Abstract
Context: Male infertility is one of the leading causes of social frustration and marginalization, mainly in the developing world. It is attributed to many factors including exposure to agropesticides such as manganese ethylenebis (dithiocarbamate) (maneb), which is one of the most frequently used fungicides in Cameroon. Previous reports support efficiency of some medicinal plants commonly used in Cameroonian folk medicine for the treatment of this disorder.
Objective: The present study was aimed at assessing the protective effect of extracts from selected plant species, namely Basella alba L. (Basellaceae) (MEBa) and Carpolobia alba G. Don (Polygalaceae) (AECa), in alleviating the maneb-induced impairment of male reproductive function in Wistar albino rats.
Materials and methods: The rats were treated with vehicle, plant extract (MEBa or AECa), maneb and maneb plus plant extract, respectively, and their fertility was assessed. Animals were thereafter sacrificed and organs (liver, kidneys and reproductive organs) were dissected out and weighed. Serum androgens together with alanine aminotransferase, liver glutathione and thiobarbituric acid reactive species (TBARS) were also measured.
Results and discussion: From this study, both plant extracts stimulated testosterone and improved fertility. Administration of MEBa plus maneb prevented fertility reduction by maneb and minimized the inhibitory effect of maneb on testosterone levels. AECa also improved fertility of the maneb-exposed rats, though without restoring testosterone levels, and other investigated parameters remained unaffected by different treatments.
Conclusion: These findings emphasized the beneficial effects of B. alba and C. alba extracts on male fertility, and suggest their protective effect against maneb-induced toxicity in male reproductive function.
Introduction
Infertility is one of the leading causes of social frustration and marginalization, particularly in developing countries. It affects approximately 15% of couples worldwide, with male infertility contributing for about half of the cases (Cui, Citation2010; Okonofua, Citation2003; Pinto-Gouveia, Citation2012). Male infertility can be caused by many factors such as infection, injury, medical treatment and exposure to environmental toxicants.
Among environmental toxicants, pesticides, which have been increasingly used worldwide, and particularly in developing countries to increase production of crops and to eliminate some diseases, are nowadays a matter of concern (Bryden et al., Citation2005; Nweke & Sanders, Citation2009). An example of pesticide is manganese ethylenebis (dithiocarbamate) (maneb), a fungicide most frequently used by farmers in West Cameroon. Maneb has been shown to decrease fertility and sperm number in male rats/rabbits, at least in part, through impairment of thyroid function and inhibition of steroid production by Leydig cells (Mallem et al., Citation2007; Manfo et al., Citation2011a, Citation2012). Moreover, our study conducted in the West Region of Cameroon revealed that farmers, who were using maneb among other agropesticides, reported more reproduction difficulties and showed androgen hormone imbalances (lower serum testosterone and higher androstenedione levels) (Manfo et al., Citation2012).
Management of male infertility involves the use of assisted reproductive technologies, synthetic molecules or herbal drugs. As these technologies and synthetic molecules are costly and have considerable drawbacks, herbal drugs, thought to be natural and safer, have been playing an important role in healthcare of the populations in the developing world (Aschwanden, Citation2001; Bandaranayake, Citation2006; Chan et al., Citation2000). Herbal drugs have even been encouraged by the World Health Organization, who urged researchers to assess their use (WHO, Citation2002). In this respect, previous studies conducted by our research team demonstrated the androgenic effect of the extracts from Basella alba L. (Basellaceae) and Carpolobia alba G. Don (Polygalaceae), therefore justifying their use by traditional healers in Cameroon to improve male fertility and virility. Both extracts significantly improved serum testosterone level and/or fertility in male rats (Manfo et al., Citation2011b; Moundipa et al., Citation1999, Citation2005, Citation2006; Nantia et al., Citation2007, Citation2012). This beneficial effect of the medicinal plants suggests their efficiency as scavengers or protective candidates against maneb-induced male infertility. The present work was thus designed to investigate whether these plant extracts could alleviate the adverse effects of maneb on male reproductive function, especially in rats.
