Abstract
Context. Cordyceps sinensis (Berk.) Sacc. (Clavicipitaceae) is a famous medicinal fungus (mushroom) in Chinese herbal medicine. Polysaccharides from Cordyceps sinensis (CSP) have been identified as active ingredients responsible for its biological activities. Although many pharmacological actions of CSP have received a great deal of attention, research in this area continues.
Objective: The current study was designed to investigate the effects of CSP on exhaustive exercise-induced oxidative stress.
Materials and methods: The mice were divided into four groups: control (C), low-dose CSP treated (LC), intermediate-dose CSP treated (IC) and high-dose CSP treated (HC). The treated groups received CSP (100, 200 and 400 mg/kg, ig), while the control group received drinking water for 28 days, followed by being forced to undergo exhaustive swimming exercise, and some biochemical parameters including superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), malondialdehyde (MDA) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) were measured using detection kits according to the manufacturers’ instructions.
Results: Compared with the C group, exhaustive swimming time was significantly prolonged in the LC, IC and HC groups (p < 0.05); SOD activities in serum, liver and muscle were significantly higher in the IC and HC groups (p < 0.05); GPx activities in serum, liver and muscle were significantly higher in the LC, IC and HC groups (p < 0.05); CAT activities in serum, liver and muscle were significantly higher in the HC groups (p < 0.05); MDA and 8-OHdG levels in serum, liver and muscle were significantly lower in the LC, IC and HC groups (p < 0.05).
Discussion and conclusion: The results obtained herein indicate that CSP could ameliorate exhaustive exercise-induced oxidative stress.
Introduction
Cells continuously produce free radicals and reactive oxygen species (ROS) as part of metabolic processes (Urso & Clarkson, Citation2003). Under normal circumstances, ROS are neutralized by an elaborate endogenous antioxidant system comprised of enzymatic and non-enzymatic antioxidants (Fan et al., Citation2011; Keong et al., Citation2006). Intense physical exercise is often associated with an increase in the production of free radicals and ROS in various tissues, which may overwhelm the capacity of the antioxidant defense systems, and the result can lead to increased oxidative stress (Powers & Jackson, Citation2008; Vollaard et al., Citation2005). It has been suggested that increased oxidative stress can lead to the destruction of tissue and cell macromolecules such as lipids, proteins and nucleic acids (Jówko et al., Citation2011). In addition, exercise-induced oxidative stress may be associated with muscle fatigue, muscle damage and a decrease in physical performance. Several lines of evidence indicate that increased antioxidant enzyme activities, increased resistance to oxidative stress and lower levels of oxidative damage may protect oxidative stress-related tissue and cell macromolecules damage (Huang et al., Citation2009; Korivi et al., Citation2012; Minato et al., Citation2003).
Cordyceps sinensis (Berk.) Sacc. (Clavicipitaceae), also known as Chinese caterpillar fungus or “DongChongXiaCao” (summer-plant, winter-worm), is a famous and highly valued medicinal fungus (mushroom) in Chinese herbal medicine (Zhang & Wu, Citation2007). For over 2000 years, Cordyceps sinensis has been commonly used in China to replenish the kidney and soothe the lung, for the treatment of fatigue, night sweating, hyposexualities, hyperglycemia, hyperlipidemia, asthenia after severe illness, respiratory disease, renal dysfunction and renal failure (Chen et al., Citation2004; Li et al., Citation2002). A number of bioactive components from Cordyceps sinensis have been reported, including polysaccharides, cordycepin and its derivative, mannitol, ergosterol, glycoproteins and peptides containing α-aminoisobutyric acid (Li & Li, Citation2009). Polysaccharides from Cordyceps sinensis (CSP) have been identified as one of the main bioactive components (Wang et al., Citation2010; Zhong et al., Citation2009). Recent studies have demonstrated the multiple pharmacological actions of CSP, including antitumor, antiinflammatory, antioxidant, antifatigue, hypoglycemic, hypolipidemic, liver protective effects and immunomodulatory properties (Kuo et al., Citation2007; Li & Li, Citation2009; Li et al., Citation2006; Wang et al., Citation2009; Zhong et al., Citation2009). In our previous studies, CSP has also been proven to have significant antifatigue effects in mice (Yan et al., Citation2012). However, the effects of CSP on exercise-induced oxidative stress are not clear. The present study is the first to investigate the effects of CSP on exhaustive exercise-induced oxidative stress by measuring antioxidant enzymes activities and malondialdehyde (MDA) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels in the serum, liver and muscle of mice.
