Abstract
Context: The search for new sources of natural antioxidants from plant material may have beneficial therapeutic potential for those diseases associated with oxidative stress. The medicinal plant Haplophyllum tuberculatum (Forsskal) A. Juss. (Rutaceae) contains phenolic compounds as main phytochemicals; however, there are no reports on its antioxidant properties.
Objective: To evaluate antioxidant and cytoprotective potential of ethanol extract of Haplophyllum tuberculatum aerial parts.
Materials and methods: Total phenol content was determined using Folin–Ciocalteu reagent; antiradical activity was measured using ORAC assay and the analysis of the major polyphenols was carried out using a HPLC-MS method. The antioxidant and cytoprotective effect were also investigated by the MTT assay and DCFH–DA method. The human astrocytoma U373-MG cell line was pretreated with ethanol extract (from 0.025 to 250 µg/mL) for 24 h, prior to 1 mM H2O2 exposure (30 min).
Results and conclusion: Total phenol content was 46.2 mg gallic acid/g sample and ORAC value was 1.283 µmol TE/mg sample. Chemical constituents were methoxyflavones, flavonols (mainly quercetin derivatives), cinnamic acids and benzoic acids. In cell system model of oxidative stress, pretreatments with ethanol extract at the concentrations of 2.5, 0.25 and 0.025 µg/mL significantly attenuated H2O2-induced loss in viability by 13.5, 17 and 20.5%, respectively. Furthermore, these ethanol extract concentrations markedly inhibited intracellular ROS production with IC50 0.026 µg/mL. These findings demonstrate the beneficial properties of ethanol extract of Haplophyllum tuberculatum aerial parts, rich in phenolic compounds, as antioxidant and radical scavenger ameliorating ROS-related processes and diseases such as several neurodegenerative disorders.
Introduction
Polyphenols are one of the most abundant, structurally varied and widely distributed naturally occurring plant products (Landete, Citation2012). Over the last few years, some of the major pharmacological research on polyphenols has focused on their antioxidant properties. Polyphenols, through different mechanisms of action including scavenging of free radicals, metal chelation and upregulation of antioxidant system, reduce the oxidative damage caused by reactive oxygen species (ROS) in biological molecules, and therefore protect from the harmful consequences of ROS action (Obrenovich et al., Citation2011). ROS cause lipid peroxidation of cell membranes, mutations in DNA, and oxidation of proteins, which can lead to irreversible cell damage and even cell death (Galli et al., Citation2005).
Brain presents particular cellular, compositional and structural features that make it susceptible to free radical-induced damage; the brain has a high content of readily oxidizable polyunsaturated fatty acid and iron that promote lipid peroxidation in membranes. Moreover, the brain contains low amounts of antioxidant defenses and it requires large amounts of oxygen to function properly (Floyd & Hensley, Citation2002). Due to all these particular features of the brain, consistent evidence implies to oxidative stress, defined as an imbalance between ROS production and antioxidant defense system, as a key underlying pathogenic mechanism in several neurodegenerative disorders including Parkinson’s and Alzheimer’s diseases (Galli et al., Citation2005). The use of antioxidants is being intensively investigated as a promising therapeutic strategy to prevent oxidative stress. In this context, polyphenols are one of the most studied groups of natural products as pharmacological antioxidant agents and the search for new sources of polyphenols constitutes one of the pillars of antioxidant research (Ebrahimi & Schluesener, Citation2012).
Haplophyllum tuberculatum (Forsskal) A. Juss. (Rutaceae) is a gland-covered perennial herb with leaves alternate and obovate-elliptic and yellow flowers with five petals. This plant grows on sandy hills and rocky slopes of arid regions from North Africa to Southwest Asia (Salvo et al., Citation2011). Haplophyllum tuberculatum has been shown to possess insect antifeedant, antifungal and antibacterial activities (Zohair et al., Citation1989). Moreover, infusions of the aerial parts of Haplophyllum tuberculatum have been extensively employed in the traditional Egyptian medicine for digestive problems including constipation and diarrhea as well as for gynecological problems and rheumatoid arthritis (Abdel-Kawy et al., Citation1989; Al-Yahya et al., Citation1992). Related to previous phytochemical assays, Haplophyllum tuberculatum is rich in lignans, flavonoids, alkaloids and essential oils (Al-Rehaily et al., Citation2001). However, although different polyphenolic compounds have been identified in Haplophyllum tuberculatum, there has been hitherto no research focused on its antioxidant properties. In our effort to deepen the pharmacological properties of this medicinal plant, we report for the first time the antioxidant activities of Haplophyllum tuberculatum extract (ethanol:water, 70:30). For this purpose, we have quantified the total phenolic compounds and identified the individual polyphenols in the ethanol extract of Haplophyllum tuberculatum and evaluated the cytoprotective activity using an in vitro model of H2O2-induced oxidative stress in astrocytes.
