Abstract
Context: The roots and rhizomes of Ligusticum porteri Coulter & Rose (Apiaceae) are widely used in Mexican folk medicine for several purposes, including painful complaints.
Objective: The main goal of this work was to demonstrate the analgesic action in mice of some preparations and major compounds from L. porteri.
Materials and methods: The extracts, aqueous (AE) and organic (OE), the essential oil (EO) and major compounds (10–316 mg/kg) from L. porteri were evaluated as potential antinociceptive agents using the acetic acid-induced writhing and hot plate tests in ICR mice.
Results: All preparations tested exhibited significant antinociceptive effect in the two animal pain models selected. AE and EO were more effective in the writhing test while OE had a better effect in the hot-plate model. On the other hand, Z-ligustilide (1) provoked an increment in the latency period to the thermal stimuli in the hot-plate test at a dose of 31.6 mg/kg, and a decrease in the number of abdominal writhes at 10 mg/kg. Z-3-butylidenephthalide (2) induced a dose-dependent antinociceptive action in the hot-plate assay; this compound was also effective for controlling the pain provoked by chemical irritation at the doses of 10 and 31.6 mg/kg. Finally, diligustilide (3) inhibited the number of writhing responses at all doses tested but was inactive in the hot-plate model.
Conclusion: The present investigation provides in vivo evidence supporting the use of L. porteri to treat painful conditions in folk medicine.
Introduction
Ligusticum porteri Coulter & Rose (Apiaceae) is one of the most commercialized medicinal herbs in Mexico and the USA. In Mexico, the plant is commonly called “chuchupate”, “raíz del cochino” and “angelica’s root”, among others. In the USA the most popular names are osha, Porter’s liquorice and bear root. According to several reports a few preparations (extract, infusion, decoction or oil) made with Osha roots’ are commonly ingested for treating respiratory illnesses, fever, gastrointestinal disorders, sore throats and rheumatism; externally, root products are used to treat aches, pain, scorpion sting and skin infections (Appelt, Citation1985; Argueta et al., Citation1994; Bye, Citation1986; Galaviz et al., Citation1994; Olivas, Citation1999; Terrell & Fennell, Citation2009; Turi & Murch, Citation2010). Several phthalides, terpenoids, phenylpropanoids and coumarins have been isolated from the organic extract of L. porteri roots (Delgado et al., Citation1988; Hou et al., Citation2004; León & Delgado, Citation2012; Reza-Garduño, Citation1987; Ríos & Delgado, Citation1999; Zschoke et al., Citation1998). The essential oil was reported to contain sabinyl acetate, Z-ligustilide (1), Z-3-butylidenephthalide (2) and sabinol as the main components (Rivero-Cruz et al., 2012). A few extracts and phytochemicals of the plant showed antihyperglycemic, hypoglycemic, sedative and spasmolytic properties (Brindis et al., Citation2011; León et al., Citation2011a).
This work was undertaken to establish the potential antinociceptive action of preparations and compounds from L. porteri in order to corroborate its use as an analgesic remedy in folk medicine. The preparations tested were chosen considering the popular intake of the crude drug.
Materials and methods
Plant material
Roots and rhizomes of L. porteri were collected in Municipio Basigochi, Chihuahua, Mexico in October 2005. A voucher specimen (Bye 31733) has been deposited at the National Herbarium (MEXU), Instituto de Biología, UNAM.
Chemicals
Morphine (MOR, 99–100.5%, purity) was obtained from Clínica del Dolor Instituto Nacional de la Nutrición Salvador Zubirán. Dipyrone hydrate (DIP, 99.0% purity) was purchased from Pisa Laboratories (Jalisco, Mexico). Acetic acid and polyoxyethylene-sorbitan monolate (Tween 80) were acquired from Sigma (St. Louis, MO). The solvents were AR grade and purchased from JT Baker-Mexico.
