Abstract
Context: Bryostatins represent an important group of pharmaceutically promising substances. These compounds are produced by commensal microorganisms naturally occurring in marine invertebrates, mainly in bryozoans. The most frequently investigated substance is bryostatin-1, which is a highly oxygenated macrolide with a polyacetate backbone.
Objective: The aim of this work was to summarize documented preclinical and clinical effects of bryostatin-class compounds.
Methods: A literature search was made of Medline and Web of Science databases in 2012.
Results and conclusion: Our review showed that bryostatins are potent agonists of protein kinase C. In addition to this, their significant antineoplastic activity against several tumor types has also been established and described. Bryostatin's anticancer activity has been proved against various cancer types. Moreover, significant results have been achieved by using bryostatin-1 in combination with other therapies, including combination with vaccine testing. Concerning other important properties that bryostatins possess, their ability to sensitize some resistant cells to chemotherapy agents, or immunoactivity and further stimulating growth of new neural connections, and enhancing effect on long-term memory are worth mentioning. In particular, some new bryostatin analogs could represent potential therapeutic agent for the treatment of cancer and other diseases in future.
Introduction
Bryostatins are defined as a class of highly oxygenated macrolides possessing a 20-membered macrolactone ring, with a wide range of biological activities including clinically proven enhancement of cytotoxicity of other agents. In recent years, the biological effects of bryostatins have become a subject widely studied by a great number of research groups. Up to now, published results have shown that bryostatin modulates the activity of protein kinase C (PKC), exerts differentiation inducing effects in various in vitro and in vivo models, and also has immunomodulatory activities, including the induction of cytokine release and expansion of tumour-specific lymphocyte populations (Schwartsmann et al., Citation2001). The levels of all bryostatins so far isolated from natural sources have always been at the minuscule level and that is why the topic of the actual source of these macrolides has been discussed for years. Moreover, there is now significant evidence for a microbial involvement (Haygood et al., Citation1999).
Sources of bryostatin
It is a well-known fact that many marine invertebrates (sponges, tunicates, bryozoans and others) are important sources of biomedicinally available natural products (Ueno et al., Citation2012) and that a number of these bioactive substances produce commensal microorganisms (bacteria, cyanobacteria, algae) living in these animals. One important group of pharmaceutically perspective substances are compounds with anti-cancer effects, especially bryostatins, which are subjected to research including functional drug development or molecular genetic methods (Dunlap et al., Citation2007).
Bryostatins represent only one group of substances separated from invertebrate animals, produced by their bacterial symbionts and considered as important sources of biomedically relevant natural products. Bacterial symbionts are highly adapted up to specific conditions and have been resistant to cultivation. Thus, it is necessary to use other, culture-independent procedures (e.g., cell separation, gene cloning) which would enable to obtain several natural products, not only bryostatins but also other potential drug substances such as halichondrin and trabectedin (ET-743) (Piel, Citation2006).
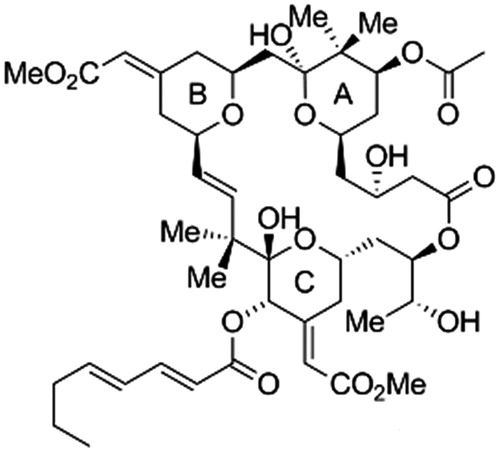
The most frequently investigated substance of bryostatin group is bryostatin-1 (), a highly oxygenated macrolide with a unique polyacetate backbone. It was originally isolated from the marine organism Bugula neritina L. (Bugulidae), often found in the form of moss-like colonies attached as a foulant to dock sides, pilings, buoys floats and vessel hulls, or occurring on rocks and shells (Banerjee et al., Citation2008). Only multigram quantities of highly pure bryostatin-1 were obtained from about 10 000 gallons (45 500 L) of Bugula neritina (Schaufelberger et al., Citation1991). Bryostatin has been extracted from bryozoan Bugula neritina since the late 1960s mainly from the area of the Gulf of California, Gulf of Mexico and from various locations on the eastern and western rims of the Pacific Ocean including Japan (Ueno et al., Citation2012). The extract analyses of 14 various marine animal species of the Gulf of Mexico have, among others, proved the occurrence of substances with bryostatin structure in other marine organisms of various taxonomy submission, which suggests that bryozoans may not necessarily be the one and only original source of bryostatins (Manning et al., Citation2005).