Materials and methods
Chemicals
The reagents used were Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), gentamicin were purchased from Invitrogen (Carlsbarg, CA). Hydrogen peroxide, dimethyl sulfoxide (DMSO), 2,7-dichlorofluorescein diacetate (DCFH-DA), 3 -(3,4-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), 2,2′-azobis(2-amidino-propane) dihydrochloride (AAPH), fluorescein and Trolox were obtained from Sigma-Aldrich (St Louis, MO).
Preparation of plant extract
The dried aerial parts of Haplophyllum tuberculatum were obtained from the mountains of Saint Katherine in Sinai (Egypt) in May 2008. A voucher specimen of Haplophyllum tuberculatum has been deposited at the Department of Plant Biology of the Faculty of Pharmacy of the University Complutense of Madrid (MAF 168120) where it was identified by Dr. Jose Pizarro.
Ground samples (500 g) were extracted in ethanol/water (70:30%), at room temperature, for 72 h. The ethanol extracts were filtered through Whatman No. 1 filter paper and then concentrated using a rotary evaporator at 55 °C. The dry extracts were stored at 4 °C until used.
Total polyphenol content
The total polyphenol content was measured using the Folin–Ciocalteu colorimetric method. Ethanol extracts (0.5 mL) were incubated with 0.5 mL of Folin–Ciocalteau reagent and 14 mL of distillated water at room temperature for 3 min. Following, 10 mL of sodium carbonate (75 g/L) were added to the mixture. After a reaction period of 1 h, absorbance was read at a wavelength of 750 nm with a spectrophotometer (UVIKON 930, Kontron Instruments, UK). The quantification of total polyphenol content was done from a standard curve of gallic acid and results were expressed as mg per g of sample (Saura-Calixto et al., Citation2007).
HPLC-MS determination
The identification of the individual polyphenolic compounds was carried out by a HPLC-MS method (Dueñas et al., Citation2004). The equipment used was a HPLC-PAD Waters system (Milford, MA) and a Hewlet Pakard 1100MS (Palo Alto, CA) chromatograph, equipped with an electrospray ionization (ESI) interface. The analytical column was a Nova-Pak C18 (300 × 3.9 mm, 4 µm). Elution was performed at ambient temperature using water/acetic acid (98:2 v/v; solution A) and water/acetonitrile/acetic acid (78:20:2 v/v/v; solution B) as mobile phase at a flow rate of 1 mL/min from the beginning to 55 min and 1.2 mL/min from this point to the end. The gradient elution was as follows: 0–55 min, 100–20% A; 55–70 min, 20–10% A; 70–80 min, 10–5% A; and 80–100 min, 100% B. Using HPLC method, the polyphenolic compounds were detected by monitoring the UV absorbance from 210 to 400 nm and using mass spectra method, by recording from an m.z of 100–2500.
Peroxyl radical scavenging ability
The scavenging ability of ethanol extracts against peroxyl radicals was measured by oxygen radical antioxidant capacity (ORAC) (Dávalos et al., Citation2004). Briefly, 20 µL of ethanol extracts samples or Trolox were incubated with 120 µL of fluorescein (70 nM) for 10 min at 37 °C. Following, 60 µL of 2,2′-azobis(2-amidino-propane) dihydrochloride (AAPH) (12 mM), which is a free radical generator, was added to the reaction mixture. The intensity of fluorescence was monitored every 56 s for 98 min using a FLUOstar Optima (BMG Labtech) fluorometer. Results, expressed as µM of Trolox equivalents (TE)/mg of sample, were calculated from the area under curve (AUC).