Preparations of herbal extracts
The roots and rhizomes were dried and grounded using a Thomas Willey laboratory mill (mesh size 2 mm). The powder of plant material (80 g) was extracted with 1000 mL of boiling water during 30 min. After filtration, the infusion was evaporated to dryness under reduced pressure to give 2.0 g of aqueous extract (AE; yield 2.5%).
The air-dried roots and rhizomes (3.0 kg) were ground into powder and exhaustively extracted with dichloromethane-methanol (1:1) at room temperature. After filtration, the extract was concentrated under reduced pressure to yield 400.0 g of organic extract (OE; yield 13.3%).
The essential oil (EO) was prepared from the ground dried crude drug (200.0 g) by hydrodistillation during 5 h using a modified Clevenger-type apparatus. The condensed material was subjected to liquid--liquid extraction with dichloromethane (3 × 500 mL); the resulting combined organic phase was dried over anhydrous sodium sulphate and the solvent was completely removed in vacuo to afford 10 mL of EO (specific gravity at room temperature 0.95 mg/mL; yield 4.75%). Sample was stored in amber bottle at 4°C before analysis. The three preparations (AE, OE and EO) were used for biological testing.
Isolation and characterization of compounds 1–3
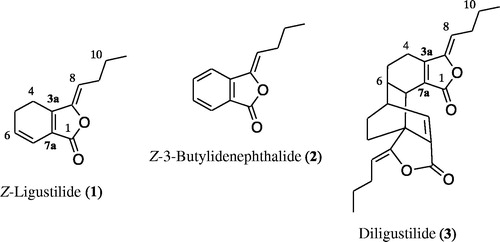
The OE (200 g) was suspended in water and sequentially partitioning using hexane, dichloromethane and ethyl acetate. The hexane soluble fraction (35 g) was subjected to open column chromatography on silica gel (Merck) eluting with a gradient of hexane-dichloromethane (from 10:0 to 0:10) and dichloromethane-methanol (from 10:0 to 0:10) to yield ten secondary fractions (F1–F10). From fraction F5 crystallized 230 mg of diligustilide (3, p.f. 127–129°C). Preparative TLC on silica gel F254 (Merck) of F3 using hexane-dichloromethane (5:5) afforded 1.0 g of Z-ligustilide (1, Rf 0.47) and 1.0 g of Z-3-butylidenephthalide (2, Rf 0.5).
Melting points were determined on a Fisher-Johns apparatus and were uncorrected. The NMR spectra were recorded on a Varian Unity Plus 400 spectrometer at 400 (1H) and 125 MHz (13C) using TMS (tetramethylsilane) as the internal standard; chemical shifts were recorded as δ values. Electron-impact mass spectra (EIMS) were obtained on a LECO Pegasus 4D-spectrometer.
Z-Ligustilide (1): 1H NMR (400 MHz, CDCl3, δ): 6.30 (dt, J = 9.6, 2.1 Hz, H-7); 6.01 (dt, J = 9.6, 4.3 Hz, H-6); 5.23 (dt, J = 9.9, 1.8 Hz, H-8); 2.60 (dt, J = 9.9, 1.8 Hz, H-4); 2.46 (ddd, J = 9.9, 2.1, 1.5 Hz, H-5); 2.38 (dt, J = 8.1, 7.5 Hz, H-9); 1.50 (dd, J = 7.5 Hz, H-10); 0.96 (t, J = 7.5 Hz, H-11). 13C NMR (125 MHz, CDCl3, δ): 167.7 (C-1), 148.5 (C-3), 147.1 (C-3a), 129.9 (C-6), 124.1 (C-7a), 117.1 (C-8), 112.9 (C-9), 28.1 (C-5), 22.5 (C-10), 18.6 (C-4), 13.8 (C-11). EIMS (m/z): 190 [M+].
