Abstract
Context. Calotropis procera (Ait.) R. Br. (Asclepiadaceae), Ficus elastica Roxb. (Moraceae) and Zingiber officinale Roscoe (Zingiberaceae) have been traditionally used to treat many diseases.
Objective: The antischistosomal activity of these plant extracts was evaluated against Schistosoma mansoni.
Materials and methods: Male mice exposed to 80 ± 10 cercariae per mouse were divided into two batches. The first was divided into five groups: (I) infected untreated, while groups from (II–V) were treated orally (500 mg/kg for three consecutive days) by aqueous stem latex and flowers of C. procera, latex of F. elastica and ether extract of Z. officinale, respectively. The second batch was divided into four comparable groups (except Z. officinale-treated group) similarly treated as the first batch in addition to the antacid ranitidine (30 mg/kg) 1 h before extract administration. Safety, worm recovery, tissues egg load and oogram pattern were assessed.
Results. Calotropis procera latex and flower extracts are toxic (50–70% mortality) even in a small dose (250 mg/kg) before washing off their toxic rubber. Zingiber officinale extract insignificantly decrease (7.26%) S. mansoni worms. When toxic rubber was washed off and ranitidine was used, C. procera (stem latex and flowers) and F. elastica extracts revealed significant S. mansoni worm reductions by 45.31, 53.7 and 16.71%, respectively. Moreover, C. procera extracts produced significant reductions in tissue egg load (∼34–38.5%) and positively affected oogram pattern.
Conclusion: The present study may be useful to supplement information with regard to C. procera and F. elastica antischistosomal activity and provide a basis for further experimental trials.
Introduction
Schistosomiasis is a chronic and debilitating disease, caused by trematode flatworms of the genus Schistosoma that exacerbates poverty (Davis, Citation2009; King & Dangerfield-Cha, Citation2008). Although close to 800 million individuals are at risk of contracting the disease and over 200 million people are thought to be infected, schistosomiasis is often neglected (Utzinger et al., Citation2009). The global burden of schistosomiasis has been estimated at 1.7–4.5 million disability-adjusted life years, but even the higher estimate might be an underestimation of the true burden (King et al., Citation2005; King & Dangerfield-Cha, Citation2008). Praziquantel (PZQ) is the only widely used drug for treatment and control of this parasitemia. Although no clinically relevant resistance to PZQ has been described to date, development of drug-resistance remains a growing threat, particularly in view of the mounting PZQ pressure (Melman et al., Citation2009). The growing need for the development of novel and inexpensive drugs against schistosomiasis has led the scientific community to intensify the search for extracts and pure compounds obtained from plants exhibiting potential schistosomicidal properties (Adamu et al., Citation2006; Perrett et al., Citation1995). The promising results obtained from plants, encourage the screening of more plants for their antischistosomal activity to come up with natural products that are more effective schistosomicides, especially in rural communities where these plants abound. Therefore, it seems important to screen and evaluate the potency of some local plants that are active on parasitic organisms.
Calotropis procera (Ait.) R. Br. (Asclepiadaceae), a wild growing tropical plant, commonly known as Arka in India, is widely distributed in Asia, Africa, South America and abundant in northeast Brazil. It is a common xerophytic plant throughout Egypt particularly in the southern extreme arid sections of the deserts and Nile regions. Calotropis procera is one of the latex-producing plants, which usually secrete milk-like fluid from a network of laticifer cells, in which subcellular organelles intensively synthesize proteins and secondary metabolites (Lopes et al., Citation2009). The biological importance of latex fluids is still unclear and knowledge of their physiological role is still limited (Ramos et al., Citation2007). Nevertheless, strong proteolytic activity was attributed to the abundant endogenous enzymes such as cysteine proteinases (EC 3.4.22.16) and chitinases (EC 3.2.1.14) that present in C. procera fluids (Freitas et al., Citation2007).