Materials and methods
Plant materials and preparation of the plant extracts
Roots of C. alba (identified by Dr Onana Jean Michel, at the Cameroonian National Herbarium, as specimen N° 30167) were harvested in 2002 in Kribi (South Region of Cameroon), dried and ground into fine powder. The powder was then macerated in distilled water, filtered and the filtrate was lyophilized to yield the extract (AECa) (Manfo et al., Citation2011b).
Fresh leaves of B. alba (identified by Dr Onana Jean Michel, at the Cameroonian National Herbarium, as specimen N° 40720) were collected in 2005 in Dschang (West Region of Cameroon), dried at room temperature and ground into powder. The methanol extract of B. alba (MEBa) was obtained through successive extraction in hexane, methylene chloride and methanol (Nantia et al., Citation2007).
The animal model
Wistar albino male and female rats (2.5-month-old, 150–200 g body weight) from the animal house of the Laboratory of Pharmacology and Toxicology (Department of Biochemistry, University of Yaoundé I, Cameroon) were used. They were handled according to ethical guidelines of the Cameroon National Veterinary Laboratory.
Experimental design
Forty male rats were divided into two batches of four groups (n = 5 animals) each. The four groups of the first batch were treated daily with MEBa suspended in 2% starch solution (2% SS), 2% SS, maneb dissolved in 0.9% NaCl, or 0.9% NaCl. MEBa and 2% SS were treated orally for 30 days (D1–D30), while maneb (4 mg/kg bwt) and 0.9% NaCl were injected intraperitoneally during the last 18 days of the experiment (Manfo et al., Citation2011a), i.e., from day 12 (D12) to D30. The animals were given food and water ad libitum, and their body weight (bwt) was recorded throughout the experiment at 4 day intervals. Also, a fertility test was performed during the last 6 days of the experiment. For the second batch, the same protocol was followed, by replacing MEBa and 2% SS with AECa (0.1 mg/kg bwt) and distilled water, respectively; and by administering the AECa for 60 days (Manfo et al., Citation2011b). summarizes the treatment procedure described so far.
Figure 1. Protocol of animal treatment for the evaluation of protective effect of AECa and MEBa against maneb toxicity. 2% SS: 2% starch solution, 10 ml/kg; 0.9% NaCl: NaCl 0.9%, 10 mL/kg bwt; MEBa: methanol extract of B. alba, 1 mg/kg; AECa: extract of C. alba, 0.1 mg/kg bwt.
Twenty-four hours following the last treatment with maneb or 0.9% NaCl, each male rat was sacrificed by decapitation, and blood collected for serum preparation. The testes, epididymides, ventral prostate, seminal vesicles, kidneys and liver were dissected out and weighed. The liver was minced and homogenized in Tris buffer (Tris-HCl 50 mM, KCl 150 mM, pH 7.4), centrifuged (10 000 × g, 4 °C, 20 min) and the supernatant (liver homogenate) was collected. The serum and liver homogenate were stored at −40 °C for biochemical analyses.
Assessment of male fertility: fecundity and fertility indices
At the 24th day (for the first batch) or 54th day (for the second batch) of the experiment, four male rats were selected randomly from each group. Each selected male rat was housed with two fertility proven female rats for 6 days (this was chosen because an estrus cycle in female rats last for 5 days). The male rats were then sacrificed as reported above, while females were housed in individual cages and their body weight was monitored for the detection of pregnancy. The pregnant rats were then followed until delivery, and the number of viable pups was recorded, and used to determine the fertility (number of viable pups delivered) and fecundity indices (percentage of male rats that fathered at least one viable pup).
Biochemical analyses
Serum parameters
The activity of alanine aminotransferase (ALT) was measured in serum according to the colorimetric method of Reitman and Frankel (Citation1957). Testosterone and androstenedione levels were quantified by 3H-radioimmunoassay (3H-RIA), as described elsewhere (Manfo et al., Citation2012; Rinaldi et al., Citation2001). Briefly, 0.25 mL of serum was extracted using diethyl ether, and the organic phase was recovered. The organic phase was evaporated, resuspended in 1 mL iso-octane and subjected to celite partition chromatography. Samples were eluted with 4 mL isooctane (fraction 1), 4 mL ethyl acetate/isooctane (13/100 v/v: fraction 2) and 5.5 mL ethyl acetate/isooctane (20/-100 v/v: fraction 3). Fractions 2 and 3, containing androstenedione and testosterone, respectively, were dried by evaporation separately and redissolved in 0.5 mL phosphate albumin buffer (0.1 M, pH 7.4, containing 0.1% bovine serum albumin) for further 3H-RIA. For estimation of recovery, 6000 dpm 3H-testosterone/androstenedione was added as internal standards prior to extraction and the radioactivity was thereafter counted in 200 µl aliquot of the redissolved androgen.