Z-3-Butylidenephthalide (2): 1H NMR (400 MHz, CDCl3, δ): 7.89 (dd, J = 7.7, 1.0 Hz, H-7); 7.68 (dd, J = 7.8, 1.0 Hz, H-5); 7.66 (dt, J = 7.8, 1.0 Hz, H-4); 7.51 (dd, J = 7.7, 0.9 Hz, H-6); 5.65 (t, J = 7.8 Hz, H-8); 2.46 (dt, J = 7.8, 7.3 Hz, H-9); 1.56 (dd, J = 7.3 Hz, H-10); 0.99 (t, J = 7.3 Hz, H-11). 13C NMR (125 MHz, CDCl3, δ): 167.4 (C-1), 145.8 (C-3), 139.6 (C-3a), 134.3 (C-5), 129.4 (C-6), 125.1 (C-7), 119.7 (C-4), 109.5 (C-8), 27.8 (C-9), 22.5 (C-10), 13.8 (C-11). EIMS (m/z): 188 [M+].
Diligustilide (3): 1H NMR (400 MHz, CDCl3, δ): 7.35 (dt, J = 9.5, 2.0 Hz, H-7′); 5.07 (dt, J = 9.9, 1.8 Hz, H-8′); 5.00 (dt, J = 9.9, 1.8 Hz, H-8); 3.25 (d, H-7); 2.99 (dd, J = 7.6, 1.0 Hz, H-6′); 2.55 (dd, J = 7.6, 1.0 Hz, H-6); 2.40 (m, H-4/4′); 2.30 (m, H-9/9′); 2.15 (m, H-5/5′); 1.56 (m, H-10/10′); 0.92 (t, 7.5 Hz, H-11/11′), 13C NMR: (125 MHz, CDCl3, δ): 168.31 (C-1), 164.75 (C-1′), 154.83 (C-3a), 150.26 (C-3′), 147.81 (C-3), 141.89 (C-7′), 133.99 (C-7′a), 126.34 (C-7a), 112.00 (C-8), 108.46 (C-8′), 47.39 (C-3′a), 41.37 (C-7), 41.26 (C-6′), 38.12 (C-6), 30.85 (C-4′), 28.76 (C-9), 27.81 (C-5), 27.28 (C-5′), 25.57 (C-9′), 22.12 (C-10/10′), 19.55 (C-4), 13.72 (C-11/11′). EIMS (m/z): 380 [M+].
Animals
ICR male mice (20–25 g) were purchased from Centro UNAM-Harlan (Harlan Mexico, S.A. de C.V.). All animals were randomly housed in a climate- and light-controlled room with a 12 h light/dark cycle and maintained on standard pellet diet and clean water ad libitum. Twelve hours prior to performing the experiments, the animals were food withheld and had free access to drinking water. After the experiments, all animals were sacrificed in a CO2 chamber.
Ethical aspects
All experimental procedures involving animals and their care were conducted in conformity with the Mexican Official Norm for Animal Care and Handing (NOM-062-ZOO-1999) and in compliance with the international rules on care and use of laboratory animals. All efforts were made to minimize the number of animals used (n = 6) as well as any discomfort (Zimmerman, Citation1983).
Drugs and treatments
MOR and DIP used as central and peripherial analgesic agents, respectively, were dissolved in vehicle (0.9% saline + Tween 80 0.2%) and administered to mice at 5 and 100 mg/kg intraperitonally (i.p.). The vehicle at the same volume was administered (10 mL/kg) orally to the control groups. All treatments (AE, OE, EO, and compounds 1–3 with a purity higher than 95% according to the NMR spectra) were suspended in vehicle for administration to mice.
Acute toxicity test
Mice were divided into control and test groups (n = 3) and treated in two phases. In the first, test groups received intragastric doses of 10, 100 and 1000 mg/kg. In the second, the animals received doses of 1600, 2900 and 5000 mg/kg, according to the Lorke method (Citation1983). The control group received the vehicle. In each phase, mice were observed daily during 14 days for mortality, toxic effects and/or changes in behavioral pattern. At the end of the each phase the animals were sacrificed in a CO2 chamber and the main organs were compared versus those of the control group (Lorke, Citation1983).