Calotropis procera has been used in Indian folk medicine for the treatment of various ailments such as rheumatism, aches, gastrointestinal disorders, leprosy, ulcers, piles and tumors (Kumar & Arya, Citation2006). The plant has also antimicrobial (Malik & Chaughati, Citation1979), antioxidant (Roy et al., Citation2005), anticancer (Ayoub & Kingston, Citation1981) and anthelmintic activities (Al-Qarawi et al., Citation2001; Iqbal et al., Citation2005). Experimentally, an ethanol extract of C. procera flowers was reported to have antipyretic, analgesic (Mascolo et al., Citation1988), anticancerogenic (Smit et al., Citation1995), antimalarial (Sharma & Sharma, Citation1999, Citation2001) and hepatoprotective (Qureshi et al., Citation2007) activities. The milky white latex of this plant has been reported to exhibit potent anti-inflammatory and analgesic, and weak antipyretic activities in various experimental models (Dewan et al., Citation2000; Kumar & Basu, Citation1994; Kumar & Arya, Citation2006). It has also been demonstrated to possess antioxidant, antihyperglycemic and hepatoprotective properties (Padhy et al., Citation2007; Roy et al., Citation2005).
Ficus elastica Roxb. (Moraceae) is a tropical evergreen tree. It is found in the Egyptian main gardens like Orman garden in Cairo and Antoniades garden in Alexandria and in many streets as used for decoration. Its constituents of coumarins, triterpenes, steroids, flavonoids and tannins as well as alkaloids characterize the plant (Mbosso et al., Citation2012). Practitioners of herbal medicine in West Africa use the plant for the treatment of muscle and joint pain. Sackeyfio and Lugeleka (Citation1986) reported anti-inflammatory activity of F. elastica aqueous extract against carrageenin-induced edema and adjuvant-induced arthritis in rats.
Ginger [Zingiber officinale Roscoe (Zingiberaceae)] has been used as a spice for over thousand years (Bartley & Jacobs, Citation2000). Ginger is said to be a native of China and India and is currently grown commercially in the warm, moist, areas of the Mediterranean (Egypt), tropical and subtropical areas of Africa, Southeast Asia, Latin America and Australia. Toxic activities or effects were recorded in the plant latex and the proteins in it such as ficin (Konno et al., Citation2004). On the other hand, the plant roots contain polyphenol compounds (6-gingerol and shogaols), which possess a high antioxidant activity (Stoilova et al., Citation2007). In addition, ginger was reported to be a detoxifying agent against alcohol abuse (Shati & Elsaid, Citation2009) and bromobenzene intoxication (El-Sharaky et al., Citation2009). Matsuda et al. (Citation2009) and Habib et al. (Citation2008) reported its antidiabetic, antihyperlipidimic and hepatic anticancer effect. Moreover, its efficacy as a molluscicide against Biomphalaria alexandrina, the snail intermediate host of S. mansoni was reported by Omran et al. (Citation2007).
Despite the favorable ethnopharmacological properties of these plant extracts, its potential activity against S. mansoni has not been previously explored. These extracts showed promising in vitro S. mansoni worm killing (an investigation by the same authors under publication). Therefore, the present study was designed to evaluate the in vivo antischistosomal activity of the extracts of C. procera stem latex and flowers, F. elastica and Z. officinale using parasitological criteria of cure (worm recovery, tissues egg load and oogram pattern), and their safety by assessing acute oral toxicity.
Materials and methods
Chemicals
Acetic anhydride, potassium hydroxide (KOH) and chloroform (BDH, England), sulphuric acid (H2SO4) and Dragendorff’s reagent (solution of potassium bismuth iodide), methanol and acetone (Sigma-Aldrich, St. Louis, MO), ferric chloride (FeCl3), hydrochloric acid (HCl) and ether (Merck, Darmstadt, Germany).
Plant collections
Calotropis procera was collected in spring (March 2009) from desert fields close to Cairo city, Egypt. Ficus elastica were collected (April 2009) from Egyptian botanical gardens, while Z. officinale rhizomes were purchased (April 2009) from the local markets. The plants were authenticated by Wafaa M. Amer, Professor of Taxonomy, Department of Botany, Faculty of Science, Cairo University, Giza, Egypt. Voucher specimens (Reg. No. C-II, F1 and G-2, respectively) were kept in the herbarium, Medicinal Chemistry Department, Theodor Bilharz Research Institute (TBRI), Giza, Egypt.