Liver parameters
The levels of glutathione (GSH) and thiobarbituric acid reactive substances (TBARS) were determined in liver homogenates using Ellman reagent (Ellman, Citation1959) and the method of Wilbur et al. (Citation1949), respectively. The latter parameters were normalized relative to homogenate protein concentrations determined as described by Gornall et al. (Citation1949), with bovine serum albumin as standard.
Statistical analyses
Data were analyzed by one way ANOVA or the Kruskal–Wallis test when appropriate. When ANOVA was significant, the Student–Newman–Keuls test was used for pair comparison between means. The analyses were performed using Statistics Package for Social Sciences (SPSS, Chicago, IL) 10.0 program for Windows. A p value of <0.05 was considered as statistically significant.
Results
Body and organ weights
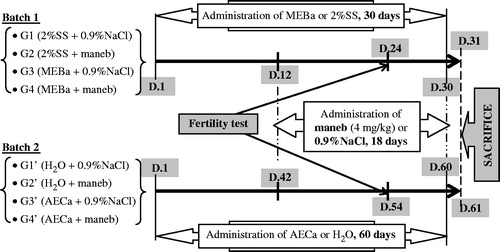
Animal body weight increased along the experiment, irrespective of the treatment. However, there was no significant difference between the groups (). The relative organ weights presented in were also not affected by maneb and/or plant extracts.
Figure 2. Relative body weight of male rats treated with MEBa (A)/AECa (B) and/or maneb. The first batch of male rats was administered MEBa and/or 2% SS for 30 days (A), while the second batch received AECa and/or distilled water for 60 days (B). Maneb and 0.9% NaCl were administered during the 18 last days of the experiment. Relative body weight of each animal was determined using the formula ((At/A0) × 100%: At and A0 represent the animal body weight at the time considered, and at the beginning of the experiment, respectively). Each data point represents the mean ± SD of five animals.
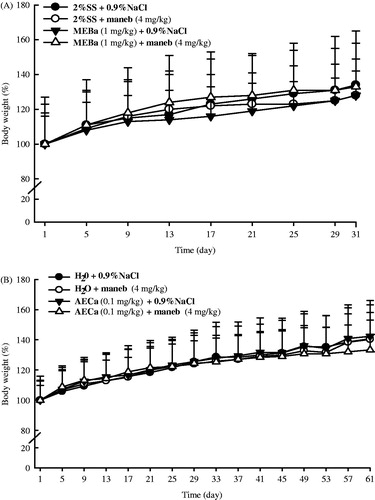
Figure 3. Relative organ weights of male rats following treatment with MEBa (A)/AECa (B) and/or maneb. The first batch of male rats was administered MEBa and/or 2% SS for 30 days (A), while the second batch received AECa and/or distilled water for 60 days (B). Maneb and 0.9% NaCl were administered during the 18 last days of the experiment. Relative body weight of each organ was determined using the formula ((b/B) × 100%: b and B represent the organ weight and animal body weight, respectively). Each data point represents the mean ± SD of five animals.
Animal fecundity and fertility
Fecundity (percentage of male rats that fathered at least one viable pup) and fertility (number of viable pups per female, or litter size) indices of male rats are shown in .
Table 1. Fecundity and fertility indices of male rats treated with MEBa (A)/AECa (B) and/or maneb.
Male rats treated with maneb for 18 days showed a 50% decrease in fecundity. When the rats were treated with maneb and MEBa, the fecundity index was 100%, indicating a recovery fecundity of 50%. Similarly, AECa administration improved fecundity in maneb-treated male rats by 25%.
