Abstract
Context: Herbal preparation of Pao pereira [Geissospermum vellosii Allem (Apocynaceae)] has long been used by oncologic patients and Integrative Medicine practitioners in South America. However, its anticancer activities have not been systematically studied.
Objective: To investigate the anticancer effects of β-carboline alkaloids-enriched extract from Pao pereira (Pao), either alone or in combination with carboplatin, in preclinical ovarian cancer models.
Materials and methods: Cytotoxicity of Pao (0–800 µg/ml) against different ovarian cancer cell lines and an immortalized epithelial cell line was detected by flow cytometry, MTT assay and colony formation in soft agar. Combination of Pao and carboplatin, a primary chemotherapeutic drug for ovarian cancer, was evaluated using Chou-Talalay’s methods. Mice bearing intraperitoneally spread ovarian cancer were treated with 20 or 50 mg/kg/day Pao by i.p. injection. Carboplatin at 15 mg/kg/week i.p. was compared and combined to Pao treatments.
Results: Pao selectively inhibited ovarian cancer cell growth with IC50 values of 180–235 µg/ml, compared to 537 µg/ml in normal cells. Pao induced apoptosis dose- and time-dependently and completely inhibited colony formation of tumor cells in soft agar at 400 µg/ml. Pao greatly enhanced carboplatin cytotoxicity, with dose reduction (DRIs) for carboplatin at 1.2–10 fold. In vivo, Pao alone suppressed tumor growth by 79% and decreased volume of ascites by 55%. When Pao was combined with carboplatin, tumor inhibition reached 97% and ascites was completely eradicated.
Discussion and conclusion: Pao possess potent antitumor activity and could enhance carboplatin effect, and therefore holds therapeutic potential in the treatment of ovarian cancer.
Introduction
Ovarian cancer causes more deaths than any other cancer of the female reproductive system. Due to lack of sufficiently accurate screening approaches in the early detection of ovarian cancer, the majority of cases (63%) are diagnosed at advanced and distant stage (Beller et al., Citation2006; Buys et al., Citation2005; Chen et al., Citation2011). These patients suffer from a dismal prognosis and severely impaired quality of life. Though primary therapy has improved 5-year survival, it has not increased the overall rate of cure (Bast, Citation2011), because more than 70% of ovarian cancer patients relapse and develop resistance to platinum- and taxane-based treatment (Beller et al., Citation2006; Monk & Coleman, Citation2009). Malignant ascites resistant to conventional chemotherapy affects 28% of ovarian cancer patients in their last period of life (Bellati et al., Citation2010). There is an urgent need for novel and effective treatment options for advanced ovarian cancer.
Numerous studies have attempted to improve the efficacy of standard platinum-based therapy for ovarian cancer by incorporating newer cytotoxic agents. Natural products have long been proven a bountiful resource for bioactive anticancer agents. Combination of natural compounds to standard chemotherapeutic drugs may exert additive or synergistic effects in killing cancer cells, therefore would achieve better therapeutic effect or allow lower and safer drug doses to be applied. One of such examples is the success of taxol as a chemotherapeutic agent, which was first isolated from the bark of the Pacific yew tree Taxaceae Taxus brevifolia Nutt. The platinum–taxol combined chemotherapy had achieved much better clinical outcomes in ovarian cancer patients than either drug alone and has become a standard regimen in treating ovarian cancer (Donaldson et al., Citation1994; du Bois et al., Citation1997; Goldberg et al., Citation1996; McGuire et al., Citation1996; Milross et al., Citation1995; Ozols, Citation1995; Pujade-Lauraine et al., Citation1997). In recent decades, numerous experimental and clinical works have been done investigating the anticancer effects of plant extracts, especially those used as folk medicines.