Cell culture and cell treatment
The U373-MG human astrocytoma cell line was cultured in DMEM supplemented with 10% FBS and gentamicin (0.5%) and grown in 96-well plates at 37 °C in 5% CO2 and 95% humidified air.
The U373-MG cells were treated in the presence of ethanol extract samples (from 250; 25; 2.5; 0.25 and 0.025 µg/mL) for 24 h prior to hydrogen peroxide exposure (1 mM, 30 min).
Ethanol extracts were dissolved in DMSO and then dilutions in medium were made. The final concentration of DMSO was <0.1%, which neither affected cell viability assays nor antiradical activity response.
Determination of cell viability
Cell viability was evaluated using MTT assay as follows: after cell treatments, U373-MG were incubated with 2 mg/mL MTT in PBS for 1 h at 37 °C; the formazan crystals were then dissolved with DMSO and the amount of reduced MTT, as reflected of cell viability, was measured at a wavelength of 550 nm using a microplate reader (Digiscan 340, Assys Hitech GMBH, Austria). Results, expressed as percentage, were determined assuming the absorbance of control cells as 100% cell viability (Mosmann, Citation1983).
Determination of intracellular ROS formation
Intracellular reactive oxygen species (ROS) generation was evaluated using 2,7′-dichlorofluorescein diacetate (DCFH–DA) assay as follows: after cell treatments, U373-MG were incubated with 0.01 M DCFH–DA in PBS-glucose for 30 min at 37 °C and then, they were washed twice with PBS-glucose. The fluorescence intensity, as reflected of intracellular ROS production, was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm using a fluorometer (FL × 800, Bio-Tek Instruments Inc., Winooski, VT). Results, expressed as percentage, were determined assuming the fluorescence of control cells as 100% ROS production (LeBel et al., Citation1992).
Statistical analysis
The data of at least three independent experiment were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s test. Results were expressed as mean ± standard deviation (SD). p Value <0.05 was considered as statistically significant.
Results and discussion
It is well known that polyphenols in general have several therapeutic properties including antioxidant activity. In the present work, the qualitative and quantitative estimation of polyphenols content in the Haplophyllum tuberculatum ethanol extract were carried out. Moreover, the antioxidant properties of this plant extract was investigated for the first time using different in vitro noncell-based and cell-based techniques.
In vitro study: total phenol content and scavenging activity
Plants are a great source of antioxidants (Landete, Citation2012) and the aqueous alcohol solvents are the most efficient solvents for the extraction of phenolic compounds (Perva-Uzunalic et al., Citation2006). The total phenol content has been previously correlated with the antioxidant activity of plants. The antioxidant activity of polyphenols is based on their redox properties; these natural products act as free radicals quenching agents through hydrogen atom and electron transfer mechanisms and as metal-ion binding agents (Obrenovich et al., Citation2011). Therefore, the total phenol content was initially estimated and further, the antioxidant potential was determined for the ethanol extract (ethanol/water; 70:30%) obtained from Haplophyllum tuberculatum aerial parts. The total phenol content, expressed as gallic acid equivalent, was 46.2 mg gallic acid/g sample.
Since one potential antioxidant mechanism of action of polyphenols involves the ability to neutralize free radicals, the scavenging activity was further investigated using ORAC assay. The ORAC assay estimates the capacity of compounds to inhibit the AAPH-radical mediated damage by measuring the fluorescence loss of fluorescein (Dávalos et al., Citation2004). The AAPH is a common lipid peroxidation radical inducer. Therefore, the ORAC assay explains the ability of antioxidant compounds to inhibit free radicals involved in the oxidation of lipid membranes.
The ORAC scavenging activity, expressed as Trolox equivalents, demonstrated that the investigated plant extract possess a strong peroxyl radical-trap activity. The value for the ORAC assay was 1.283 µmol TE/mg sample. Many previous studies have identified phenol compounds as hydrogen donors (Obrenovich et al., Citation2011). Therefore, the scavenging activity of ethanol plant extracts may be due, at least in part, to the content in phenol compounds with antioxidant activity through hydrogen transfer mechanisms.