Antinociceptive activity
Acetic acid-induced writhing test
The peripheral antinociceptive activity of the preparations (AE, OE, EO and compounds 1–3) was studied by acetic acid-induced writhing test according to previously described protocols. Briefly, mice were divided into control, reference drug and test groups (n = 6). After 30 min of oral administration of the treatments or vehicle each mouse received acetic acid (0.6%) i.p. The reference drug was administered 15 min before injection of acetic acid. Writhings (abdominal contraction, elongation of body and extension of the hind limb) were counted cumulatively during 30 min (Al-Chaer & Traub, Citation2002, Arendt-Nielsen & Chen, Citation2003).
Hot-plate test
The hot-plate test was performed using a conventional thermal apparatus (Le 7406, Panlab Technology Bioresearch). Mice were placed into an acrylic cylinder on the heated surface (55.5 ± 0.2 °C); the time between placements of the mouse in the platform and shaking/licking of the paws or jumping was recorded as the latency period. Mice were treated by oral administration with vehicle, samples (AE, OE, EO and compounds 1–3) or the reference drug (MOR) 30 min before the thermal noxious stimuli and the latency period was recorded at 0, 30, 60, 90, 120 and 150 min following treatments (n = 6). A cut-off of period 30 s was maintained to avoid injury to the paw of mice.
Statistical analysis
All results are presented as the mean ± SEM (n = 6). The statistical significance among the groups was assessed using the one way analysis of variance (ANOVA) followed by Dunnett's post hoc test using GraphPad software Inc., San Diego, CA. p < 0.05 = *, p < 0.01 = ** and p < 0.001 = *** are considered statistically significant.
Results
Phytochemical study
Z-Ligustilide (1), Z-3-butylidenephthalide (2) and diligustilide (3) () from L. porteri were isolated using conventional chromatographic as previously described (Brindis et al., Citation2011; León et al., 2011b). The isolated compounds were analyzed by MS and NMR and identified by comparison of their spectral data with those of authentic samples (Brindis et al., Citation2011; Delgado et al., Citation1988; Reza-Garduño, Citation1987; Ríos & Delgado, Citation1999). The corresponding NMR properties are summarized in the Materials and methods section.
Acute toxicity
Oral administration of AE or EO, at doses in the range of 10–5000 mg/kg did not produce visible toxic effects, changes on behavior or body weight of treated mice after 14 days. Furthermore, no lesions or bleedings were observed in macroscopic morphology on internal organs such as lungs, kidneys, liver, heart and stomach. Since no death or damage was observed throughout the experiment, the LD50 was greater than 5000 mg/kg for both herbal preparations assayed.