Pao pereira [Geissospermum vellosii Allem (Apocynaceae)] (Pao) extract, an herbal preparation of the bark of the Amazonian tree Pao, has been used traditionally as folk medicine in South American to treat a variety of ailments including cancer. A number of compounds have been identified and described for antiviral, antiplasmodial and antiparasitic activities from Pao extracts. However, the anticancer active components have not been reported to our knowledge. The reported active components were from extracts of plants of the same genus, mainly indole alkaloids and beta-carboline alkaloids. As early as in 1959, three alkaloids were isolated from Geissospermum leave (Vellozo) Baillon: geissoschizoline, apogeissoschizine and geissospermine, but without testing their antitumor activities (Puiseux et al., Citation1959). Later, Steele et al. (Citation2002) reported isolation of the three indole alkaloids and a β-carboline alkaloid flavopereirine from the bark of Geissospermum sericeum and their antiplasmodial activities. Reina et al. (Citation2012) reported seven indole alkaloids from the leaves and three from the bark of Geissospermum reticulatum and the antiparasitic activities of both the extract and each alkaloid. Mbeunkui et al. (Citation2012) isolated a new indole alkaloid along with four known indole alkaloids from the bark of Geissospermum vellosii and described their antiplasmodial activities. However, the anticancer activities of the components were not tested in these studies.
It has been reported the DNA damaging and anticancer activities of flavopereirine along with a few other β-carboline alkaloids (Beljanski & Beljanski, Citation1982, Citation1986; Beljanski et al., Citation1993). We also have reported anticancer activities of beta-carboline alkaloids; however, by using other sources and synthetic compounds (Cao et al., Citation2004; Chen et al., Citation2005). The evidence of Pao anticancer activity is suggestive, however, has been only anecdotal. The anticancer activity of Pao has not yet thoroughly tested. To date the only published study on Pao anticancer activity indicated that Pao suppressed prostate cancer cells (Bemis et al., Citation2009). In this study, we investigated the anticancer activity of Pao in the treatment of ovarian cancer in preclinical models, either used alone or in combination with carboplatin.
Materials and methods
Study materials, cell lines and viability assay
Pao extract was provided by Natural Source International (New York, NY). Aqueous alcoholic extraction from the bark of Pao yielded a proprietary extract which on spray drying yields a free flowing powder containing flavopereirine. Pao was prepared in DMSO and diluted with sterile water. Carboplatin (Sigma, St. Louis, MO) was prepared in sterile water and stock at −20 °C.
Human ovarian cancer cell lines OVCAR-5 and OVCAR-8 were obtained from the American Type Culture Collection (Manassas, VA), SHIN-3 was donated by Dr. Perter Eck at the National Institutes of Health (Imai et al., Citation1990). Immortalized human epithelial cells MRC-5 was provided by Dr. Sittampalam at the University of Kansas Medical Center. All the cells were cultured at 37 °C in 5% CO2/95% air in recommended growth media containing 10% fetal calf serum.
Cells in exponential growth phase were exposed to serial dilutions of Pao, carboplatin or the combination of the two for 48 h. Control cells were treated with 1% DMSO which was equivalent to the DMSO amount in the Pao working solution. Then cells were changed into fresh media containing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and were incubated for 4 h. The colorimetric MTT assay assessed relative proliferation, based on the ability of living, but not dead cells to reduce MTT to formazan (Cole, Citation1986; Denizot & Lang, Citation1986). Cells did not reach plateau phase during the incubation period. Fifty percent inhibitory concentration (IC50) was defined as the concentration of drug that inhibited cell growth by 50% relative to vehicle-treated control. Pilot experiments for each cell line were performed to optimize cell density and assay duration and to center drug dilution series approximately on the IC50.
Flow cytometry for detection of apoptosis versus necrosis
Cells were exposed to various concentrations of Pao for 48 h. Cells were washed in PBS, resuspended in binding buffer and subjected to FITC-conjugated annexin V and propidium iodide (PI) double staining according to the manufacturer’s protocol (BD Biosciences, San Jose, CA). Cells were analyzed by flow cytometry. Annexin V positive and Annexin V-PI double positive cells were identified as apoptotic cells. PI positive cells were identified as necrotic cells.
Anchorage-independent colony formation assay
Anchorage-independent colony formation assay in soft agar was utilized to determine long-term survival of tumor cells and survival of tumorigenic cancer cells in vitro after the treatments. In 6-well plates, SHIN-3 cells (5000 cells per well) were seeded in the upper layer containing 0.5% agar, DMEM medium and 10% FBS, with or without 400 μg/ml Pao. The solid agar base (lower layer) contained 0.75% agar in complete medium with or without 400 μg/ml Pao, respectively. Cells were incubated for 20 days. Colonies were visualized by crystal violet staining and counted.
