from oxidative damage against T1D and it is speculated that protective effects of lichen extract are a consequence of its ability to reduce MDA formation and increase associated antioxidants. In conclusion, we suggest that it may be a chemopharmaceutical molecule of interest against T1D.
Declaration of interest
This investigation was supported by Atatürk University (BAP-2004/172 and 2008/76). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
We are grateful to volunteers for the blood samples.
References
- Aebi H. (1984). Catalase in vitro. In: Packer L, ed. Methods in Enzymology. Orlando, FL: Academic Press, 1–126
- Al-Shebly MM, Mansour MA. (2012). Evaluation of oxidative stress and antioxidant status in diabetic and hypertensive women during labor. Oxid Med Cell Long 2012:1–6
- Albertini RJ, Anderson D, Douglas GR, et al. (2000). IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat Res 463:111–72
- Allen J, Bradley RD. (2011). Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J Altern Complement Med 17:827–33
- Archer D, Connerton I, MacKenzie D. (2008). Filamentous fungi for production of food additives and processing aids. Food Biotechnol 111:99–147
- Aslan A. (2000). Lichens from the regions of Artvin, Erzurum, and Kars (Turkey). Israel J Plant Sci 48:143–8
- Aslan A, Yazıcı K, Karagöz Y. (2002). Lichen flora of the Murgul district, Artvin, Turkey. Israel J Plant Sci 50:77–81
- Baka S, Ekonomopoulou MT, Kosmidis C, et al. (2009). Cytogenetic effects of recombinant interferon-gamma on lymphocyte cultures from patients with non-small cell lung cancer. Cancer Genet Cytogenet 193:38–43
- Banu BS, Devi KD, Mahboob M, Jamil K. (2001). In vivo genotoxic effect of zinc sulfate in mouse peripheral blood leukocytes using comet assay. Drug Chem Toxicol 24:63–73
- Bezivin C, Tomasi S, Lohezic-Le Devehat F, Boustie J. (2003). Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 10:499–503
- Bhatti R, Sharma S, Singh J, Ishar MP. (2011). Ameliorative effect of Aegle marmelos leaf extract on early stage alloxan-induced diabetic cardiomyopathy in rats. Pharm Biol 49:1137–43
- Bonassi S, Znaor A, Ceppi M, et al. (2007). An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis 28:625–31
- Bulum T, Duvnjak L. (2011). Relationship between nonalcoholic fatty liver disease markers and renal function in patients with type 1 diabetes. Acta Med Croatica 65:6–10
- Cansaran D. (2010). Türkiye’de bazı liken türlerindeki usnik asitin hplc yöntemi ile değerlendirilmesi ve antimikrobiyal aktiviteleri. Türk Hijyen ve Deneysel Biyoloji Dergisi 66:153–60
- Cansaran D, Atakol O, Halıcı MG, Aksoy A. (2007). HPLC analysis of the usnic acid in some Ramalina species from Anatolia and investigation of their antimicrobial activities. Pharm Biol 45:77–81
- Carlberg I, Mannervik B. (1972). Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–80
- Cernescu I, Tarţău L, Macavei A, Lupuşoru CE. (2011a). Effects of a Cetraria islandica extract in monotherapy and in association with magnesium in an experimental-induced hepatopathy model. Revista medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi 115:1195–9
- Cernescu I, Tarţău L, Macavei A, Lupuşoru CE. (2011b). Experimental research on the effects of a Cetraria islandica extract on oxidative stress in laboratory animals. Revista medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi 115:899–904
- Chistiakov DA, Sobenin IA, Bobryshev YV, Orekhov AN. (2012). Mitochondrial dysfunction and mitochondrial DNA mutations in atherosclerotic complications in diabetes. World J Cardiol 4:148–56
- Cinkilic N, Kiyici S, Celikler S, et al. (2009). Evaluation of chromosome aberrations, sister chromatid exchange and micronuclei in patients with type-1 diabetes mellitus. Mutat Res 676:1–4
- Dobrila-Dintinjana R, Vanis N, Dintinjana M, Radić M. (2012). Etiology and oncogenesis of pancreatic carcinoma. Coll Antropol 36:1063–7
- Duh PD, Tu YY, Yen GC. (1999). Antioxidant activity of water extract of harng jyur (Chrysanthemum morifolium Ramat). LWT-Food Sci Technol 32:269–77
- Dülger B, Gücin F, Aslan A. (1998). Cetraria islandica (L.) Ach. likeninin antimikrobiyal aktivitesi. Turkish J Biol 22:111–18
- Dumitrache LC, Hu L, Son MY, et al. (2011). Trex2 enables spontaneous sister chromatid exchanges without facilitating DNA double-strand break repair. Genetics 188:787–97
- Evans HJ, O’Riordan ML. (1975). Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. Mutat Res 31:135–48
- Fenech M. (1993). The cytokinesis–block micronucleus technique, a detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res 285:35–44