Figure 1. Structure of bryostatin-1 (Trost et al., Citation2011).
Pharmacological studies
It should be noted that bryostatin exhibited some extremely interesting biological activities from the very beginning of the testing. The fundamental part of the study of bryostatin is the investigation of pharmacokinetics, tissue distribution, metabolism and elimination from organism. The research was undertaken in mice, using [C26-H3]-labeled bryostatin-1 (Zhang et al., Citation1996) and its results suggest that bryostatin-1 is relatively stable in vivo and widely distributed in the lungs, liver, gastrointestinal tract (GIT) and fatty tissue. According to the ascertained concentration of bryostatin in the GIT, along with the fecal excretion, the results also indicate the possibility of its enterohepatic circulation. Though the excretion through feces was delayed, bryostatin was rapidly excreted through urine.
The impact of bryostatin-1 on reproductive parameters in female rats was investigated by intravenous administration to females on gestation days 6–15 at concentrations of 4.0, 8.0 and 16.0 µg/kg on a daily basis (Jiangbo et al., Citation2010). After that, fetuses were examined for external, visceral and skeletal malformations and as a result, maternal toxicity of bryostatin-1 in dose 8.0 µg/kg, embryotoxicity at 16.0 µg/kg and fetotoxicity at 4.0 µg/kg were evaluated. However, these experiments did not reveal any teratogenic effect of bryostatin-1 in rats. Thus, specific parameters were evaluated during this study and findings were as follows: total weight gains were significantly different in rats between the bryostatin-1 treated (8.0 and 16.0 µg/kg) and non-treated groups. The mortality of embryos, i.e., resorption of embryos and death of fetus rates were significantly higher in animals upon bryostatin-1 treatment (16 µg/kg) in comparison with the control group. The fetal weight and fetal body length were found to be significantly lower in bryostatin-1 treated animals compared to the control group.
Anticancer effects of bryostatin
In the previous years, a number of preclinical studies have been reported, which have contributed to the understanding of the role of bryostatin in various malignancies. Basically, most, but not all, of bryostatins’ pharmacological effects are attributed to its interaction with the diacylglycerol (DAG) binding site of the C-1, C-19 and C-26 regulatory domain of PKC (Trindade-Silva et al., Citation2010). PKC exists in 10 isoforms, variously regulated by DAG, Ca2+ and phospholipid, and it is likely that when bryostatin-1 binds to the cysteine-rich domains of PKC, similar changes occur, though specific differences may well occur with different PKCs (Newman, Citation2012). Wender and co-workers (Citation1999) have proposed a pharmacophoric hypothesis that bryostatin macrolactones exhibit high affinities for PKC isozymes because they compete with phorbol ester for its binding site on PKC, and stimulate kinase activity in vitro and in vivo. Unlike the phorbol esters, they do not act as tumor promoters.