Antinociceptive activity
Acetic acid-induced writhing test
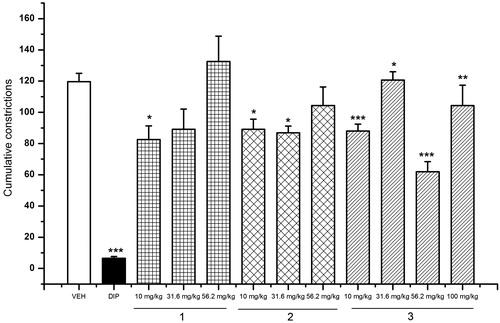
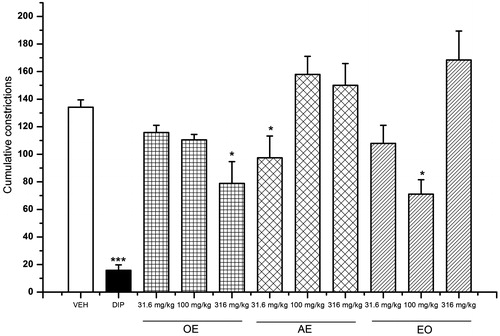
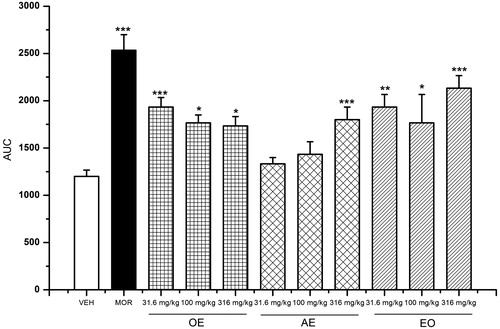
The effects of the three preparations of L. porteri on the writhing response in mice are shown in . The OE, AE and EO caused significant inhibition of the stretching response induced by acetic acid in the writhing model. The best effect of each preparation was achieved at the doses of 316 (30.23% of inhibition), 31.6 (33.33% of inhibition) and 100 mg/kg (45.23% of inhibition), respectively.
Figure 2. Antinociceptive activity of L. porteri herbal preparations: organic extract (OE), aqueous extract (AE) and essential oil (EO) in the writhing test. DIP: dipyrone (100 mg/kg). Data are presented as mean ± SEM (n = 6). *p < 0.05, **p < 0.01 and ***p < 0.001, Dunnett’s post hoc test compared with control group.
Hot-plate test
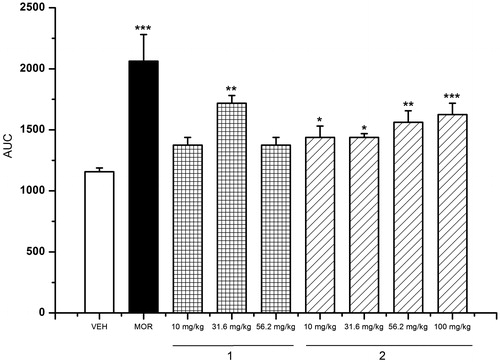
The second model used to evaluate antinociceptive activity was the hot-plate test. The experimental evidence obtained in this study () revealed that the three herbal preparations were active, increasing significantly the latency of response to thermal stimuli; the effect was not dose-dependent. OE and AE showed the best effect at the doses of 31.6 and 316 mg/kg, while the EO was active at the three doses tested (31.6, 100 and 316 mg/kg); the positive control MOR (5 mg/kg) showed the highest prolongation of the latency period.
Figure 3. Antinociceptive activity of L. porteri herbal preparations: organic extract (OE), aqueous extract (AE) and essential oil (EO) in the hot-plate test. MOR: morphine (5 mg/kg). Data are presented as mean ± SEM (n = 6). *p < 0.05, **p < 0.01 and ***p < 0.001, Dunnett’s post hoc test compared with control group.
Since the major components of EO and OE were Z-ligustilide (1), Z-3-butylidenephthalide (2) and diligustilide (3), they were included in the study and tested in both models of nociception. The results showed that (1) provoked an increment in the latency period to the thermal stimuli in the hot-plate test at the dose of 31.6 mg/kg () and a decrease in the number of abdominal writhes at 10 mg/kg (). On the other hand, Z-3-butylidenephthalide (2) induced a dose-dependent antinociceptive action (); this compound was also effective for controlling the pain provoked by chemical irritation at the doses of 10 and 31.6 mg/kg, being inactive at the other doses tested. Finally, diligustilide (3) inhibited the number of writhing responses at all doses tested (10–100 mg/kg; ), but was inactive in the hot-plate assay.