- Fraga CG, Tappel AL. (1988). Damage to DNA concurrent with lipid peroxidation in rat liver slices. Biochem J 252:893–6
- Freysdottir J, Omarsdottir S, Ingólfsdóttir K, et al. (2008). In vitro and in vivo immunomodulating effects of traditionally prepared extract and purified compounds from Cetraria islandica. Int Immunopharmacol 8:423–30
- Gülçin I, Oktay M, Küfrevioğlu OI, Aslan A. (2002). Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J Ethnopharmacol 79:325–9
- Halici M, Odabasoglu F, Suleyman H, et al. (2005). Effects of water extract of Usnea longissima on antioxidant enzyme activity and mucosal damage caused by indomethacin in rats. Phytomedicine 12:656–62
- Han C. (2011). Studies on tea and health. Wei Sheng Yan Jiu 40:802–5
- Imperatore G, Boyle JP, Thompson TJ, et al. (2012). Projections of type 1 and type 2 diabetes burden in the US population aged <20 years through 2050 dynamic modeling of incidence, mortality, and population growth. Diabetes Care 35:2515–20
- Kakarla P, Vadluri G, Reddy Kesireddy S. (2005). Response of hepatic antioxidant system to exercise training in aging female rat. J Exp Zool Part A Comp Exp Biol 303:203–8
- Kedziora-Kornatowska K, Czuczejko J, Pawluk H, et al. (2004). The markers of oxidative stress and activity of the antioxidant system in the blood of elderly patients with essential arterial hypertension. Cell Mol Biol Lett 9:635–41
- Kosanić M, Ranković B. (2011). Lichen as possible sources of antioxidants. Pak J Pharm Sci 24:165–70
- Kostić N, Caparević Z, Ilić S. (2007). Antioxidant status in type II diabetes mellitus patients with or without microvascular complications. Srp Arh Celok Lek 135:143–6
- Kotan E, Alpsoy L, Anar M, et al. (2011). Protective role of methanol extract of Cetraria islandica (L.) against oxidative stress and genotoxic effects of AFB1 in human lymphocytes in vitro. Toxicol Ind Health 27:599–605
- Lao-ong T, Chatuphonprasert W, Nemoto N, Jarukamjorn K. (2012). Alteration of hepatic glutathione peroxidase and superoxide dismutase expression in streptozotocin-induced diabetic mice by berberine. Pharm Biol 50:1007–12
- Madrigal-Santillán E, Morales-González JA, Vargas-Mendoza N, et al. (2010). Antigenotoxic studies of different substances to reduce the DNA damage induced by aflatoxin B1 and ochratoxin A. Toxins 2:738–57
- Malerbi FEK, Negrato CA, Gomes MB. (2012). Assessment of psychosocial variables by parents of youth with type 1 diabetes mellitus. Diabet Met Syndrome 4:48–52
- McConkey D, Hartzell P, Jondal M, Orrenius S. (1989). Inhibition of DNA fragmentation in thymocytes and isolated thymocyte nuclei by agents that stimulate protein kinase C. J Biol Chem 264:13399–402
- Misra HP, Fridovich I. (1972). The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–5
- Mitrović T, Stamenković S, Cvetković V, et al. (2011). Antioxidant, antimicrobial and antiproliferative activities of five lichen species. Int J Mol Sci 12:5428–48
- Moller P, Knudsen LE, Loft S, Wallin H. (2000). The comet assay as a rapid test in biomonitoring occupational exposure to DNA-damaging agents and effect of confounding factors. Cancer Epidemiol Biomarker Prev 9:1005–15
- Moreira LL, Dias T, Dias LG, et al. (2011). Propolis influence on erythrocyte membrane disorder (hereditary spherocytosis), a first approach. Food Chem Toxicol 49:520–6
- Murri M, Garcia-Delgado R, Alcázar-Ramirez J, et al. (2010). Assessment of cellular and plasma oxidative stress in SAHS patients before and after continuous positive airway pressure treatment. Clin Lab 56:397–406
- Nader MA, El-Agamy DS, Suddek GM. (2010). Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch Pharm Res 33:637–43
- Naka KK, Papathanassiou K, Bechlioulis A, et al. (2012). Determinants of vascular function in patients with type 2 diabetes. Cardiovasc Diabetol 11:127–36
- Odabasoglu F, Cakir A, Suleyman H, et al. (2006). Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J Ethnopharmacol 103:59–65
- Országhová Z, Liptáková A, Muchová J, et al. (2009). Influence of pyridoxylidene aminoguanidine on biomarkers of the oxidative stress and selected metabolic parameters of rats with diabetes mellitus. Gen Physiol Biophys 28:347–55
- Palazzo RP, Bagatini PB, Schefer PB, et al. (2012). Genomic instability in patients with type 2 diabetes mellitus on hemodialysis. Revista Brasileira de Hematologia e Hemoterapia 34:31–5
- Perez-Gutierrez RM, Damian-Guzman M. (2012). Meliacinolin, a potent α-glucosidase and α-amylase inhibitor isolated from Azadirachta indica leaves and in vivo antidiabetic property in streptozotocin-nicotinamide-induced type 2 diabetes in mice. Biol Pharm Bull 35:1516–24