This hypothesis has led to the synthesis of simplified analogs, based on the modification of the A-ring and B-ring of bryostatin. In continuous elucidation of the structure activity relationship (SAR) of the bryostatins, simplified B-ring analogs have been synthesized (Wender et al., Citation2006). The synthesis of B-ring analogs lacking the A-ring has provided evidence that a B-ring ester can influence the selective activation of PKC isozymes. Late-stage diversification through cross-metathesis led to the synthesis of five additional analogs. The synthesis of analogs mimicking the A-ring functionality of bryostatin-1 and bryostatin-2 has been accomplished through an efficient and multiply convergent route utilizing a key Prins cyclization. This strategy has led to the generation of 14 new diversified analogs, uncovering a previously unknown hotspot for PKC affinity at the C7 position. This position was also found to have implications for selectivity and efficacy against various cancer cells. A potential geminal dimethyl shielding effect was discovered in the A-ring through the synthesis of a C8 gem-dimethyl C7 hydroxyl analog; this position might influence selective PKC activation (Wender & Verma, Citation2008).
Several studies focused on bryostatins’ anticancer effects have been published by the research group of Ramzi M. Mohammad. They evaluated activity of bryostatin-1 in combination with dolastatin 10 (a natural product derived from the marine mollusk, Dolabella auricularia) and its structural modification, auristatin PE, on a human B-cell chronic lymphocytic leukemia cell line (WSU-CLL) (Mohammad et al., Citation1998a). These tests were carried out in a severe combined immune deficient (SCID) mouse xenograft model bearing this cell line. The combination of bryostatin-1 with auristatin PE in treated mice was successful – all animals were free of tumors (five of five) in 150 days and were considered cured. In contrast, the combination of bryostatin-1 with dolastatin 10 was effective only partially – 2 cured mice of 5 experimental animals. This might suggest a synergetic effect of bryostatin-1 and other used agents, with auristatin PE combination was considered as more efficient. Another reported study from the same laboratory, involving WSU-CLL-bearing SCID mice, showed that the anticancer activity of bryostatin-1 is highly dose- and schedule-specific (Mohammad et al., Citation1998b). The best results were obtained when 2-chlorodeoxyadenosine (2-CdA) was given at 30 mg/kg/injection/day after bryostatin-1 at 5 μg/kg/injection/day. Reversal of administration or 2-CdA alone was not active at all. Bryostatin-1 on its own was active, but not as good as the combination. The only reported cure of WSU-CLL-bearing SCID mice (5/5) was achieved with auristatin PE (1.5 mg/kg iv) followed by bryostatin 1 (75 μg/kg ip) every other day, repeated three times.
The effect of bryostatin on a line derived from primary human pancreatic adenocarcinoma was also tested (Mohammad et al., Citation1999a). Human pancreatic cell line (KCl-MOH1) grows well in tissue culture and also forms tumors in the SCID mice when implanted subcutaneously and in orthotopic sites. The combination of bryostatin-1 and gemcitabine (or spongistatin and gemcitabine) was effective; mice treated with these substance combinations produced remissions in only one of seven animals. In addition, bryostatin has another remarkable effect: it sensitizes some resistant cells to chemotherapy agents. For example, Mohammad et al. (Citation1999b) studied fludarabine-resistant WSU-CLL cell line, which was isolated from a patient with advanced CLL and was refractory to chemotherapy including fludarabine. It was observed that bryostatin-1 sensitizes WSU-CLL cells and enhances apoptosis. The treatment with bryostatin-1 followed by fludarabine resulted in a higher antitumor activity compared to either agent alone, in combination, or the reverse addition of these agents. Results of this study also suggest the possibility of common pathway of interaction between bryostatin-1 and purine analogs. Fludarabine was used both in vitro and in vivo in animal studies and when used sequentially after bryostatin-1, it resulted in significantly higher rates of growth inhibition, improved animal survival and consequently showed the importance of combination of the agents and their order. The application of fludarabine (100 nM) and bryostatin-1 (10 nM for 72 h) and also concurrent addition of bryostatin-1 and fludarabine resulted in significantly higher rates of growth inhibition. In contrast, this result was not found by using reverse addition of these two substances and by using the agents alone. Bryostatin was also tested in the sphere of investigation of other anticancer treatment methods, one of which was a combination with vaccine application (Parviz et al., Citation2003). 4T1 or 4T07-IL-2-vaccine-sensitized draining lymph node (DLN) cells, activated ex vivo with bryostatin-1 and ionomycin and cultivated in culture, induced complete tumor regressions when adoptively transferred to 4T1 tumor-bearing mice. Vaccination alone with either 4T1, 4T1-IL-2 or 4T07-IL-2 was not effective.