Plants extraction
Calotropis procera stem latex and flowers extraction
The latex of C. procera was collected from the stem near the buds of the plant and was dried on glass plates under the shade at room temperature for at least 15 days, then scratched from the glass plates and with the help of a mortar it was ground to small granules, and extracted with water. The extract was then concentrated to dryness in a vacuum rotary evaporator and stored at 4 °C until used. About 100 g of powdered C. procera flowers was mixed with 1000 ml of distilled water in a 2-liter flask and boiled for 1.5 h. Following cooling to 40 °C, the “brew” was filtered using Whatman No. 1 filter paper. The filtrate was then concentrated to dryness in a vacuum rotary evaporator, washed with chloroform to extract and purify lipids and was stored at 4 °C until used.
Ficus elastica extraction
The latex of F. elastica was collected from the stems of the plant, dried as described for stem latex of C. procera and extracted with water. The resulting water extract was filtered using Whatman No. 1 filter paper and concentrated to dryness under vacuum using a rotary evaporator, then the extract stored at 4 °C for subsequent experiments.
Removal of toxic rubber
The toxic rubber materials of C. procera and F. elastica dry latex were washed out by extraction with aqueous methanol (85%). Then, the extract was filtrated and dried under vacuum. The dry residue obtained was washed again with boiled acetone. The clean, rubber-free acetone-insoluble portion was used in all subsequent experiments. This procedure eliminates acetone-soluble molecules while retaining almost all proteins.
Zingiber officinale extraction
The dried powdered rhizomes (500 g) were extracted using ether. The extracts were concentrated under reduced pressure using a rotary evaporator at temperature not >50 °C and were kept at 4 °C until used for phytochemical and pharmacological studies.
Phytochemical screening
The different extracts were subjected to qualitative phytochemical screening using the standard procedures for the identification of the phytoconstituents. All extracts were tested for tannins, flavonoids, saponins, sterols, terpenes and alkaloids (Harborne, Citation1998; Siddiqui et al., Citation2009).
Animals and infection
Male Swiss albino mice (CD-1 strain) obtained from Schistosome Biological Supply Center (SBSC) at TBRI, Giza, Egypt and weighing 18–20 g were used in this study. The animals were maintained on a standard commercial pellet diet and were kept in air-conditioned animal house at 20–22 °C. All experimental procedures described below were carried out in accordance with the guidelines of institutional animal ethics committee. Mice were infected with 80 ± 10 S. mansoni cercariae per mouse (provided by SBSC of TBRI) according to the method described by Liang et al. (Citation1987).
Acute toxicity study
Adult normal male mice were used to study acute oral toxicity (Bruce, Citation1985) of test plant extracts. For each plant extract, mice were divided equally into five groups, each of 6 mice and were given the extract at increasing oral doses of 100, 200, 400, 800 and 1600 mg/kg, respectively. Animals were observed individually after dosing at least once during the first 30 min, periodically during the first 24 h, with special attention given during the first 4 h. In addition, the general behavior of animals, signs of discomfort or any nervous manifestations were observed for further up to 14 days following treatment.
Animal groups
Before removal of toxic rubber
Schistosoma mansoni-infected mice were classified into three groups; one of them was left without treatment as respective control. While the other two groups were given C. procera stem latex and flower extracts orally in a dose of 250 mg/kg (the lowest test dose survived animals to the time of sacrifice) divided equally on five consecutive days as a suspension with gum acacia in normal saline.
After removal of rubber
A group of S. mansoni-infected mice was divided equally into two batches. The first batch was divided into five groups. The first one was left without treatment as respective untreated control, while groups from the second to the fifth were given extracts of stem latex and of flower (C. procera), F. elastica and Z. officinale orally in a dose of 1500 mg/kg divided equally on three consecutive days. The second batch of mice was divided into four groups. The first one was left without treatment as untreated control. While groups from the second to the fourth were given extracts of stem latex and of flower (C. procera) and F. elastica orally in a dose of 1500 mg/kg divided equally on three consecutive days after 1 h of the antacid ranitidine (30 mg/kg) administration. Animals of all infected groups were treated with plant extracts 7 weeks after infection and were killed by rapid decapitation 9-weeks post-infection.