The fertility index, calculated by taking into consideration the number of females that delivered at least one pup, was significantly higher in male rats treated with MEBa or AECa (p < 0.05), and this improvement was also observed when animals were administered a plant extract and maneb.
Liver and serum biochemical parameters of toxicity
shows liver TBARS and GSH levels of male rats. When compared to respective control group, neither maneb nor the plant extracts affected the two parameters. Likewise, serum alanine aminotransferase was not affected ().
Figure 4. Liver TBARS and GSH levels after treatment of male rats with MEBa (A)/AECa (B) and/or maneb. The first batch of male rats was administered MEBa and/or 2% SS for 30 days (A), while the second batch received AECa and/or distilled water for 60 days (B). Maneb and 0.9% NaCl were administered during the 18 last days of the experiment. The levels of thiobarbituric acid reactive substances (TBARS) and glutathione (GSH) are expressed as mean ± SD of five animals.
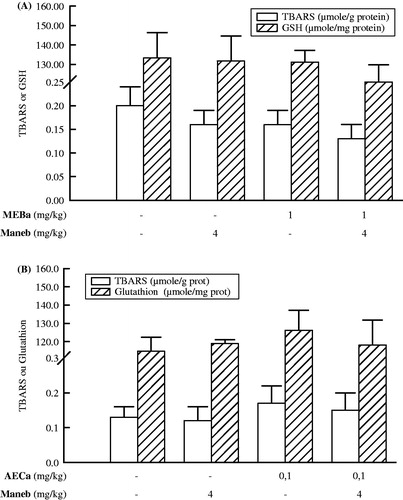
Table 2. Serum alanine aminotransferase activity (ALT) of male rats following evaluation of the protective effect of MEBa (A) and AECa (B).
Serum androgen hormones
Serum androstenedione and testosterone levels of male rats at the end of follow-up are shown in and , respectively. Administration of AECa extract to the rats for 60 days induced a significant increase in the androstenedione levels (p < 0.01), relative to the control treated with distilled water (). However, the latter parameter was not affected by MEBa and maneb.
Figure 5. Serum androstenedione levels in male rats treated with MEBa (A)/AECa (B) and/or maneb. The first batch of male rats was administered MEBa and/or 2% SS for 30 days (A), while the second batch received AECa and/or distilled water for 60 days (B). Maneb and 0.9% NaCl were administered during the 18 last days of the experiment. Data are expressed as mean ± SD of five animals per group. *p < 0.01, compared with the corresponding control group (group receiving neither plant extract nor maneb) (Student–Newman–Keuls test).
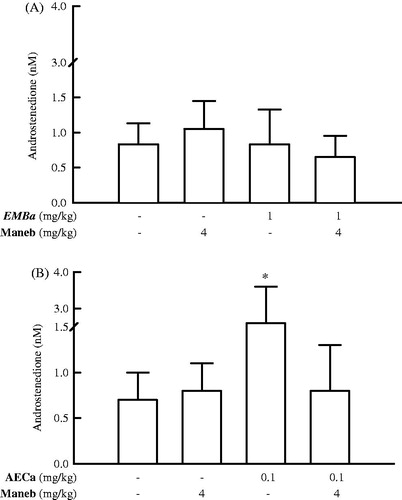
Figure 6. Serum testosterone levels of male rats treated with MEBa (A)/AECa (B) and/or maneb. The first batch of male rats was administered MEBa and/or 2% SS for 30 days (A), while the second batch received AECa and/or distilled water for 60 days (B). Maneb and 0.9% NaCl were administered during the 18 last days of the experiment. Data represent mean ± SD of five animals. ns: nonsignificant; *p < 0.05 and **p < 0.02, compared with the corresponding control group (group receiving neither plant extract nor maneb) (Student–Newman–Keuls test).