Interestingly, there are members of the bryostatin family with different antitumor activity. Kraft et al. (Citation1996) studied the ability of bryostatins marked as 1, 5 and 8 to inhibit the growth of murine melanoma K1735-M2. Bryostatins 1, 5 and 8 induced equivalent inhibition of melanoma growth, but bryostatins 5 and 8 induced less weight loss than bryostatin-1 (p < 0.001).
Bryostatin-1 was tested in combination chemotherapy with cyclophosphamide, hydroxydoxorubicin, vincristine and prednisone (Huang et al., Citation2007). These treatments were administered to tumor-bearing SCID mice weekly for up to four cycles. Currently, the feasibility of H-1 and IT magnetic resonance spectroscopy (MRS) for in vivo detection of response to combination chemotherapy was evaluated. Results showed that H-1 and P-31 MRS can detect in vivo therapeutic response of non-Hodgkin's lymphoma (NHL) tumors. Treated tumors exhibited significant changes in appropriate metabolite level. The synergistic activity of bryostatin-1, another PKC activator phorbol-12-myristate-13-acetate (PMA) and the synthetic tellurium compound (AS101) on human myelocytic leukemia cell differentiation in vitro, and in a mouse model was also tested (Hayun et al., Citation2007). The synergistic effect of the combination of bryostatin-1 with AS101 or with a low concentration of PMA both in vitro and in vivo was detected. Results showed that such a combination led to the differentiation of HL-60 cell line to macrophage-like cells. Also, the survival of treated mice was significantly increased. The effect of the application of bryostatin-1 alone (similarly as AS101 alone) was reduced. The application of AS101 and bryostatin-1 synergistically increased p21 (waf1) expression levels which were necessary for HL-60 cell differentiation.
The sequential use of vincristine (VCR) and bryostatin-1 also resulted in significant antitumor activity in diffusing large cell lymphoma (DLCL) model (Al-Katib, Citation1998). A cell line from a patient with relapsed DLCL was used for this investigation. Cell line WSU-DLCL2 (a mature B-cell line with some chromosomal aberrations) grows in liquid culture and forms s.c. tumors in SCID mice. Treatment of WSU-DLCL2 cells with bryostatin-1 led to reversion of the multidrug resistance phenotype within 24 h. The increase of [H3]vincristine accumulation in cells treated with bryostatin-1 was proved. The application of alone tested antitumor agent bryostatin-1, as well as VCR, doxorubicin and 1-β-d-arabinofuranosylcytosine showed no clinically significant activity to WSU-DLCL2-bearing SCID mice. The 24 h pretreatment with bryostatin-1 prior to other cytotoxic agents caused significant increase in antitumor activity of VCR, but not 1-β-d-arabinofuranosylcytosine. A possible mechanism of bryostatin-1 antitumor effect was deduced from this study. The decrease in the expression of β-glycoprotein in WSU-DLCL2 tumors removed from bryostatin-1-treated animals was statistically significant (p < 0.001). Competitive RT-PCR assay revealed decrease of mdr1 RNA expression 24 h after bryostatin-1 treatment. It suggests that down-regulation of mdr1 caused by bryostatin-1 might be one possible mechanism of enhanced vincristine effect.
In another study, immunotherapy using DLN (draining lymph nodes) cells was examined (Baldwin et al., Citation1997). Vital RT-2 glioma cells were injected into the rat hind foot, after 10 days the tumor draining lymph nodes (DLN) were obtained. These DLN cells were suspended in culture medium supplemented with interleukin-2 (IL-2) and incubated (for 18 h) with bryostatin-l and ionomycin to stimulate expansion. Ex vivo expanded DLN cells were applied intravenously into rats with a 7-day intracerebral RT-2 glioma. The result of these experiments was not significantly increasing survival, but tumors were smaller, showed no necrosis, and appeared to be less infiltrative. By intravenous application of ex vivo DLN cells expanded into three-day intracerebral RT-2 glioma models, tumors were almost entirely eliminated and animals survived. Thus, within this immunotherapy in pretreated cells the used substances were capable of limiting the progression of large gliomas and eradicate small ones.