Assessment of parasitological criteria
To recover mature and immature worms for subsequent counting and determination of sex, hepatic and mesenteric vessels of animals were perfused according to Duvall and DeWitt (Citation1967). The number of ova per gram of liver or intestinal tissues (tissue egg load) was counted according to the method of Kamel et al. (Citation1977), in which a piece of small intestinal or hepatic tissue was weighed before digestion in 5% KOH. The percentage of different egg developmental stages (oogram pattern) was studied according to the method of Pellegrino et al. (Citation1962), in which eggs at different stages of maturity were identified (from I to IV) according to the size of the embryo and were counted. In addition, mature eggs containing fully developed miracidium and dead eggs (granular, dark and semitransparent) were also counted in three fragments of small intestine and the mean number of each stage was calculated.
Statistical analysis
Results are expressed as mean ± SEM. Differences between means in different groups were analyzed statistically for significance using Student’s t-test and p value of 0.05 or less was considered significant.
Results and discussion
Phytochemical screening studies
Preliminary phytochemical screening of the tested extracts revealed the presence of low concentrations of tannins, sterols, terpenes (C. procera flowers) and alkaloids in Z. officinale. In addition, moderate and high concentrations of tannins, flavonoids, saponins and terpenes were identified in Z. officinale. On the other hand, none of these secondary six metabolites was found in the stem latex of C. procera and F. elastica (). The concentrations of the most active compounds were recorded to be present in the water (C. procera flowers) and Z. officinale ether extracts. These results are in agreement with those of Atta et al. (Citation2010) and Murti et al. (Citation2010).
Table 1. Phytochemical screening of selected plant extracts.
Safety and in vivo efficacy against S. mansoni
Oral administration of water extracts of stem latex and flowers of C. procera in a total dose of 250 mg/kg produced percentage mortality of 50–70% (data not shown) without any significant change in the parasitological parameters assessed (). In this regard, El-Badwi et al. (Citation1998) reported that the whole latex of C. procera has been described as a rich source of toxic compounds. Also, Konno et al. (Citation2004) suggested that plant latex and the proteins in it such as papain, ficin and bromelain, all possess toxic activities or effects.
Table 2. Effect of C. procera stem latex and flower extracts oral administration (50 mg/kg for five consecutive days) on worm burden, egg count and the percentage of egg developmental stages (oogram pattern) in S. mansoni-infected mice 2-week post-treatment.
When the latex was washed off, it lost toxicity, hence, upon oral administration of water extracts of stem latex and flowers of C. procera. F. elastica and ether extract of Z. officinale at any dose level up to the highest dose tested (1600 mg/kg) did not reveal symptoms of morbidity or mortality. These results showed that, at single dose, there are no adverse effects of plant extracts used indicating that the medium lethal dose (LD50) should be >1600 mg/kg and the tested plants are safe for use (). Therefore, 500 mg/kg body weight was used as a therapeutic dose. Although a numerical decrease (7.26–17.71%) in total number of worms was recorded (), yet no statistically significant changes were recorded in the total number of worms, tissue egg load and % egg developmental stages after the oral administration of plant extracts. Concerning Z. officinale, results are in agreement with those of Sanderson et al. (Citation2002) who reported that neither oral nor subcutaneous administration of 150 mg/kg of ethyl acetate extract of ginger produced any significant reduction in S. mansoni worm numbers compared with untreated controls. Conversely, Mostafa et al. (Citation2011) reported that worm burden and the egg density in liver and faeces of mice treated with ginger were fewer than in non-treated ones.
Table 3. Acute toxicity of the test plant extracts administered orally to mice.
Table 4. Effect of C. procera stem latex and flower, Ficus elastica and Zingiber officinale extracts oral administration (500 mg/kg for three consecutive days) on worm burden, ova count and the percentage of egg developmental stages (oogram pattern) in S. mansoni-infected mice 2-week post-treatment.