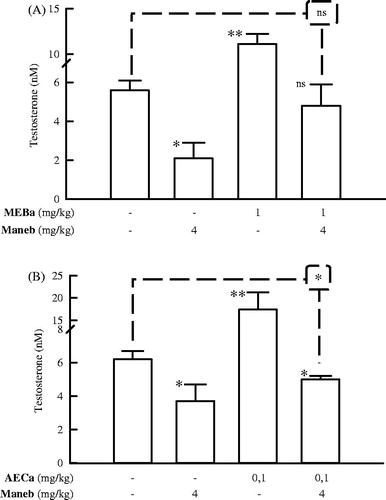
Administration of MEBa and AECa to male rats induced 97% and 180% (p < 0.01) increase in the testosterone level, respectively; while maneb treatment decreased the latter hormone by 40–63% (p < 0.05) (). When the same animals were administered a pesticide and MEBa, the maneb inhibitory effect was alleviated (p > 0.5 versus control) (). Co-administration of AECa and maneb to rats also reduced the impact of maneb on testosterone, though the latter parameter remains significantly lower compared to the control group (p < 0.05 versus control treated with H2O + 0.9% NaCl) ().
Discussion
The present study was aimed at evaluating the protective effect of MEBa and AECa against maneb-induced toxicity, with particular interest in fertility, androgen levels, organs and body weight, and some biochemical parameters of toxicity. We administered MEBa (1 mg/kg bwt/day, for 30 days), AECa (0.1 mg/kg bwt, for 60 days) and maneb (4 mg/kg bwt/day, for 18 days) to rats as defined by our previous studies. MEBa and AECa stimulated testosterone release after 30 and 60 days of treatment, respectively, while 18-day administration of maneb resulted in the inhibition of androgen/testosterone production (Manfo et al., Citation2011a,Citationb; Nantia et al., Citation2007). In addition, AECa stimulated production of androstenedione, a testosterone precursor.
Animal hepatic GSH and TBARS, body weight and organ weights were neither affected by maneb nor the extracts. Fecundity index of the male rats (percentage of male rats that fathered at least one viable pup) was reduced by 50% when treated with maneb, suggesting a decrease in rat fertility, which might be attributed to alteration of sperm quality by the pesticide, as reported earlier in rabbits (Mallem et al., Citation2007). Similarly, Al-Thani et al. (Citation2003) observed impaired spermatogenesis coupled with the reduction in the number of females fertilized by male mice treated with the pesticide amitraz. A decline in fertility was also observed by Joshi et al. (Citation2007), Yu et al. (Citation2009) and Shalaby et al. (Citation2010) after the administration of chlorpyrifos, carbendazim or methomyl pesticides to male rats. Maneb-induced infertility was associated to serum testosterone inhibition. Testosterone is the main androgen implicated in spermatogenesis regulation (although other factors such as FSH, IGF, TGF-β, are also involved in the latter physiological process), and inhibition of its synthesis by maneb (as reported by Manfo et al., Citation2011a) may thus contribute to the reduction of fertility in male rats (Oatley & Brinster, Citation2008; Pryor et al., Citation2000; Verhoeven et al., Citation2010). Correlation between the inhibition of testosterone by pesticide and impaired fertility (semen quality) is further illustrated by investigations on dimethoate and deltamethrin exposure (Afifi et al., Citation1991; Farag et al., Citation2007; Oda & El-Maddawy, Citation2012). The adverse effects induced by maneb are thus of great issue in the preservation of human health and fertility, considering its frequent use by farmers of west Cameroon and elsewhere to improve food production (Manfo et al., Citation2012).