Discussion
Investigation of the acute toxicity is the first step in the pharmacological investigations of herbal drugs. Lorke’s method is perhaps the most widely used approach since the experiments are carried out with a minimum number of experimental animals (Lorke, Citation1983). Previously, we demonstrated (Déciga-Campos et al., Citation2007) that an OE of L. porteri showed mild toxicity (LD50 = 1085 mg/kg). However, in this occasion, the most widely used preparations, namely AE and EO (Terrell & Fennell, Citation2009), were not tested. In the present study, it was found that AE and EO are safer (LD50 > 5000 mg/kg) since they did not provoke mortality or visible changes in behavior (i.e., ataxia, hyperactivity, hypoactivity, etc.), body weight or macroscopic morphology of heart, liver, kidney and lung. Since “osha” is commonly made into a tea with boiling water, chewed or inhaled (Terrell & Fennell, Citation2009) to treat several sorts of sores, its consumption in popular medicine might not be dangerous. However, caution should be taken with preparations including organic solvents.
In order to assess the potential analgesic action of L. porteri preparations and compounds, two widely used pain models were employed. The first one, the acetic acid-induced writhing test, is a classical visceral pain test useful to detect painful complaints associated to inflammatory disorders (Al-Chaer & Traub, Citation2002). On the other hand, the hot-plate test is a suitable method for to evaluate central antinociceptive activity. In addition, this model measures animal behavior and has good sensitivity and specificity (Arendt-Nielsen & Chen, Citation2003). The applications of these assays suggest that phytopreparations and compounds from L. porteri evaluated possessed antinociceptive properties that are mediated both by central and peripheral mechanisms. Compound 3 were the most active in the acetic acid-induced writhing test and could exert its activity predominantly by peripheral mechanism, while 1 and 2 seems to combine both central and peripheral mode of actions.
Du and collaborators (2007) and Wang et al. (Citation2010) demonstrated that Z-ligustilide (1) attenuated pain behavior induced by acetic acid or formalin. In addition, it was established in vitro that the inflammatory action of 1 involved the inhibition of NF-κB and MAPK signal pathways in a dose-dependent manner (Chung et al., Citation2012). Altogether, these results corroborate our findings regarding the antinociceptive action of 1 in both pain models; furthermore, the moderate sedative properties of Z-ligustilide (1) (León et al., 2011a) could enhance its analgesic action.
Regarding compounds 2 and 3, no antinociceptive or anti-inflammatory action has been previously reported and the mechanism underplayed their antinociceptive action remains an open question.
In conclusion, it was demonstrated that the preparations (AE, OE, EO) and isolates 1–3 from L. porteri showed antinociceptive properties associated with central and peripheral mechanisms in animal models. These pharmacological actions are not associated with toxic effects since the infusion and essential oil were innocuous when tested by the Lorke method. The main outcomes of this work provide rational evidence for the use of L. porteri to treat some painful disorders in Mexican folk medicine.
Declaration of interest
This research was supported by grants from Consejo Nacional de Ciencia y Tecnología grant (CONACyT, Proyecto 99395) and DGAPA-UNAM grants (IN-212913). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
The technical assistance of Araceli Pérez-Vásquez is recognized. K.J. acknowledges a fellowship from CONACyT to carry out graduate studies.