The laticifer fluid of C. procera and F. elastica is rich in proteins (cysteine proteases in particular) and there is evidence that they are involved in the pharmacological properties of the latex (Freitas et al., Citation2007; Konno et al., Citation2004). The cysteine proteases found in the latices and extracts of laticiferous plant fruits all have a neutral pH optimum of ∼7, with a range, for example in the case of ficin, in which some activity is retained from pH 4–8.5 (Robbins, Citation1930). The author added that fruit derived, secreted cysteine proteases cannot work effectively at lower or higher pH (although some intracellular cysteine proteases have lower optimal pHs), hence an expected antitrematodal activity has been considered a remote possibility in the trematode model flatworms of S. mansoni. Huet et al. (Citation2006) reported that the effect of acidity on cysteine proteases is irreversible. In view of the above-mentioned information, results from this work did not reveal any activity against S. mansoni when the C. procera latex and flowers as well as the latex of F. elastica were given orally. However, when acid secretion was temporarily blocked by giving the antacid ranitidine 1 h before the plant extracts (), a significant reduction in total worm burden has been observed that was highest with C. procera flowers (p < 0.001; 53.7%) followed by C. procera stem latex (p < 0.001; 45.31%). Meanwhile, F. elastica latex showed less significant worm reduction (p < 0.05; 16.71%). With C. procera stem or flower extracts, both worm sex was more or less equally insulted with evident uncoupling that reached almost 50% of the total number of worms (). Several studies showed the anthelmintic activity of C. procera latex and flowers against Haemonchus contortus, the gastrointestinal nematodes of sheep (Al-Qarawi et al., Citation2001; Iqbal et al., Citation2005). Meanwhile, the parasiticidal activity of latex of some other species of Ficus was attributed to the presence of ficin (Pistelli et al., Citation2000).
Table 5. Effect of C. procera stem latex and flower and Ficus elastica extracts oral administration (500 mg/kg for 3 days + ranitidine 30 mg/kg for 3 days) on total males, females and worm burden in S. mansoni-infected mice 2-week post-treatment.
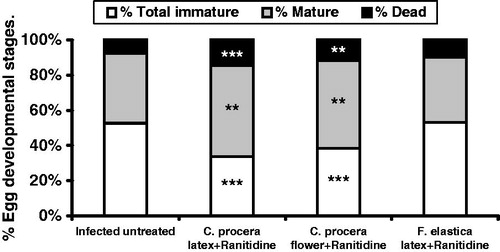
In the present study, data revealed significant reduction (p < 0.05) in both the hepatic (38.3%) and intestinal (37.6%) tissue egg loads upon administration of C. procera flower extract post-ranitidine. Meanwhile, this reduction was noticed only in the intestinal tissue egg load (34.9%) in C. procera stem latex-treated group in comparison with infected untreated control (). These findings were accompanied with significant decrease in the percentage of total immature eggs (p < 0.001; 35.9% for C. procera stem latex and 27.3% for flowers) and a significant increase in the percentage of both mature (p < 0.01; 30.3% for C. procera stem latex and 25.9% for flowers) and dead eggs (p < 0.001; 88.8% for C. procera stem latex and p < 0.01; 53.3% for flowers) compared with the infected untreated control (). These results are in consistence with Pellegrino et al. (Citation1962) who stated that if viable ova of all developmental stages are still present after specific chemotherapy in experimental schistosomiasis, the drug is considered inactive. They also added that a dose level of the drug was considered to have a definite action against S. mansoni when the oogram showed an increase (from ∼35% to 50% or more) in mature ova with the absence of one or more of the immature stages of ova. On the other hand, F. elastica showed insignificant changes in tissue egg load and percentage egg developmental stages (). In this context, Seif el-Din et al. (Citation2006) and Ebeid et al. (Citation2007) recorded that administration of the antioxidants β-carotene or N-acetylcysteine (NAC) produced significant reduction in S. mansoni worm burden accompanied with significant increase in the percentage of dead ova and a decrease in the percentage of mature ova stages. Moreover, Seif el-Din et al. (Citation2011) reported that treatment with NAC alone increased the percentage of dead ova and enhanced the decrease in total number of worms and tissue egg loads when used in addition to the anti-malarial drug artemether. Accordingly, the antischistosomal effects of C. procera and F. elastica could be attributed to their anthelmintic activity (Al-Qarawi et al., Citation2001; Iqbal et al., Citation2005; Pistelli et al., Citation2000), antioxidative and anti-inflammatory properties of the phytochemical constituents and that of the cysteine proteases of these extracts (Kumar & Arya, Citation2006; Roy et al., Citation2005; Sackeyfio & Lugeleka, Citation1986).