Other biological effects of bryostatin
In addition to the interaction with PKCs, a very significant amount of evidence has been accumulated that suggests that bryostatin-1, and by inference, other similar compounds, with the possible exception of the 20-deoxy class such as bryostatin-13, can function as very potent immunostimulants. Thus, resting T cells and neutrophils are activated both in vitro and in vivo, and in clinical trials a rise of circulating levels of tumor necrosis factor-α (TNF-α), which is normally produced by the body following immunostimulation, has been shown (Newman, Citation2012). In experiments with immunosuppressed rats treated with bryostatin-1, it was proved that such animals had a partial (50%) protection against Pneumocystis carinii infection (Oz et al., Citation2000). In contrast, three other immunomodulators used in the study, namely recombinant FLT3 ligand (FLT3L), recombinant granulocyte colony-stimulating factor (G-CSF) and recombinant interleukin-15 (IL-15), provided no protection against P. carinii infection.
A different study concerning other than anticancer effects of bryostatin was focused on its possible impact on memory functions (Kuzirian et al., Citation2006). For the purpose of this study marine opisthobranch gastropod mollusk Hermissenda was used; a species of small, brightly colored, sea nudibranch mollusks from the family Glaucidae. Bryostatin in low concentrations from 0.1 to 0.5 ng/mL enhanced memory acquisition in Hermissenda, while concentrations higher than 1.0 ng/mL exhibited no recall of the associative training. The effects of two training events (TEs) with paired conditioned and unconditioned stimuli standardly evoked only short-term memory (STM) lasting 7 min. When bryostatin was added concurrently, it enhanced the long-term memory (LTM) that lasted about 20 h. The effects of both 4- and 6-paired TEs (which by themselves did not generate LTM) were also enhanced by bryostatin to induce a consolidated memory (CM) that lasted at least 5 days. The standard positive 9-TE regime typically produced a CM lasting at least 6 days. Low concentrations of bryostatin (<0.5 ng/mL) elicited no demonstrable enhancement of CM from 9-TEs. Specimen exposed to bryostatin concentrations higher than 1.0 ng/mL exhibited no behavioral learning. Another part of the study was the use of sharp-electrode intracellular recordings of type-B photoreceptors in the eyes from animals conditioned in vivo with bryostatin. It revealed changes in input resistance and an enhanced long-lasting depolarization (LLD) in response to light. Also, quantitative immunocytochemical measurements using an antibody specific for the PKC-activated Ca2+/GTP-binding protein calexcitin showed enhanced antibody labeling with bryostatin. The authors claimed that pathways responsible for the enhancement effects induced by bryostatin were putatively mediated by PKC.
Bryostatin was also investigated as a potential HIV inhibitor. Mehla et al. (Citation2010) found that bryostatin revealed receptor independent antiviral activity against R5- and X4-tropic viruses and partly via transient decrease in CD4/CXCR4 expression. Bryostatin, applied at low nanomolar concentrations, also strongly reactivated latent viral infection in monocytic and lymphocytic cells via activation of PKC-α and -δ (PKC inhibitors such as rottlerin and GF109203X abrogated the bryostatin effect). Bryostatin specifically modulated novel PKC (nPKC). Notably, bryostatin was considered as non-toxic in vitro and was unable to provoke T-cell activation. The impact of bryostatin on the HIV life-cycle may be important for the treatment of HIV, especially by purging latent virus from different cellular reservoirs such as brain and lymphoid organs. It could be used in order to broaden the inhibitory range and effectiveness of current antiretrovirals in clinical applications.