As a contribution towards solving the toxicity posed by maneb, we co-administered the pesticide with MEBa or AECa (plant extracts that exhibit an androgenic effect) (Manfo et al., Citation2011a,Citationb; Nantia et al., Citation2007). When co-administrated with maneb, the MEBa improved fertility index and prevents testosterone inhibition by maneb. This suggests the protective effect of MEBa vis-à-vis maneb toxicity. Early reports on MEBa supported its positive effect to enhance fecundity/fertility in normal male rats and in rats perinatally exposed to the antiandrogen flutamide (Nantia et al., Citation2012). Such observations were made by Rezvanfar et al. (Citation2008), who showed attenuation of cyclophosphamide antiandrogenic and antifertility effects (alteration of testosterone, sperm and fertility) when the chemical was co-administered to rats with Satureja khuzestanica (Lamiaceae) essential oil. Fertility improvement by MEBa may be related to its ability to stimulate production of estradiol (Nantia et al., Citation2011), which protects testicular cells against reactive oxygen species (ROS) and enhances sperm motility (Carreau & Hess, Citation2010; Carreau et al., Citation2010; Hamden et al., Citation2008). The protective effect of B. alba against maneb-induced toxicity may also be due to the presence of antioxidant compounds in the extract. In fact, phytochemical analyses of B. alba conducted in Cameroon (Nantia et al., Citation2013) and elsewhere (Luo et al., Citation2011; Ng et al., Citation2000; Raju et al., Citation2007; Sato et al., Citation2002; Yu et al., Citation2005) revealed the presence of chemicals, or a group of chemicals, with proven antioxidant activity, such as phenol compounds, carotenoids, ascorbic acid, saponins, coumarins and limonoids. Our research group (Nantia et al., Citation2013) also demonstrated the stimulatory effect of MEBa on the testicular antioxidant system. Improving testicular antioxidant status by MEBa may consequently contribute to the elimination of ROS and thereby alleviate maneb-induced reproductive toxicity. Other examples of natural products with protective effect on chemical-induced testicular toxicity include soybean isoflavones (genistein and daidzein), caffeic acid phenethyl ester and Panax ginseng C.A. Mey (Araliaceae) extract (Abdallah et al., Citation2012; Jang et al., Citation2011; Yousef et al., Citation2003).
AECa administration to maneb-treated male rats improved their fertility (fecundity and fertility index), without restoration of serum testosterone levels. Similarly, administration of Paullinia cupana var. sorbilis (Sapindaceae) extract to rats alleviated cadmium-induced testicular inflammation, with no androgenic effect in Cd-exposed animals (Leite et al., Citation2010). Although 52% recovery of the serum testosterone levels in the animals co-treated with maneb and AECa during the current study may not entirely rule out the implication of AECa androgenic effect in protecting animal fertility from maneb, this observation suggests the action of the plant extract through other factors implicated in male reproductive function regulation, such as modulation of paracrine growth factors, and stimulation of the testicular antioxidant defense system (Carreau et al., Citation2010; Hamden et al., Citation2008). Possible antioxidant properties of AECa is supported by the identification of saponins – a group of chemical exhibiting antioxidant properties – from the roots of C. alba (Luo et al., Citation2011; Mitaine-Offer et al., Citation2002; Tapondjou et al., Citation2011).
Concluding remarks
Taken altogether, the results of this study suggest a protective effect of MEBa and AECa against maneb-induced alteration of male reproductive function. Identification of groups of compounds with proven antioxidant properties in the investigated medicinal plants suggests a mechanism through improvement of the antioxidant status, besides the possible contribution of the androgenic effect. However, further investigations will attempt to clarify the implicated mechanism of action, and to identify the pure compounds exhibiting the pharmacological effects.
Declaration of interest
The authors report no declarations of interest. This work was supported by the SCAC (Service d’Action Culturelle et de Coopération), French Embassy in Cameroon, and the Universities of Yaoundé I and Buea (Cameroon).
References
- Abdallah FB, Fetoui H, Zribi N, et al. (2012). Protective role of caffeic acid on lambda cyhalothrin-induced changes in sperm characteristics and testicular oxidative damage in rats. Toxicol Ind Health 28:639–47
- Afifi NA, Ramadan A, El-Aziz MI, Saki EE. (1991). Influence of dimethoate on testicular and epididymal organs, testosterone plasma level and their tissue residues in rats. Deutsch Tierarztliche Wochenschrift 98:419–23
- Al-Thani RK, Al-Thani AS, Elbetieha A, Darmani H. (2003). Assessment of reproductive and fertility effects of amitraz pesticide in male mice. Toxicol Lett 138:253–60
- Aschwanden C. (2001). Herbs for health, but how safe are they? News Features Bull World Health Org 79:691–2.