Identification of polyphenols
Previous phytochemical studies have indicated the presence of alkaloids (Al-Rehaily et al., Citation2001), lignans (Sheriha & Abouamer, Citation1984) and essential oils (Al-Burtamani et al., Citation2005) in Haplophyllum tuberculatum. Since one of the objectives of the present study is to investigate the antioxidant properties of the Haplophyllum tuberculatum ethanol extract and there is a strong relation between antioxidant potential of plant extract and polyphenols, we have further analysed the composition of individual polyphenol compounds.
Therefore, by using a HPLC-MS method, the polyphenols contained in the ethanol extract obtained from the aerial parts of Haplophyllum tuberculatum were identified (): benzoic acids [vanillic acid (4)], cinnamic acids [caffeic acid derivatives (1, 2), ferulic acid (7), ferulic acid derivative (3), p-OH cinnamic acids (5)], flavones [trihydroxy methoxyflavone (6), methoxy luteolin glucoside (9), methoxy apigenin glucoside (15), methoxy luteolin (17), and other flavone derivatives (11, 12, 13, 14, 19)] and flavonols [syringetin (8), quercetin glucuronide (10), quercetin derivatives (16, 18)].
Figure 1. HPLC–MS profile of the polyphenols obtained from ethanol extract of Haplophyllum tuberculatum aerial parts at 254 nm, 280 nm and 340 nm.
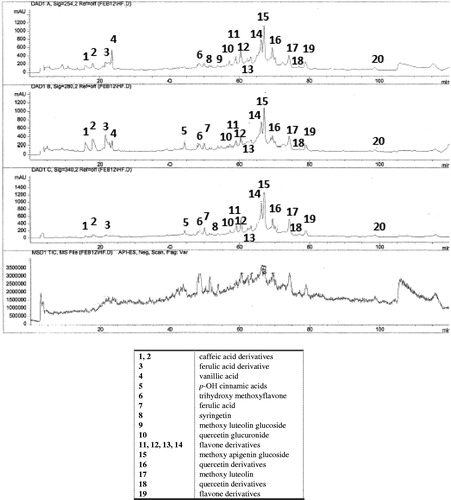
Thus, the analysis of polyphenolic profile of the ethanol extract of Haplophyllum tuberculatum demonstrated the presence of a great variety of compounds with chemical structure of phenol, being flavones and flavonols the majority types. Flavones and flavonols are a pharmacological interesting chemical group for their antioxidant properties (Pietta, Citation2000). These flavonoids types act as free radical scavengers of hydroxyl radical, peroxyl radical and super oxide anion, among others, and they also act as metal-ion quenching agents and modulating the endogenous antioxidant system (Obrenovich et al., Citation2011).
Effect on cell viability
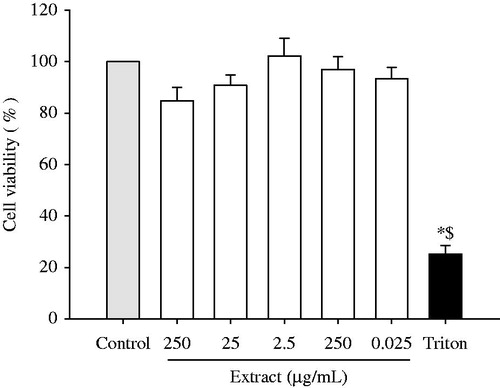
The range of concentrations where this plant extract did not exhibit cytotoxicity in U373-MG cells was first established. As shown in , none of the tested concentrations (from 0.025 to 250 µg/mL) induced a significant loss in cell viability compared to control cells. Therefore, this range of concentrations was selected for evaluating the possible potential effect of the Haplophyllum tuberculatum ethanol extract under oxidative stress conditions.
Figure 2. Effect of Haplophyllum tuberculatum ethanol extract on cell viability. U373-MG cells were treated with plant extract (range of concentrations from 0.025 to 250 µg/mL) for 24 h. Triton X-100 was employed as negative control. Results were expressed as mean of the percentage of control cells (100%) ± standard deviation (S.D.). *p < 0.05 versus control cells; $p < 0.05 versus ethanol extracts at all assayed concentrations.