Western blot
Forty micrograms of protein were loaded for SDS-polyacrylamide gel electrophoresis. Western blots were performed routinely, with specific primary and secondary antibodies from Cell Signaling Technology Inc. (Danvers, MA): rabbit anti-poly-(ADP-ribose)-polymerase (PARP) (1:2000), rabbit anti-caspase-3 (1:1000), rabbit anti-capase-8 (1:1000), mouse anti-β-actin (1:1000) and goat anti-rabbit or anti-mouse IgG (1:5000). The secondary antibodies were conjugated with horseradish peroxidase. Blots were developed using chemiluminescent substrate Pierce ECL2 (Thermo Scientific, Waltham, MA).
Intraperitoneal ovarian cancer mouse model
SHIN-3 cells were inoculated intraperitoneally (2.6 × 106/mouse) into nude mice. Seven days after tumor cell inoculation, treatment began with i.p. (intraperitoneal) injection of carboplatin (Cp, 15 mg/kg weekly), Pao (20 or 50 mg/kg daily, dissolved in DMSO and diluted with sterile water to contain 5% DMSO in the working solution), the respective combination of Cp and Pao, and 5% DMSO as control. After 23 days of treatment, mice were euthanized. All tumor lesions in the peritoneal cavity were collected and weighed. Ascites was collected, and non-blood cells were counted in ascitic fluids as an index reflecting tumor cells in ascitic fluids. Major organs such as liver, kidney and spleen were fixed in 4% formaldehyde and subjected to histological analysis for any damage due to potential drug toxicity.
Data analysis
MTT data were normalized to their corresponding controls for each condition (drug, cell type) and were expressed as percentage viability. Dose reduction index (DRI) for carboplatin were calculated by the equation DRIICx = (DCp/DCp+Pao), where DCp is the dose of carboplatin alone required to produce an ICx level of cytotoxicity, and the divisor DCp+Pao is the dose of carboplatin needed to produce the same ICx level of cytotoxicity when it is combined with Pao (at a given molar ratio). DRICp is defined with respect to carboplatin. SPSS15.0 (SPSS Inc., Chicago, IL) was used for additional statistical analysis.
Results
Effect of Pao pereira extract (Pao) against ovarian cancer cells
Human ovarian cancer cell lines (SHIN-3, OVCAR-5 and OVCAR-8) were compared to an immortalized non-tumorigenic epithelial cell line (MRC-5) for sensitivity to Pao. The dose-response curves showed that cancer cells were more sensitive to Pao treatment than the non-cancerous cells MRC-5 (). The IC50 values for the cancer cells ranged from 180 to 235 µg/ml, while the IC50 to the non-cancerous cells was 537 µg/ml, almost two-fold higher ().
Figure 1. Cytotoxicity of Pao in ovarian cancer cells. (A) Dose–response curves of ovarian cancer cells and non-cancerous cells to Pao. Human ovarian cancer cells SHIN-3, OVCAR-5 and OVCAR-8 were exposed to serial concentrations of Pao for 48 h, and cell viabilities were detected by MTT assay. An immortalized epithelial cell MCR-5 was subjected to the same treatment. IC50 was defined as the concentration of drug that inhibited cell growth by 50% relative to the vehicle-treated control. All values are expressed as means ± SD of three independent experiments each done in triplicates. (B) Colony formation of SHIN-3 cells in soft agar with and without Pao treatment. Five thousand SHIN-3 cells per well in 6-well plate were either treated with 400 µg/ml Pao (Pao) or vehicle containing DMSO (Control). No colonies were formed in the Pao-treated cells. All values are expressed as means ± SD of three independent experiments.
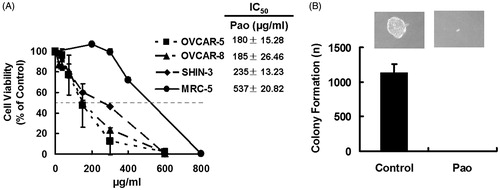
Colony formation in soft agar was used to assess long-term survival of tumorigenic cancer cells, which has been positively correlated to in vivo tumorigenicity of the cancer cells in animal models (Eagle et al., Citation1970; Zeng et al., Citation2012). As shown in , vehicle-treated SHIN-3 cells formed colonies at a rate of 23% (1135/5000). Pao at 400 µg/ml completely inhibited formation of colonies of these cells, indicating no survival of tumorigenic cancer cells with this treatment.