References
- Al-Chaer ED, Traub RJ. (2002). Biological basis of visceral pain: Recent developments. Pain 96:221–5
- Appelt GD. (1985). Pharmacological aspects of selected herbs employed in Hispanic folk medicine in the San Luis Valley of Colorado, USA: I. Ligusticum porteri (osha) and Matricaria chamomilla (manzanilla). J Ethnopharmacol 13:51–5
- Arendt-Nielsen L, Chen ACN. (2003). Lasers and other thermal stimulators for activation of skin nociceptors in human. Clin Neurophysiol 33:259–68
- Argueta VA, Cano AL, Rodarte ME. (1994). Atlas de las plantas de la medicina tradicional mexicana. Instituto Nacional Indigenista. México
- Brindis F, Rodriguez R, Bye R, et al. (2011). (Z)-3-Butylidenephthalide from Ligusticum porteri, an alpha-glucosidase inhibitor. J Nat Prod 74:314–20
- Bye RA. (1986). Medicinal plants of the Sierra Madre: Comparative study of Tarahumara and Mexican market plants. Econ Bot 40:103–24
- Chung JW, Choi RJ, Seo EK, et al. (2012). Anti-inflammatory effects of (Z)-ligustilide through suppression of mitogen-activated protein kinases and nuclear factor-κB activation pathways. Arch Pharm Res 35:723–32
- Déciga-Campos M, Rivero-Cruz I, Arriaga-Alba M, et al. (2007). Acute toxicity and mutagenic activity of Mexican plants used in traditional medicine. J Ethnopharmacol 110:334–42
- Delgado G, Reza-Garduño RG, Toscano RA, et al. (1988). Secondary metabolites from the roots of Ligusticum porteri (Umbelliferae). X-Ray structure of Z-6,6′,7,3a′-diligustilide. Heterocycles 27:1305–12
- Du J, Yu Y, Ke Y, et al. (2007). Ligustilide attenuates pain behavior induced by acetic acid or formalin. J Ethnopharmacol 112:211–14
- Galaviz A, Galaviz C, Galaviz L, Duarte C. (1994). Flora medicinal Pima de Yecora, Sonora. Flora medicinal indígena de México: Treinta y cinco monografías del atlas de las plantas de la medicina tradicional mexicana. México: Instituto Nacional Indigenista
- Hou YZ, Zhao GR, Yang J, et al. (2004). Protective effect of Ligusticum chuanxiong and Angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Sci 75:1775–86
- León A, Toscano RA, Tortoriello J, Delgado G. (2011a). Phthalides and other constituents from Ligusticum porteri; sedative and spasmolytic activities of some natural products and derivatives. Nat Prod Res 25:1234–42
- León A, Chávez MI, Delgado G. (2011b). 1H and DOSY NMR spectroscopy analysis of Ligusticum porteri rhizome extracts. Magn Reson Chem 49:469–76
- León A, Delgado G. (2012). Diligustilide: Enantiomeric derivatives, absolute configuration and cytotoxic properties. J Mex Chem Soc 56:222–6
- Lorke D. (1983). A new approach to practical acute toxicity testing. Arch Toxicol 54:275–87
- Olivas M. (1999). Plantas Medicinales del Estado de Chihuahua. México: CEB, Vol. 114
- Reza-Garduño RG. (1987). Aislamiento y caracterización de los ftálidos de Ligusticum porteri C&R (Umbelliferae). Licenciatura, Facultad de Química, Universidad Nacional Autónoma de México
- Ríos MY, Delgado G. (1999). Lewis acid catalyzed transformations of Z-ligustilide. Rev Soc Quim Mex 43:127–32
- Rivero-Cruz I, Juárez K, Zuluaga M, et al. (2012). Quantitative HPLC analyses for determining two of the major active phthalides from Ligusticum porteri roots. J AOAC Int 95:84–91
- Terrell B, Fennell A. (2009). Oshá (Bear root). Ligusticum porteri J. M. Coult & Rose var. porteri. Native Plants 10:111–18
- Turi C, Murch SJ. (2010). The genus Ligusticum in North America: An ethnobotanical review with special emphasis upon species commercially known as ‘Osha’. Herbal Gram 89:40–51
- Wang J, Du J, Wang Y, et al. (2010). Z-Ligustilide attenuates lipopolysaccharide-induced proinflammatory response via inhibiting NF-κB pathway in primary rat microglia. Acta Pharmacol Sin 31:791–7
- Zimmerman M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–10
- Zschoke S, Liu JH, Stuppner H, Bauer R. (1998). Comparative study of roots of Angelica sinensis and related umbelliferous drugs by thin layer chromatography, high-performance liquid chromatography, and liquid chromatography–mass spectrometry. Phytochem Anal 9:283–90