Protective effect of plant extracts
High concentrations of hydrogen peroxide produce toxic effects through the generation of free radicals, being an important cause of cell damage that occurs in cells of nervous system such as astrocytes during the pathological development of several neurodegenerative diseases including Parkinson’s and Alzheimer’s diseases (Klein & Ackerman, Citation2003). High levels of hydrogen peroxide have been found in post-mortem brain samples of patients with some of these CNS-disorders (Tabner et al., Citation2005). Therefore, the interaction with hydrogen peroxide constitutes one of the key targets approaches for the prevention and treatment of neurodegenerative diseases associated with oxidative stress (Klein & Ackerman, Citation2003).
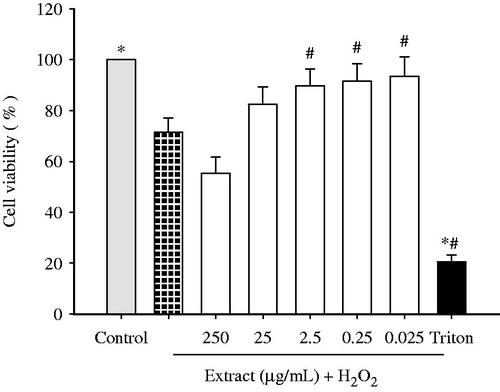
The potential protective effect of different concentrations of the ethanol extract obtained from the aerial parts of Haplophyllum tuberculatum against hydrogen peroxide has been evaluated measuring the cell viability and the proliferation by the MTT assay. As shown in , when U373-MG cells were exposed to hydrogen peroxide (1 mM) for 30 min, the cell viability significantly decreased by over 30% compared to control cells. However, pretreatments with the ethanol extract of Haplophyllum tuberculatum (250, 25, 2.5, 0.25 and 0.025 µg/mL) for 24 h, prior to H2O2 exposure, increased cell viability. Particularly, the concentrations of 2.5; 0.25 and 0.025 µg/mL restored significantly cell viability to almost untreated control levels by 13.5, 17 and 20.5%, respectively.
Figure 3. Protective effect of Haplophyllum tuberculatum ethanol extract against H2O2-induced loss in viability. U373-MG cells were treated with plant extract (range of concentrations from 0.025 to 250 µg/mL) for 24 h, prior to 1 mM H2O2 exposure (30 min). Triton X-100 was employed as negative control. Results were expressed as a mean of the percentage of control cells (100%) ± standard deviation (S.D.). *p < 0.05 versus control cells; #p < 0.05 versus H2O2.
Effect of plant extracts on intracellular ROS production
Oxidative stress is defined as an imbalance between the intracellular ROS production and the antioxidative defense mechanisms. H2O2 play a role in normal cellular signaling and function and it is involved in the generation of other dangerous ROS including hydroxyl radical (Tabner et al., Citation2005).
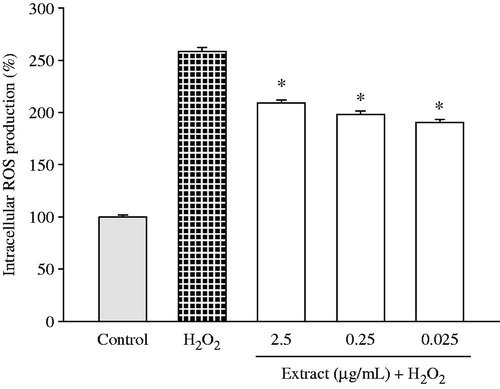
The effect on oxidative stress-induced ROS production was then evaluated using DCFH-DA assay. The astrocytes treated with H2O2 (1 mM, 30 min) showed a significant increase in the production of intracellular ROS (1.5-fold versus control) (). However, pretreatment of the ethanol extract obtained from Haplophyllum tuberculatum aerial parts (concentrations 2.5, 0.25 and 0.025 µg/mL, 24 h) inhibited the increase in the levels of intracellular ROS H2O2-induced in astrocytes (IC50 values 0.026 µg/mL), and therefore reducing the oxidative stress.
Figure 4. Protective effect of Haplophyllum tuberculatum ethanol extract against H2O2-induced intracellular ROS production. U373-MG cells were treated with plant extract (0.025, 0.25 and 0.025 µg/mL) for 24 h, prior to 1 mM H2O2 exposure (30 min). Results were expressed as a mean of the percentage of control cells (100%) ± standard deviation (S.D.). *p < 0.05 versus H2O2.