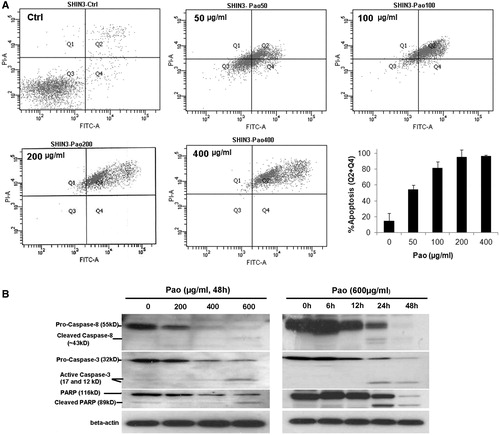
Apoptosis was detected in SHIN-3 cells treated with Pao. Flow cytometry demonstrated that the percentage of cells positive with Annexin V/PI straining increased from 5.01% in vehicle-treated control cells to 95% in 200 or 400 μg/ml Pao treated cells (). Apoptosis was induced dependent on Pao concentrations and was the predominant form of cell death induced by Pao. Necrosis contributed less than 15% of total cell death at all treatment conditions (). In consistence, western blot analysis detected extensive cleavage of caspase-8 and caspase-3 and PARP in Pao treated SHIN-3 cells. Cleavages of these molecules indicated apoptosis and were dependent on the concentrations of Pao and the time of treatment ().
Figure 2. Apoptosis in ovarian cancer cells induced by Pao. (A) Flow-cytometry detection of apoptotic cells. Representative flow cytometry graphs were shown. SHIN-3 cells were treated with Pao at 0, 50, 100, 200 or 400 μg/ml for 48 h, and then subjected to FITC affiliated Annexin-V and PI double staining and flow cytometry. FITC positive cells (Q4, early apoptosis) and FITC/PI double positive cells (Q2, late apoptosis) were identified as apoptotic cells. The apoptosis rate was represented in the bar graph which presents means ± SD of three independent experiments. (B) Cleavage of caspase-8, caspase-3 and PARP in SHIN-3 cells treated with Pao. Cells were treated with Pao at indicated concentrations and time. The dose-dependent and time-dependent cleavage of caspase-8, caspase-3 and PARP were detected by western blots.
Potentiation of carboplatin effect against ovarian cancer cells by combination with Pao
With the positive results that Pao induced preferential ovarian cancer cell death, we then evaluated the combination effect of Pao with the conventional chemotherapeutic drug carboplatin. After determined the dose–response relationships for Pao (), the dose–response relationships for carboplatin (Cp) cytotoxicity were established in SHIN-3, OVCAR-5 and OVCAR-8 cells (, dotted lines). Chou-Talalay’s constant ratio design was used to systematically examine combination dose–response relationships between carboplatin and Pao. Ratio of Cp: Pao was chosen as IC50Cp:IC50Pao. Combination data were presented in terms of carboplatin concentration. If the Cp + Pao combinations were more potent than carboplatin as a single agent, then the dose–response curves would be shifted leftward relative to curves generated with carboplatin alone. Alternatively, a right shift would indicate that Pao pairing with carboplatin was less potent (antagonistic) with respect to carboplatin mono-therapy. The results showed an unambiguous leftward shift in the dose–response curves in Cp + Pao combinations for all cell lines compared to the corresponding curves with carboplatin as a single agent ().
Figure 3. Effect of Pao and carboplatin combinations on ovarian cancer cells. (A–C) Ovarian cancer cells were treated with carboplatin (Cp, dotted line) and the combination of carboplatin and Pao (Cp + Pao, solid line) for 48 h. The combination took the molar ratio of IC50Pao:IC50Cp. Cell viabilities were plotted against carboplatin concentrations. All values are expressed as means ± SD of three independent experiments each done in triplicates. (D) Dose reduction index (DRI) for carboplatin across the fraction affected (fa) when Pao was combined.