Clinical studies
Advances in deep-sea collection and aquaculture technology have led to a significant number of compounds derived from marine organisms entering preclinical and early clinical evaluation as anticancer candidates (da Rocha et al., Citation2001). Also, bryostatin-1 has already been used in a number of clinical studies. In the past 10 years, more than 20 clinical trials have been conducted with bryostatin-1 in monotherapy or in combination with clinically used cytotoxic drugs. The spectrum of diagnoses included different tumor types, such as sarcoma, melanoma, ovaria, cervical, neck and head carcinoma, esophageal, gastric, pancreatic, renal cell carcinoma, as well as a variety of leukemias, e.g., chronic lymphocytic leukemia, non-Hodgkin’s lymphoma, or multiple myeloma (Newman, Citation2012). Induction of cell differentiation in patients with refractory chronic lymphocytic leukemia after bryostatin-1 administration was also reported (Schwartsmann et al., Citation2001). In contrast, bryostatin-1 showed no significant objective antitumour activity in phase I and II trials in patients with advanced solid tumors, including renal-cell and non-small-cell lung cancer and malignant melanoma (Jayson et al., Citation1995; Prendiville et al., Citation1993; Propper et al., Citation1998).
Based on conflicting results of objective responses observed in phase I studies in a broad spectrum of tumors, a number of phase II studies of bryostatin-1 using various infusion regimens were conducted in both solid and hematological malignancies. To date, phase II studies of bryostatin-1 have failed to demonstrate any clinically meaningful activity. Despite the lack of significant clinical benefit observed when used as a single agent, additional upcoming results revealed synergistic action between bryostatin-1 and conventional cytotoxic drugs, which triggered several phase I studies of bryostatin in combination with cytotoxic agents (Banerjee et al., Citation2008). These include cisplatin, vinca alkaloids (vincristine), paclitaxel, nucleosides (fludarabine, gemcitabine), cytokines (interleukin-2, GM-CSF), or protein kinase inhibitor temsirolimus (Newman, Citation2012).
After more than 30 phase I and II clinical trials in a variety of cancers, alone and in combination with other chemotherapy agents, bryostatin has not been effective enough to progress to phase III clinical trials as a cancer treatment. Nevertheless, interestingly, based on striking positive results produced by bryostatin in central nervous system models in rodents, a human Alzheimer’s disease phase II clinical trial has been initiated with bryostatin-1 (Trindade-Silva et al., Citation2010). Most recently, bryostatin has been proposed as a possible therapy for human immunodeficiency virus (HIV) (Mehla et al., Citation2010). Although current antiviral therapy is effective against HIV, the disease cannot be cured because of latent viruses persisting in resting cells. On the basis of in vitro studies, bryostatins may be effective in activating latent viruses so they can be purged from cellular reservoirs, exposed to antiviral therapy, and eliminated (Trindade-Silva et al., Citation2010).
However, some negative aspects of bryostatin therapy appeared during clinical studies. The main toxic effects of bryostatin-1 in initial clinical trials were myalgia, local phlebitis, fatigue, nausea and vomiting, and thrombocytopenia (Schwartsmann et al., Citation2001). Moreover, although it is based solely on in vitro cell line studies, there is a risk of bryostatin-1 tumor-inducing capability. Thus, care might have to be taken in choosing patients for bryostatin treatment (Newman, Citation2012).
In conclusion, monotherapy with bryostatin does not appear to be optimal or efficacious, except perhaps in a very selective cohort of leukemia patients; the combination with cytotoxic drugs seems to have greater potential for its clinical use. However, recent approval by the FDA for a phase II clinical trial of bryostatin-1 in Alzheimer’s patients (identifier number: NCT00606164), shows that bryostatin in one version or another may yet have therapeutic potential [National Institutes of Health (NIH), Citation2012].
The future of bryostatins’ clinical use
Although total synthesis of naturally occurring bryostatins has been achieved and is steadily being improved, it is not yet practical for industrial production. Synthesis of simplified structural analogs of bryostatins is currently an active area of research. Analogs that are more synthetically accessible, yet retain biological activity, have been attained and may ultimately prove clinically useful (Trindade-Silva et al., Citation2010). All these analogs are bound strongly to a mixture of PKC isozymes, and several show significant growth inhibitory activity against human cancer cell lines in vitro (Schwartsmann et al., Citation2001). It is more likely that instead of continued clinical development of bryostatin-1, more potent bryostatin analogs (e.g., bryologs) will enter the clinical area for further testing. Indeed, the most recent paper by Wender group (DeChristopher et al., Citation2012) provides the first in vivo validation that the bryostatin analog, “picolog”, at a concentration of 100 µg/kg is a potential therapeutic agent for the treatment of cancer and other diseases.