Figure 1. Effect of C. procera stem latex and flower and Ficus elastica extracts oral administration (500 mg/kg for 3 days + ranitidine 30 mg/kg for 3 days) on hepatic and intestinal tissue egg loads in S. mansoni-infected mice 2-week post-treatment. Values are expressed as means ± SEM. *p < 0.05 (significant difference compared with infected control group).
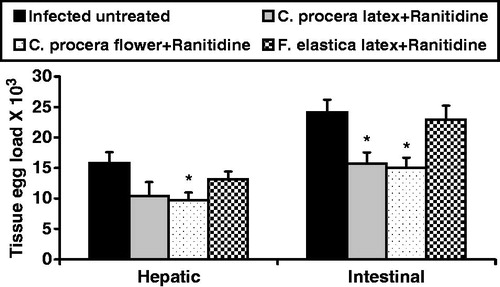
Figure 2. Effect of C. procera stem latex and flower and Ficus elastica extracts oral administration (500 mg/kg for 3 days + ranitidine 30 mg/kg for 3 days) on the percentage of egg developmental stages (oogram pattern) in S. mansoni-infected mice 2-week post-treatment. Values are expressed as means ± SEM. **p < 0.01, ***p < 0.001 (significant difference compared with infected control group).
Conclusions
The stem latex and flowers of C. procera were found toxic before rubber removal even in low doses. The ether extract of Z. officinale has no antischistosomal activity, while results of the C. procera stem latex and flowers and latex of F. elastica (after washing off toxic rubber materials) clearly demonstrate promising antischistosomal activity when used 1 h after administration of the antacid ranitidine. These effects could be due to an antischistosomal, antioxidant or anti-inflammatory activity of their content of cysteine proteases, tannins, flavonoids, sterols and terpenes. However, further detailed studies are required to identify their chemical nature and the possibility that further fractionation may lead to a proper lead antischistosomal of natural origin.
Declaration of interest
There are no conflicts of interest. The authors alone are responsible for the content and writing of the article.
This work is part of the research project [Development of new compounds with effect on schistosomiasis (bilharzia); 2009–2012] funded by the Swedish Research Council [Swedish-MENA Research Links (SRL-MENA)], Contract No. (348-2008-6102), Swedish PI Prof. Olov Sterner, Chemistry Department, Lund University, Sweden and Egyptian PI Prof. Sanaa Botros, Pharmacology Department, Theodor Bilharz Research Institute, Giza, Egypt.
Acknowledgements
The authors would like to thank Dr. Wafaa M. Amer, Professor of Taxonomy, Department of Botany, Faculty of Science, Cairo University, Giza, Egypt for plant authentication.
References
- Adamu SU, Kela SL, Suleiman MM. (2006). Antischistosomal properties of extracts of Jatropha curcas (L) on Schistosoma mansoni infection in mice. Afr J Tradit Complement Altern Med 3:37–41
- Al-Qarawi AA, Mahmoud OM, Sobaih MA, et al. (2001). A preliminary study on the anthelmintic activity of Calotropis procera latex against Haemonchus contortus infection in Najdi sheep. Vet Res Commun 25:61–70
- Atta AH, Elkoly TA, Mouneir SM, et al. (2010). Hepatoprotective effect of methanol extracts of Zingiber officinale and Cichorium intybus. Indian J Pharm Sci 72:564–70
- Ayoub SMN, Kingston DGI. (1981). Screening of plants used in Sudan folk medicine for anticancer activity. Fitoterapia 52:281–4
- Bartley J, Jacobs A. (2000). Effects of drying on flavour compounds in Australian grown ginger (Zingiber officinale). J Sci Food Agric 80:209–15
- Bruce RD. (1985). An up-and-down procedure for acute toxicity testing. Fundam Appl Toxicol 6:151–7
- Davis A. (2009). Schistosomiasis. In: Cook GC, Zumla AI, eds. Manson’s Tropical Diseases, 22nd ed. London: Saunders, 1413–56
- Dewan S, Sangraula H, Kumar VL. (2000). Preliminary studies on the analgesic activity of latex of Calotropis procera. J Ethnopharmacol 73:307–11
- Duvall RH, DeWitt WB. (1967). An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg 16:483–6