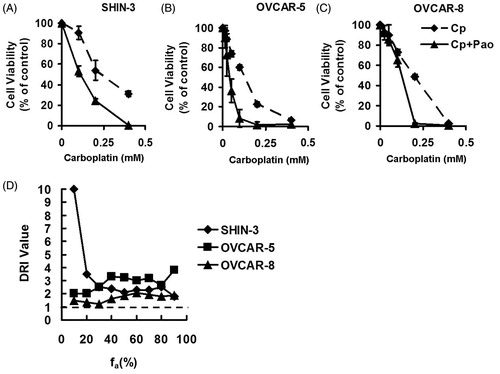
In order to evaluate whether Pao could potentiate carboplatin effect on ovarian cancer cells, dose reduction index (DRI) was evaluated against carboplatin. A DRI of >1 would indicate potentiation of carboplatin effect by pairing with Pao, and a DRI of <1 would be interpreted as an antagonistic combination. In all cell lines tested, DRI values for carboplatin were >1 () across the desired levels of effect (fa, fraction affected). Reduction in carboplatin doses ranged from 1.2- to 10-fold when Pao was combined, depending on cell lines and the aimed level of effect. These data unequivocally support the conclusion that carboplatin effect was enhanced when Pao was combined, and the concentration of carboplatin can be decreased to produce an equitoxic effect on ovarian cancer cells when Pao was combined.
In vivo tumor inhibitory effect of Pao either alone or in combination with carboplatin
An intraperitoneally implanted SHIN-3 tumor model was used to evaluate the effect of Pao and carboplatin plus Pao (Cp + Pao) treatment. Compare to subcutaneous tumor model, the intraperitoneal tumor model better mimics clinical conditions of human ovarian cancer, especially in peritoneal metastasis and ascitic fluid formation, which are common in patients with advanced ovarian cancer.
As shown in , Pao treatment alone decreased tumor weight by 58% and 79% at the daily dose of 20 or 50 mg/kg, respectively, compared to DMSO-treated controls. Carboplatin at the dose of 15 mg/kg/week decreased tumor weight by 45%. By combining Pao to carboplatin, the tumor inhibitory effect was dramatically enhanced. Tumor weight decreased 89% (Cp + 20 mg/kg Pao) and 97% (Cp + 50 mg/kg Pao) relative to control. The enhancement was significant compared to either Pao or carboplatin single-drug treatment.
Figure 4. Effect of Pao and the combination of Pao and carboplatin against ovarian cancer in an intraperitoneal mouse model. SHIN-3 ovarian cancer cells (2.6 × 106 cells) were intraperitoneally inoculated into nude mice. Seven days after tumor cell inoculation treatment commenced with i.p. injection of Pao at 20 mg/kg/day (Pao20) or 50 mg/kg/day (Pao50), carboplatin 15 mg/kg/week (Cp), and respective combinations of Pao and carboplatin (Cp + Pao20 and Cp + Pao50). Control mice were injected with 5% DMSO. Treatment lasted for 23 days. (A) Total tumor weight. (B) Volume of ascites. (C) Number of non-blood cells in the ascitic fluids, shown as an index of tumor cells in the ascites. (D) Cleavage of capase-3 and PARP in tumor samples from different treatment groups. (E) H&E staining of major organs from different treatment groups. Kidney, liver and spleen were collected from each treatment group and fixed in 4% formaldehyde and later subjected to histological analysis. (*p < 0.05, **p < 0.01, ***p < 0.001 relative to control group; #p < 0.05, ##p < 0.01, ###p < 0.001 relative to carboplatin treated group.)
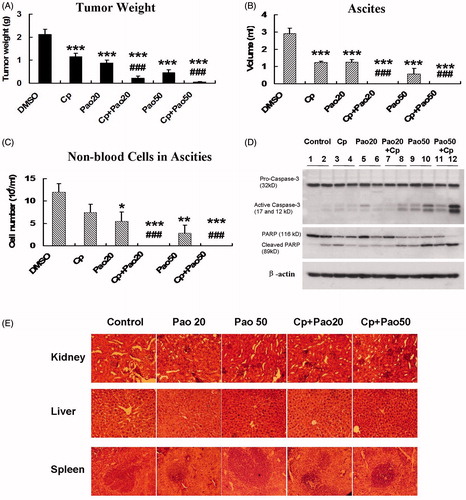
Excessive amount of ascitic fluid was formed in mice of control group at the end point of the experiment (3 ml ascites/mouse in average). Pao treatment alone significantly decreased the volume of ascitic fluid to an average of 1.3 ml/mouse at 20 mg/kg and to 0.6 ml/mouse at 50 mg/kg (p < 0.001). These effects were comparable to carboplatin. By combining Pao to carboplatin at either 20 or 50 mg/kg Pao, formation of ascetic fluid was completely eradicated ().