Declaration of interest
The authors report no conflicts of interest.
Acknowledgements
We thank Mgr. Jitka Chromeckova and Mr. Willem Westra for improving the English of this paper. The study was supported by project GACR P503/12/0337. Final processing of this study was supported by project CZ.1.07/2.3.00/09.0076.
References
- Al-Katib AM, Smith MR, Kamanda WS, et al. (1998). Bryostatin 1 down-regulates mdr1 and potentiates vincristine cytotoxicity in diffuse large cell lymphoma xenografts. Clin Cancer Res 4:1305–14
- Baldwin NG, Rice CD, Tuttle TM, et al. (1997). Ex vivo expansion of tumor-draining lymph node cells using compounds which activate intracellular signal transduction. I. Characterization and in vivo anti-tumor activity of glioma-sensitized lymphocytes. J Neurooncol 32:19–28
- Banerjee S, Wang Z, Mohammad M, et al. (2008). Efficacy of selected natural products as therapeutic agents against cancer. J Nat Prod 71:492–6
- DeChristopher BA, Fan AC, Felsher DW, Wender PA. (2012). “Picolog,” a synthetically-available bryostatin analog, inhibits growth of MYC-induced lymphoma in vivo. Oncotarget 3:58–66
- Dunlap WC, Battershill CN, Liptrot CH, et al. (2007). Biomedicinals from the phytosymbionts of marine invertebrates: A molecular approach. Methods 42:358–76
- Haygood MG, Schmidt EW, Davidson SK, Faulkner DJ. (1999). Microbial symbionts of marine invertebrates: Opportunities for microbial biotechnology. J Mol Microbiol Biotechnol 1:33–43
- Hayun M, Okun E, Hayun R, et al. (2007). Synergistic effect of AS101 and bryostatin-1 on myeloid leukemia cell differentiation in vitro and in an animal model. Leukemia 21:1504–13
- Huang MQ, Nelson DS, Pickup S, et al. (2007). In vivo monitoring response to chemotherapy of human diffuse large B-cell lymphoma xenografts in SCID mice by 1H and 31P MRS. Acad Radiol 14:1531–9
- Jayson GC, Crowther D, Prendiville J, et al. (1995). A phase-I trial of bryostatin 1 in patients with advanced malignancy using a 24-hour intravenous infusion. Br J Cancer 72:461–8
- Jiangbo Z, Xuying W, Yuping Z, et al. (2010). Toxicity of bryostatin-1 on the embryo-fetal development of Sprague-Dawley rats. Birth Defects Res B Dev Reprod Toxicol 89:171–4
- Kraft AS, Woodley S, Pettit GR, et al. (1996). Comparison of the antitumor activity of bryostatins 1, 5, and 8. Cancer Chemother Pharmacol 37:271–8
- Kuzirian AM, Epstein HT, Gagliardi CJ, et al. (2006). Bryostatin enhancement of memory in Hermissenda. Biol Bull 210:201–14
- Manning TJ, Land M, Rhodes E, et al. (2005). Identifying bryostatins and potential precursors from the bryozoan Bugula neritina. Nat Prod Res 19:467–91
- Mehla R, Bivalkar-Mehla S, Zhang R, et al. (2010). Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. PLoS ONE 5:e11160. Available from: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0011160 [last accessed 19 Nov 2012]
- Mohammad RM, Varterasian ML, Almatchy VP, et al. (1998a). Successful treatment of human chronic lymphocytic leukemia xenografts with combination biological agents auristatin PE and bryostatin 1. Clin Cancer Res 4:1337–43
- Mohammad RM, Katato K, Almatchy VP, et al. (1998b). Sequential treatment of human chronic lymphocytic leukemia with bryostatin 1 followed by 2-chlorodeoxyadenosine: Preclinical studies. Clin Cancer Res 4:445–53