- Bandaranayake WM. (2006). Quality control screening toxicity and regulation of herbal drugs. In: Ahmad I, Aqil F, Owais M, eds. Modern Phytomedicine. Turning Medicinal Plants into Drugs, Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA, 25–57
- Bryden PA, McKnight RH, Westneat SC. (2005). Using U.S. Poison Control Center records to identify bystander pesticide exposures: A one-year surveillance of four southeastern states. J Agric Saf Health 11:159–66
- Carreau S, Hess RA. (2010). Oestrogens and spermatogenesis. Phil Trans R Soc B 365:1517–35
- Carreau S, Wolczynski S, Galeraud-Denis I. (2010). Aromatase, oestrogens and human male reproduction. Phil Trans R Soc B 365:1571–9
- Chan AW, Luetjens CM, Dominko T, et al. (2000). Transgenic ICSI reviewed: Foreign DNA transmission by intracytoplasmic sperm injection in rhesus monkey. Mol Reprod Dev 56:325–8
- Cui W. (2010). Mother or nothing: The agony of infertility. Bull World Health Org 88:881–2
- Ellman GL. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–7
- Farag AT, El-Aswad AF, Shaaban NA. (2007). Assessment of reproductive toxicity of orally administered technical dimethoate in male mice. Reprod Toxicol 23:232–8
- Gornall AG, Bardwill GS, David MM. (1949). Determination of serum protein by means of biuret reactions. J Biol Chem 177:751–66
- Hamden K, Silandre D, Delalande C, et al (2008). Protective effects of estrogens and caloric restriction during aging on various rat testis parameters. Asian J Androl 10:837–45
- Jang M, Min JW, In JG, Yang DC. (2011). Effects of red ginseng extract on the epididymal sperm motility of mice exposed to ethanol. Int J Toxicol 30:435–42
- Joshi SC, Mathur R, Gulati N. (2007). Testicular toxicity of chlorpyrifos (an organophosphate pesticide) in albino rat. Toxicol Ind Health 23:439–44
- Leite RP, Wada RS, Monteiro JC, et al. (2010). Protective effect of Guaraná (Paullinia cupana var. sorbilis) pre-treatment on cadmium-induced damages in adult Wistar testis. Biol Trace Elem Res 141:262–74
- Luo H, Huang J, Liao WG, et al. (2011). The antioxidant effects of garlic saponins protect PC12 cells from hypoxia-induced damage. Br J Nutr 105:1164–72
- Mallem L, Keck G, Franck M, Boulakoud MS. (2007). Effects of Maneb on thyroid and fertility of rabbit (in French). Revue Méd Vét 158:452–7
- Manfo FPT, Chao W-F, Moundipa PF, et al. (2011a). Effects of maneb on testosterone release in male rats. Drug Chem Toxicol 34:120–8
- Manfo FPT, Moundipa PF, Déchaud H, et al. (2012). Effect of agropesticides use on male reproductive function: A study on farmers in Djutitsa (Cameroon). Environ Toxicol 27:423–32
- Manfo FPT, Nantia EA, Tchana AN, et al. (2011b). Evaluation of the effect of Carpolobia alba (Polygalaceae) aqueous extract on male reproductive function. J Appl Anim Res 39:80–4
- Mitaine-Offer AC, Miyamoto T, Khan IA, et al. (2002). Three new triterpene saponins from two species of Carpolobia. J Nat Prod 65:553–7
- Moundipa FP, Beboy ESN, Zelefack F, et al. (2005). Effects of Basella alba and Hibiscus macranthus extracts on testosterone production by adult rat and bull Leydig cells. Asian J Androl 7:411–17
- Moundipa FP, Silvère N, Kamtchouing P, et al. (1999). Effects of aqueous extracts of Hibiscus macranthus and Basella alba in mature rat testis function. J Ethnopharmacol 65:133–9
- Moundipa FP, Silvère N, Kamtchouing P, et al. (2006). Effects of extracts from Hibiscus macranthus and Basella alba and mixture on testosterone production in vitro in adult rat testes slices. Asian J Androl 8:111–14
- Nantia AE, Manfo TPF, Beboy ESN, Moundipa FP. (2013). In vitro the antioxidant activity of the methanol extract of Basella alba L. (Basellaceae) in rat testis homogenate. Oxid Antioxid Med Sci 2:131--6