Non-blood cells in the ascitic fluid were counted as an estimation of tumor cells in the ascites. The results were shown as cell numbers/ml of ascites. Whereas carboplatin at the used does did not significantly reduce the number of cells in ascites, Pao showed a strong effect in decreasing cell numbers/ml of ascites by 54% and 77% at the indicated doses (). As there was no ascitic fluid in the combination treatment groups, the cell numbers in the ascites were shown as zero in .
Taken together, Pao not only inhibited ovarian cancer growth, but also inhibited ascites formation and reduced tumor cells presenting in the ascites. When Pao was combined with the conventional chemo-drug carboplatin, the anti-tumor effect was dramatically enhanced.
Proteins were isolated from tumor samples of the treated and control mice. Western blot analysis showed massive cleavage of caspase-3 and PARP in Pao and Cp + Pao treatment groups at either high or low doses of Pao (). These results confirmed the in vitro data that Pao induced apoptosis in tumor cells.
All mice did not show observable toxicity associated with the treatments. At the end of the experiment, major organs (kidney, liver and spleen) were subjected to H&E staining and histological analysis. No tissue damages were detected in the treatment groups, and there was not significant differences between control group and treated groups (). These data demonstrated that Pao at the used doses was low-toxic either alone or combined with carboplatin.
Discussion
Pao extract is one of the many herbal remedies that oncology patients are using. It contains the alkaloid flavopereirine (also called PB-100), as well as many other kinds of indole and β-carboline alkaloids (Puiseux et al., Citation1959; Reina et al., Citation2012; Steele et al., Citation2002). According to anecdotal reports and some inconclusive evidence, flavopereirine or Pao may be of benefit for the treatment of malaria, cancer (including prostate cancer, glioblastoma and leukemia), HIV/AIDS, herpes simplex and hepatitis C (Beljanski & Beljanski, Citation1982; Beljanski et al., Citation1993; Bemis et al., Citation2009; Reina et al., Citation2012; Steele et al., Citation2002). However, Pao is commonly administered together with other herbal remedies, including ginkgo (Ginkgo biloba) and the indole alkaloid alstonine, which may have antipsychotic properties. The scientific basis for using Pao extract in cancer treatment has not been rigorously tested. Here, we investigated the anti-ovarian cancer activity of Pao extract either alone, or in combination with the front-line chemotherapy carboplatin, using rigorous preclinical cancer models. Our data clearly showed that P. pereira extract exhibited a substantial inhibitory effect against ovarian cancer cells, both in vitro and in vivo.
Inherent or acquired drug resistance has been a pressing problem in the success of ovarian cancer therapy. Combination of platinum-based chemotherapy with other anticancer drugs has been an effective way to improve therapeutic outcome (Bell-McGuinn et al., Citation2011; Fu et al., Citation2012; Weroha et al., Citation2011). The activities of Pao extract is of interest. Despite the inherent carboplatin sensitivity of the cell lines, Pao increased chemosensitivity of all tested ovarian cancer cells and synergized with carboplatin. The in vitro results were consistent with in vivo inhibition of tumor growth and ascites formation in ovarian cancer-bearing mice. Remarkably, with Pao and carboplatin combination treatment, almost complete tumor inhibition (97%) was achieved, and the ascites was completely diminished. This effect was not achieved by carboplatin treatment alone.
Moreover, our data showed Pao had relatively low toxicity toward normal cells. The low toxicity was evident in mice treated with Pao that major organ toxicities were absent. In addition, by the dose-reduction effect, Pao could lower down the dose of carboplatin in achieving an equitoxic effect with higher carboplatin dose alone, therefore may allow decrease of carboplatin toxicity. Collectively, the antitumor activities, synergy with carboplatin and low-toxicity with Pao suggested that this herbal preparation could potentially offer therapeutic benefit to ovarian cancer patients.