Conclusions
The current work for the first time provides evidence of the antioxidant activity and protective effect against oxidative stress of Haplophyllum tuberculatum extracts, attributed at least in part to its high level in polyphenolic compounds. Because the strong antioxidants properties and astrocytes protective effect under oxidative stress demonstrated by Haplophyllum tuberculatum extracts, this medicinal plant may have a promising therapeutic option for the prevention of different neurodegenerative diseases including Alzheimer and Parkinson’s diseases.
Declaration of interest
The authors report no conflicts of interest.
Acknowledgements
The authors thank Drs I. Estrella and T. Hernández from Instituto de Ciencia y Tecnología de Alimentos y Nutrición (CSIC), Madrid, Spain for technical assistance with HPLC analysis.
References
- Abdel-Kawy MA, El-Kashoury EA, El-Fishawy AM, et al. (1989). Alkaloids and lignans of Haplophyllum tuberculatum (Forssk.). Egypt J Pharmaceut Sci 30:299–308
- Al-Burtamani SK, Fatope MO, Marwah RG, et al. (2005). Chemical composition, antibacterial and antifungal activities of the essential oil of Haplophyllum tuberculatum from Oman. J Ethnopharmacol 96:107–12
- Al-Rehaily AJ, Tawfeq AA, Mohammad SA, et al. (2001). Alkaloids from Haplophyllum tuberculatum. Phytochemistry 57:597–602
- Al-Yahya MA, Al-Rehaily AJ, Mohammed SA, et al. (1992). New alkaloid from Haplophyllum tuberculatum. J Nat Prod 55:899–903
- Dávalos A, Gómez-Cordovés C, Bartolomé B. (2004). Extending applicability of the oxygen radical absorbance capacity (ORAC-Fluorescein) assay. J Agr Food Chem 52:48–54
- Dueñas M, Hernández T, Estrella I. (2004). Phenolic composition of the cotyledon and the seed coat of lentils (Lens culinaris L.). Eur Food Res Tech 219:116–23
- Ebrahimi A, Schluesener H. (2012). Natural polyphenols against neurodegenerative disorders: Potentials and pitfalls. Ageing Res Rev 11:329–45
- Floyd RA, Hensley K. (2002). Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging 23:795–807
- Galli F, Piroddi M, Annetti C, et al. (2005). Oxidative stress and reactive oxygen species. Contrib Nephrol 149:240–60
- Klein JA, Ackerman SL. (2003). Oxidative stress, cell cycle, and neurodegeneration. J Clin Inves 111:785–93
- Landete JM. (2012). Updated knowledge about polyphenols: Functions, bioavailability, metabolism, and health. Crit Rev Food Sci Nutr 52:936–48
- LeBel CP, Ischiropoulos H, Bondy SC. (1992). Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–31
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Meth 65:55–63
- Obrenovich ME, Li Y, Parvathaneni K, et al. (2011). Antioxidants in health, disease and aging. CNS Neurol Disord Drug Targets 10:192–207
- Perva-Uzunalic A, Skerget M, Knez Z, et al. (2006). Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem 96:597–605
- Pietta PG. (2000). Flavonoids as antioxidants. J Nat Prod 63:1035–42
- Salvo G, Manafzadeh S, Ghahremaninejad F, et al. (2011). Phylogeny, morphology, and biogeography of Haplophyllum (Rutaceae), a species-rich genus of the Irano-Turanian floristic region. Taxon 60:513–27
- Saura-Calixto F, Serrano J, Goñi I. (2007). Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem 101:492–501
- Sheriha GM, Abouamer K. (1984). Lignans of Haplophyllum tuberculatum. Phytochemistry 23:151–3
- Tabner BJ, El-Agnaf OM, Turnbull S, et al. (2005). Hydrogen peroxide is generated during the very early stages of aggregation of the amyloid peptides implicated in Alzheimer disease and familial British dementia. J Biol Chem 280:35789–92
- Zohair HM, Hamed JJ, May A, Ali ZS. (1989). Insecticidal effects of Haplophyllum tuberculatum against Cluex quinquefasciatus. J Crude Drug Res 27:17–21