- Perry P, Wolff S. (1974). New Giemsa method for the differential staining of sister chromatids. Nature 251:156–8
- Piwowar A, Knapik-Kordecka M, Warwas M. (2007). AOPP and its relations with selected markers of oxidative/antioxidative system in type 2 diabetes mellitus. Diabet Res Clin Pract 77:188–92
- Prabhavathy Das G, Pasha Shaik A, Jamil K. (2006). Cytotoxicity and genotoxicity induced by the pesticide profenofos on cultured human peripheral blood lymphocytes. Drug Chem Toxicol 29:313–22
- Ren MR, Hur JS, Kim JY, et al. (2009). Anti-proliferative effects of Lethariella zahlbruckneri extracts in human HT-29 human colon cancer cells. Food Chem Toxicol 47:2157–62
- Ross KA. (2011). Evidence for somatic gene conversion and deletion in bipolar disorder, Crohn's disease, coronary artery disease, hypertension, rheumatoid arthritis, type-1 diabetes, and type-2 diabetes. BMC Med 9:12
- Russo A, Piovano M, Lombardo L, et al. (2006). Pannarin inhibits cell growth and induces cell death in human prostate carcinoma DU-145 cells. Anticancer Drugs 17:1163–9
- Safonova MY, Sakanyan EI, Lesiovskaya EE. (1999). Cetraria islandica (L) Ach., khimicheskiyj sostav i perspektivih primeneniya medicine. Rastiteljnihe Resursih 35:106–15
- Sardas S, Yilmaz M, Öztok U, et al. (2001). Assessment of DNA strand breakage by comet assay in diabetic patients and the role of antioxidant supplementation. Mutat Res 490:123–9
- Satoh K. (1978). Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta 90:37–43
- Senchilo VI, Senchilo YV. (2004). Medicinal Plants of Belarus [in Russian]. Minsk: Belarus State University
- Shailey S, Basir SF. (2012). Strengthening of antioxidant defense by Azadirachta indica in alloxan-diabetic rat tissues. J Ayurveda Integ Med 3:130–5
- Sheth FJ, Patel P, Vaidya ADB, et al. (2006). Increased frequency of sister chromatid exchanges in patients with type II diabetes. Curr Sci 90:236–40
- Simões-Ambrosio L, Gregório L, Sousa J, et al. (2010). The role of seasonality on the inhibitory effect of Brazilian green propolis on the oxidative metabolism of neutrophils. Fitoterapia 81:1102–8
- Singh NP, McCoy MT, Tice RR, Schneider EL. (1988). A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–91
- Stoppa GR, Cesquini M, Roman EA, et al. (2006). Aminoguanidine prevented impairment of blood antioxidant system in insulin-dependent diabetic rats. Life Sci 78:1352–61
- Tan ALY, Forbes JM, Cooper ME. (2007). AGE, RAGE, and ROS in diabetic nephropathy. Semin Nephrol 27:130--43
- Tanaka M, Kuei CW, Nagashima Y, Taguchi T. (1988). Application of antioxidative maillard reaction products from histidine and glucose to sardine products. Bull Japan Soc Sci Fish 54:1409--14
- Tapiero H, Townsend D, Tew K. (2004). The role of carotenoids in the prevention of human pathologies. Biomed Pharmacother 58:100–10
- Valente MJ, Baltazar AF, Henrique R, et al. (2011). Biological activities of Portuguese propolis, protection against free radical-induced erythrocyte damage and inhibition of human renal cancer cell growth in vitro. Food Chem Toxicol 49:86–92
- Varga T, Somogyi A, Barna G, et al. (2011). Higher serum DPP-4 enzyme activity and decreased lymphocyte CD26 expression in type 1 diabetes. Pathol Oncol Res 17:925–30
- Vartia K. (1973). Antibiotics in lichens. In: Ahmadjian V, Hale ME, eds. The Lichens. New York: Academic Press, Inc., 547–61
- Vitturi DA, Sun CW, Harper VM, et al. (2013). Antioxidant functions for the hemoglobin β93 cysteine residue in erythrocytes and in the vascular compartment in vivo. Free Radic Biol Med 55:119–29
- Widmer I, Dal Grande F, Excoffier L, et al. (2012). European phylogeography of the epiphytic lichen fungus Lobaria pulmonaria and its green algal symbiont. Mol Ecol 21:5827–44
- Yagi K. (1984). Assay for blood plasma or serum. Methods Enzymol 105:328–31
- Yang H, Fan S, Song D, et al. (2013). Long-term streptozotocin-induced diabetes in rats leads to severe damage of brain blood vessels and neurons via enhanced oxidative stress. Mol Med Rep 7:431–40
- Yavari A, Azizova G. (2012). Effect of oxidative stress on immunological parameters in type 2 diabetes mellitus in the Azerbaijan Republic. Diabet Met Syndrome, Clin Res Rev 6:195–8
- Yousefi M, Rahimi H, Barikbin B, et al. (2011). Uric acid, a new antioxidant in patients with pemphigus vulgaris. Ind J Dermatol 56:278--81
- Zambare VP, Christopher LP. (2012). Biopharmaceutical potential of lichens. Pharm Biol 50:778–98