- Nantia AE, Moundipa FP, Beboy ESN, et al. (2007). Study of the androgenic effect of the methanol extract of Basella alba L. (Basellaceae) on the male rat reproductive function. Andrologie 17:129–33
- Nantia EA, Manfo PFT, Beboy NE, et al. (2012). Effect of methanol extract of Basella alba L. (Basellaceae) on the fecundity and testosterone level in male rats exposed to flutamide in utero. Andrologia 44:38–45
- Nantia EA, Travert C, Manfo FPT, et al. (2011). Effects of the methanol extract of Basella alba L (Basellaceae) on steroid production in Leydig cells. Int J Mol Sci 12:376–84
- Ng TB, Liu F, Wang ZT. (2000). Antioxidative activity of natural products from plants. Life Sci 66:709–23
- Nweke CO, Sanders III HW. (2009). Modern environmental health hazards: A public health issue of increasing significance in Africa. Environ Health Perspect 117:863–70
- Oatley JM, Brinster RL. (2008). Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol 24:263–86
- Oda SS, El-Maddawy ZKh. (2012). Protective effect of vitamin E and selenium combination on deltamethrin-induced reproductive toxicity in male rats. Exp Toxicol Pathol 64:813–19
- Okonofua F. (2003). New reproductive technologies and infertility rreatment in Africa. Afr J Reprod Health 7:7–8
- Pinto-Gouveia J, Galhardo A, Cunha M, Matos M. (2012). Protective emotional regulation processes towards adjustment in infertile patients. Hum Fertil 15:27–34
- Pryor JL, Hughes C, Foster W, et al. (2000). Critical windows of exposure for children's health: The reproductive system in animals and humans. Environ Health Perspect 108:491–503
- Raju M, Varakumar S, Lakshminarayana R, et al. (2007). Carotenoid composition and vitamin A activity of medicinally important green leafy vegetables. Food Chem 101:1598–605
- Reitman S, Frankel S. (1957). Colorimetric method for determination of serum oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
- Rezvanfar MA, Sadrkhanlou RA, Ahmadi A, et al. (2008). Protection of cyclophosphamide-induced toxicity in reproductive tract histology, sperm characteristics, and DNA damage by an herbal source; evidence for role of free-radical toxic stress. Human Exp Toxicol 27:901–10
- Rinaldi S, Déchaud H, Biessy C, et al. (2001). Reliability and validity of commercially available, direct radioimmunoassays for measurement of blood androgens and estrogens in postmenopausal women. Cancer Epidemiol Biomarkers Prev 10:757–65
- Sato T, Nagata M, Engle ML. (2002). Evaluation of antioxidant activity of indigenous vegetables from South and Southeast Asia. JIRCAS Research Highlights, 10–11
- Shalaby MA, El Zorba HY, Ziada RM. (2010). Reproductive toxicity of methomyl insecticide in male rats and protective effect of folic acid. Food Chem Toxicol 48:3221–6
- Tapondjou LA, Nyaa LB, Tane P, et al. (2011). Cytotoxic and antioxidant triterpene saponins from Butyrospermum parkii (Sapotaceae). Carbohydr Res 346:2699–704
- Verhoeven G, Willems A, Denolet E, et al. (2010). Androgens and spermatogenesis: Lessons from transgenic mouse models. Phil Trans R Soc B 365:1537–56
- WHO. (2002). Traditional Medicine Strategy 2002–2005. World Health Organization, Geneva, 61p
- Wilbur KM, Bernhein F, Shapiro OW. (1949). The thiobarbituric acid reagent as a test for the oxidation of unsaturated fatty acid by various agents. Arch Biochem Biophy 24:305–13
- Yousef MI, El-Demerdash FM, Al-Salhen KS. (2003). Protective role of isoflavones against the toxic effect of cypermethrin on semen quality and testosterone levels of rabbits. J Environ Sci Health B 38:463–78
- Yu G, Guo Q, Xie L, et al. (2009). Effects of subchronic exposure to carbendazim on spermatogenesis and fertility in male rats. Toxicol Ind Health 25:41–7
- Yu J, Wang L, Walzem RL, et al. (2005). Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J Agric Food Chem 53:2009–14