- Mohammad RM, Li Y, Mohamed AN, et al. (1999a). Clonal preservation of human pancreatic cell line derived from primary pancreatic adenocarcinoma. Pancreas 19:353–61
- Mohammad RM, Limvarapuss C, Hamdy N, et al. (1999b). Treatment of a de novo fludarabine resistant-CLL xenograft model with bryostatin 1 followed by fludarabine. Int J Oncol 14:945–50
- National Institutes of Health (NIH): Safety, efficacy, pharmacokinetics, and pharmacodynamics study of bryostatin 1 in patients with Alzheimer's disease. Available from: http://clinicaltrials.gov/ct2/show/NCT00606164 [last accessed 19 Nov 2012]
- Newman DJ. (2012). The bryostatins. In: Cragg GM, Kingston DGI, Newman DJ, eds. Anticancer Agents from Natural Products. Boca Raton: Taylor and Francis, 199–218
- Oz HS, Hughes WT, Rehg JE, Thomas EK. (2000). Effect of CD40 ligand and other immunomodulators on Pneumocystis carinii infection in rat model. Microb Pathog 29:187–90
- Parviz M, Chin CS, Graham LJ, et al. (2003). Successful adoptive immunotherapy with vaccine-sensitized T cells, despite no effect with vaccination alone in a weakly immunogenic tumor model. Cancer Immunol Immunother 52:739–50
- Piel J. (2006). Bacterial symbionts: Prospects for the sustainable production of invertebrate-derived pharmaceuticals. Curr Med Chem 13:39–50
- Prendiville J, Crowther D, Thatcher N, et al. (1993). A phase-I study of intravenous bryostatin-1 in patients with advanced cancer. Br J Cancer 68:418–24
- Propper DJ, Macaulay V, O'Byrne KJ, et al. (1998). A phase II study of bryostatin 1 in metastatic malignant melanoma. Br J Cancer 78:1337–41
- da Rocha AB, Lopes RM, Schwartsmann G. (2001). Natural products in anticancer therapy. Curr Opin Pharmacol 1:364–9
- Schaufelberger DE, Koleck MP, Beutler JA, et al. (1991). The large-scale isolation of bryostatin 1 from Bugula neritina following current good manufacturing practices. J Nat Prod 54:1265–70
- Schwartsmann G, da Rocha AB, Berlinck RG, Jimeno J. (2001). Marine organisms as a source of new anticancer agents. Lancet Oncol 2:221–5
- Trindade-Silva AE, Lim-Fong GE, Sharp KH, Haygood MG. (2010). Bryostatins: Biological context and biotechnological prospects. Curr Opin Biotech 21:834–42
- Trost BM, Yang H, Dong G. (2011). Total syntheses of bryostatins: Synthesis of two ring-expanded bryostatin analogues and the development of a new-generation strategy to access the C7–C27 fragment. Chemistry 17:9789–805
- Ueno S, Yanagita RC, Murakami K, et al. (2012). Identification and biological activities of bryostatins from Japanese Bryozoan. Biosci Biotechnol Biochem 76:1041–3
- Wender PA, Hinkle KW, Koehler MF, Lippa B. (1999). The rational design of potential chemotherapeutic agents: Synthesis of bryostatin analogues. Med Res Rev 19:388–407
- Wender PA, Horan JC, Verma VA. (2006). Total synthesis and initial biological evaluation of new B-ring-modified bryostatin analogues. Org Lett 8:5299–302
- Wender PA, Verma VA. (2008). The design, synthesis, and evaluation of C7 diversified bryostatin analogues reveals a hot spot for PKC affinity. Org Lett 10:3331–4
- Zhang X, Zhang R, Zhao H, et al. (1996). Preclinical pharmacology of the natural product anticancer agent bryostatin 1, an activator of protein kinase C. Cancer Res 56:802–8