- Ebeid FA, Ezzat AR, Badawy AA, Seif el-Din SH. (2007). Enhancement role of β-carotene against experimental schistosomiasis. Egypt J Schistosomiasis Infect Endem Dis 29:67–90
- El-Badwi Samia MA, Adam SE, Shigidi MT, Hapke HJ. (1998). Studies on laticiferous plants: Toxic effects in goats of Calotropis procera latex given by different routes of administration. Dtsch Tierarztl Wochenschr 105:425–7
- El-Sharaky AS, Newairy AA, Newairy AA, et al. (2009). Protective effect of ginger extract against bromobenzene-induced hepatotoxicity in male rats. Food Chem Toxicol 47:1584–90
- Freitas CD, Oliveira JS, Miranda MR, et al. (2007). Enzymatic activities and protein profile of latex from Calotropis procera. Plant Physiol Biochem 45:781–9
- Habib SH, Makpol S, Abdul Hamid NA, et al. (2008). Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics (Sao Paulo) 63:807–13
- Harborne JB. (1998). Phytochemical Methods: A guide to modern techniques of plant analysis. In: The Terpenoids. 3rd ed. London: Chapman and Hall Ltd., 114–18
- Huet TJ, Looze Y, Bartik K, et al. (2006). Structural characterization of the papaya cysteine proteinases at low pH. Biochem Biophys Res Commun 341:620–6
- Iqbal Z, Lateef M, Jabbar A, et al. (2005). Anthelmintic activity of Calotropis procera (Ait.) Ait. F. flowers in sheep. J Ethnopharmacol 102:256–61
- Kamel IA, Cheever AW, Elwi AM, et al. (1977). Schistosoma mansoni and S. haematobium infections in Egypt: Technique for recovery of worms at necropsy. Am J Trop Med Hyg 26:696–701
- King CH, Dangerfield-Cha M. (2008). The unacknowledged impact of chronic schistosomiasis. Chronic Ills 4:65–79
- King CH, Dickman K, Tisch DJ. (2005). Reassessment of the cost of chronic helminthic infection: A meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 365:1561–9
- Konno K, Hirayama C, Nakamura M, et al. (2004). Papain protects papaya trees from herbivorous insects: Role of cysteine proteases in latex. Plant J 37:370–8
- Kumar VL, Arya S. (2006). Medicinal uses and pharmacological properties of Calotropis procera. In: Govil JN, ed. Recent Progress in Medicinal Plants. Texas: Studium Press, 373–88
- Kumar VL, Basu N. (1994). Anti-inflammatory activity of the latex of Calotropis procera. J Ethnopharmacol 44:123–5
- Liang YS, John BI, Boyd DA. (1987). Laboratory cultivation of schistosome vector snails and maintenance of schistosome life cycles. Proceed 1st Sino-American Symp 1:34–48
- Lopes KLB, Thadeo M, Azevedo AA, et al. (2009). Articulated laticifers of vegetative organs Mandevilla atroviolaceae (Apocynaceae, Apocynoideae). Botany 87:202–9
- Malik NN, Chaughati MID. (1979). Antimicrobial activity of Calotropis procera: A preliminary study. Pak J Sci 31:127–9
- Mascolo N, Sharma R, Jain SC, Capasso F. (1988). Ethnopharmacology of Calotropis procera flowers. J Ethnopharmacol 22:211–21
- Matsuda A, Wang Z, Takahashi S, et al. (2009). Upregulation of mRNA of retinoid binding protein and fatty acid binding protein by cholesterol enriched-diet and effect of ginger on lipid metabolism. Life Sci 84:903–7
- Mbosso EJ, Nguedia JC, Meyer F, et al. (2012). Ceramide, cerebroside and triterpenoid saponin from the bark of aerial roots of Ficus elastica (Moraceae). Phytochemistry 83:95–103
- Melman SD, Steinauer ML, Cunningham C, et al. (2009). Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl Trop Dis 3:e504
- Mostafa OM, Eid RA, Adly MA. (2011). Antischistosomal activity of ginger (Zingiber officinale) against Schistosoma mansoni harbored in C57 mice. Parasitol Res 109:395–403