Pao induced predominant apoptotic cell death in ovarian cancer cells as shown with our flow cytometry data, which is a powerful tumor-suppressive pathway potentially depleting stem-like and progenitor cancer cell pools (Chao et al., Citation2012; Chen, Citation2012). Consistent with this potential, our data showed Pao completely inhibited the in vitro tumorigenic capacity of ovarian cancer cells in soft agar. However, as this plant preparation contains a complex mixture of natural compounds, multiple molecular targets and pathways might be involved. As the antitumor activity can be tracked down to purified compounds in future studies, the molecular mechanism of action can be further elucidated. With the elucidation of its mechanism(s) of action, it is also expected to add to our understanding to the preferential cytotoxicity toward cancer cells versus normal cells.
Preponderance of bioactive compounds is often found in medical plant and herbal mixtures, making them a superb source for discovery of novel drug leads. The indole and β-carboline alkaloids-enriched Pao extract could contain compounds that possess potent anticancer activity. The data presented herein is the initial step in identifying of the anticancer activity of Pao. Active components could be isolated and developed for optimizing efficacy, toxicity and other profiles that could lead to anticancer drug development.
Declaration of interest
The authors report no declaration of interest.
Acknowledgements
This study was supported by a grant from the Beljanski Foundation and the plant extract was provided by Natural Source International Ltd. The authors have no affiliation or financial relationship with the sponsor. Neither the Beljanski Foudation nor Natural Source International Ltd. played a role in the design and conduction of the study and preparation of the manuscript.
References
- Bast RC Jr. (2011). Molecular approaches to personalizing management of ovarian cancer. Ann Oncol 22:viii5–15
- Beljanski M, Beljanski MS. (1982). Selective inhibition of in vitro synthesis of cancer DNA by alkaloids of beta-carboline class. Exp Cell Biol 50:79–87
- Beljanski M, Beljanski MS. (1986). Three alkaloids as selective destroyers of cancer cells in mice. Synergy with classic anticancer drugs. Oncology 43:198–203
- Beljanski M, Crochet S, Beljanski MS. (1993). PB-100: A potent and selective inhibitor of human BCNU resistant glioblastoma cell multiplication. Anticancer Res 13:2301–8
- Bell-McGuinn KM, Matthews CM, Ho SN, et al. (2011). A phase II, single-arm study of the anti-alpha5beta1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer. Gynecol Oncol 121:273–9
- Bellati F, Napoletano C, Ruscito I, et al. (2010). Complete remission of ovarian cancer induced intractable malignant ascites with intraperitoneal bevacizumab. Immunological observations and a literature review. Invest New Drugs 28:887–94
- Beller U, Quinn MA, Benedet JL, et al. (2006). Carcinoma of the vulva. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 95:S7–27
- Bemis DL, Capodice JL, Desai M, et al. (2009). Beta-carboline alkaloid-enriched extract from the amazonian rain forest tree pao pereira suppresses prostate cancer cells. J Soc Integr Oncol 7:59–65
- Buys SS, Partridge E, Greene MH, et al. (2005). Ovarian cancer screening in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial: Findings from the initial screen of a randomized trial. Am J Obstet Gynecol 193:1630–9
- Cao R, Chen Q, Hou XR, et al. (2004). Synthesis, acute toxicities, and antitumor effects of novel 9-substituted beta-carboline derivatives. Bioorg Med Chem 12:4613–23
- Chao MP, Majeti R, Weissman IL. (2012). Programmed cell removal: A new obstacle in the road to developing cancer. Nat Rev Cancer 12:58–67
- Chen F. (2012). JNK-induced apoptosis, compensatory growth, and cancer stem cells. Cancer Res 72:379–86