- Murti Y, Yogi B, Pathak D. (2010). Pharmacognostic standardization of leaves of Calotropis procera (Ait.) R. Br. (Asclepiadaceae). Int J Ayurveda Res 1:14–17
- Omran NEE, El-Nouby KA, Shoheib ZS, Kabbash AM. (2007). Molluscicidal effects of some plant extracts on Biomphalaria alexandrina, the intermediate host snail of Schistosoma mansoni in Egypt. J Egypt Ger Soc Zool 53: Invertebrate, Zoology, 1–29
- Padhy BM, Srivastava A, Kumar VL. (2007). Calotropis procera latex affords protection against carbon tetrachloride induced hepatotoxicity in rats. J Ethnopharmacol 113:498–502
- Pellegrino J, Oliveira CA, Faria J, Cunha AS. (1962). New approach to the screening of drugs in experimental schistosomiasis mansoni in mice. Am J Trop Med Hyg 11:201–15
- Perrett S, Whitfield PJ, Sanderson L, Bartlett A. (1995). The plant molluscicide Millettia thonningii (Leguminosae) as a topical antischistosomal agent. J Ethnopharmacol 47:49–54
- Pistelli L, Chiellini EE, Moselli I. (2000). Flavonoids from Ficus pumila. Biochem Syst Ecol 28:287–9
- Qureshi AA, Prakash T, Patil T, et al. (2007). Hepatoprotective and antioxidant activities of flowers of Calotropis procera (Ait) R. Br. in CCl4 induced hepatic damage. Indian J Exp Biol 45:304–10
- Ramos MV, Freitas CDT, Stanisçuaski F, et al. (2007). Performance of distinct crop pests reared on diets enriched with latex proteins from Calotropis procera: Role of laticifer proteins in plant defense. Plant Sci 173:349–57
- Robbins B. (1930). A proteolytic enzyme in ficin, the anthelmintic principle of leche de higueron. J Biol Chem 87:251–7
- Roy S, Sehgal R, Padhy BM, Kumar VL. (2005). Antioxidant and protective effect of Calotropis procera against alloxan-induced diabetes in rats. J Ethnopharmacol 102:470–3
- Sackeyfio AC, Lugeleka OM. (1986). The anti-inflammatory effect of a crude aqueous extract of the root bark of “Ficus elastica” in the rat. Arch Int Pharmacodyn Ther 281:169–76
- Sanderson L, Bartlett A, Whitfield PJ. (2002). In vitro and in vivo studies on the bioactivity of a ginger (Zingiber officinale) extract towards adult schistosomes and their egg production. J Helminthol 76:241–7
- Seif el-Din SH, Al-Hroob AM, Ebeid FA. (2011). Schistosoma mansoni. N-acetylcysteine downregulates oxidative stress and enhances the antischistosomal activity of artemether in mice. Exp Parasitol 128:230–5
- Seif el-Din SH, Ebeid FA, Badawy AA, Ezzat AR. (2006). Protective effects of β-carotene; N-acetyl-l-cysteine with and without praziquantel treatment in Schistosoma mansoni-infected mice. Egypt J Schistosomiasis Infect Endem Dis 28:67–90
- Sharma P, Sharma JD. (1999). Evaluation of in vitro schizontocidal activity of plant parts of Calotropis procera -- an ethnobotanical approach. J Ethnopharmacol 68:83–95
- Sharma P, Sharma JD. (2001). In vitro hemolysis of human erythrocytes – by plant extracts with antiplasmodial activity. J Ethnopharmacol 74:239–43
- Shati AA, Elsaid FG. (2009). Effects of water extracts of thyme (Thymus vulgaris) and ginger (Zingiber officinale Roscoe) on alcohol abuse. Food Chem Toxicol 47:1945–9
- Siddiqui S, Verma A, Rather AA, et al. (2009). Preliminary phytochemical analysis of some important medicinal and aromatic plants. Adv Biol Res 3:188–95
- Smit HF, Woerdenbag HJ, Singh RH, et al. (1995). Ayurvedic herbal drugs with possible cytostatic activity. J Ethnopharmacol 47:75–84
- Stoilova I, Krastanov A, Stoyanova A, et al. (2007). Antioxidant activity of a ginger extract (Zingiber officinale). Food Chem 102:764–70
- Utzinger J, Raso G, Brooker S, et al. (2009). Schistosomiasis and neglected tropical diseases: Towards integrated and sustainable control and a word of caution. Parasitology 136:1859–74