- Chen H, Hardy TM, Tollefsbol TO. (2011). Epigenomics of ovarian cancer and its chemoprevention. Front Genet 2:67
- Chen Q, Chao RH, Chen HS, et al. (2005). Antitumor and neurotoxic effects of novel harmine derivatives and structure--activity relationship analysis. Int J Cancer 114:675–82
- Cole SP. (1986). Rapid chemosensitivity testing of human lung tumor cells using the MTT assay. Cancer Chemother Pharmacol 17:259–63
- Denizot F, Lang R. (1986). Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89:271–7
- Donaldson KL, Goolsby GL, Wahl AF. (1994). Cytotoxicity of the anticancer agents cisplatin and taxol during cell proliferation and the cell cycle. Int J Cancer 57:847–55
- du Bois A, Luck HJ, Merer W, et al. (1997). Carboplatin/paclitaxel versus cisplatin/paclitaxel as first-line chemotherapy in advanced ovarian cancer: An interim analysis of a randomized phase III trial of the Arbeitsgemeinschaft Gynakologische Onkologie Ovarian Cancer Study Group. Semin Oncol 24:S15-44–52
- Eagle H, Foley GE, Koprowski H, et al. (1970). Growth characteristics of virus-transformed cells. Maximum population density, inhibition by normal cells, serum requirement, growth in soft agar, and xenogeneic transplantability. J Exp Med 131:863–79
- Fu S, Hennessy BT, Ng CS, et al. (2012). Perifosine plus docetaxel in patients with platinum and taxane resistant or refractory high-grade epithelial ovarian cancer. Gynecol Oncol 126:47–53
- Goldberg JM, Piver MS, Hempling RE, Recio FO. (1996). Paclitaxel and cisplatin combination chemotherapy in recurrent epithelial ovarian cancer. Gynecol Oncol 63:312–17
- Imai S, Kiyozuka Y, Maeda H, et al. (1990). Establishment and characterization of a human ovarian serous cystadenocarcinoma cell line that produces the tumor markers CA-125 and tissue polypeptide antigen. Oncology 47:177–84
- Mbeunkui F, Grace MH, Lategan C, et al. (2012). In vitro antiplasmodial activity of indole alkaloids from the stem bark of Geissospermum vellosii. J Ethnopharmacol 139:471–7
- McGuire WP, Hoskins WJ, Brady MF, et al. (1996). Cyclophosphamide and cisplatin versus paclitaxel and cisplatin: A phase III randomized trial in patients with suboptimal stage III/IV ovarian cancer (from the Gynecologic Oncology Group). Semin Oncol 23:40–7
- Milross CG, Peters LJ, Hunter NR, et al. (1995). Sequence-dependent antitumor activity of paclitaxel (taxol) and cisplatin in vivo. Int J Cancer 62:599–604
- Monk BJ, Coleman RL. (2009). Changing the paradigm in the treatment of platinum-sensitive recurrent ovarian cancer: From platinum doublets to nonplatinum doublets and adding antiangiogenesis compounds. Int J Gynecol Cancer 19:S63–7
- Ozols RF. (1995). Combination regimens of paclitaxel and the platinum drugs as first-line regimens for ovarian cancer. Semin Oncol 22:1–6
- Puiseux F, LeHir A, Goutarel R, et al. (1959). On the alkaloids of “pao-pereira”, Geissospermum laeve (Vellozo) Baillon. Note III. Geissoschizoline, apogeissoschizine and geissospermine. Ann Pharm Fr 17:626–33
- Pujade-Lauraine E, Guastalla JP, Weber B, et al. (1997). Efficacy and safety of the combination paclitaxel/carboplatin in patients with previously treated advanced ovarian carcinoma: A multicenter French Groupe des Investigateurs Nationaux pour l'Etude des Cancers Ovariens phase II study. Semin Oncol 24:S15-30–35
- Reina M, Ruiz-Mesia W, Lopez-Rodriguez M, et al. (2012). Indole alkaloids from Geissospermum reticulatum. J Nat Prod 75:928–34
- Steele JC, Veitch NC, Kite GC, et al. (2002). Indole and beta-carboline alkaloids from Geissospermum sericeum. J Nat Prod 65:85–8
- Weroha SJ, Oberg AL, Ziegler KL, et al. (2011). Phase II trial of lapatinib and topotecan (LapTop) in patients with platinum-refractory/resistant ovarian and primary peritoneal carcinoma. Gynecol Oncol 122:116–20
- Zeng G, Cai S, Liu Y, Wu GJ. (2012). METCAM/MUC18 augments migration, invasion, and tumorigenicity of human breast cancer SK-BR-3 cells. Gene 492:229–38