Materials and methods
Plant material
The dried Cordyceps sinensis mycelium was purchased from Tong-Ren-Tang Pharmaceutical Group (Beijing, China) in September 2011 and authenticated by Yiyang Zhang, a biologist of Jilin University (Changchun, China). A voucher specimen (No. NC084321) has been deposited in Plant Herbarium, Jilin University.
Chemicals and reagents
The detection kits (special for animal testing) including malonaldehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) were purchased from BioSino Bio-technology and Science Inc (Beijing, China). 8-Hydroxy-2′-deoxyguanosine (8-OHdG) enzyme-linked immunosorbent assay (ELISA) kit (special for animal testing) was purchased from Fangcheng Biotechnology Co., Ltd. (Beijing, China). All other chemicals and reagents used were of analytical grade and MilliQ grade water was used.
Extraction and purification of polysaccharides
Crude polysaccharides from Cordyceps sinensis were extracted by the method of Wu et al. (Citation2006) with slight modification. Dried Cordyceps sinensis mycelium were extracted with ethanol (95% and 85%, respectively) to defat and decolorize, and then extracted with aqueous 75% ethanol overnight. After centrifugation (6700 rpm, 30 min), the residue was dried naturally and then extracted with 0.05 M phosphate buffer (pH 7.0) for 10 h at 80 °C, and the process of extraction was repeated. After centrifugation (6700 rpm, 30 min), the extracting liquids were combined, removed solvents under reduced pressure and dialyzed. The nondialyzable phase was diluted with 95% EtOH, and the resulting precipitate was collected by centrifugation, washed three times with acetone and dried. The dried crude polysaccharides was then dissolved in 10 mM Tris–HCl buffer (pH 8.4) for fast performance liquid chromatography (FPLC) with DEAE cellulose column.
The purified polysaccharides were prepared as described previously (Li & Li, Citation2009; Li et al., Citation2006). In belief, the concentrated fractions were applied onto a Sephacryl S-300 column equlibrated with 0.2 M NaCl in 10 mM Tris–HCl (pH 8.0). Elution was carried out with the same solution with a flow rate of 0.2 mL/min, and the amount of polysaccharides was determined. The fractions of first-peak were pooled and dialyzed extensively against 10 mM Tris--HCl (pH 8.0). The purified polysaccharides from Cordyceps sinensis (CSP), with a molecular weight of 210 kDa, were obtained. CSP contains glucose, mannose and galactose in a ratio of 1:0.6:0.75.
Selection of animal and care
Male Kunming mice (18–22 g) were obtained from the Vital River Lab Animal Technology Co., Ltd. (Beijing, China). They were housed in a controlled environment with temperature maintained at 22 ± 1 °C and humidity at 50 ± 5% under a 12:12 h light-dark cycle. Standard pellets (purchased from Xincaihong Feed Co., Ltd., Beijing, China) and water were provided ad libitum. All animal experiments were conducted according to the “Principles of Laboratory Animal Care” (World Health Organization Chronicle, 1985), and approved by the Institutional Animal Ethics Committee of University of International Business and Economics.
Exhaustive swimming exercise
The mice were allowed to acclimate to the laboratory environment for 1-week period before the experiments. After this period, the mice were randomly divided into four groups (n = 8 in each group), i.e., control (C) group, low-dose CSP treated (LC) group, intermediate-dose CSP treated (IC) group and high-dose CSP treated (HC) group. The treated groups were received CSP (100, 200 and 400 mg/kg, ig) dissolved in 1.0 mL of drinking water, and the control group received drinking water (1.0 mL, ig) for 28 days. After 28 days, exhaustive swimming exercise was performed by our previously described method (Yan et al., Citation2012). In brief, the mice swam with tin wire of 7% body weight tied to their tails in an acrylic plastic tank (50 cm × 50 cm × 40 cm) filled with 30 cm depth of water maintained at 25 ± 1 °C. The mice were assessed to be exhausted when they failed to rise to the surface of water to breathe within a 10-s period (Bing & Zhaobao, Citation2010; Cai et al., Citation2010; Tan et al., Citation2012). The doses of CSP and 28-day treatment time used in this study were confirmed to be suitable and effective in tested mice, according to preliminary experiments.
Analysis of biochemical parameters
The mice were anesthetized with ethyl ether and sacrificed immediately after the exhaustive swimming exercise. The blood was collected and the serum was isolated for use, while the liver and gastrocnemius muscle tissues were quickly removed, washed with physiological saline and homogenized in ice-cold buffer. The antioxidant enzymes (SOD, GPx and CAT) activities, MDA and 8-OHdG levels were measured using detection kits according to the manufacturers’ instructions.
Statistical analysis
The data are expressed as mean ± SD. Statistical analysis was performed by one-way ANOVA followed by least-significant difference (LSD). A difference was considered significant when p < 0.05.
Results
Effects of CSP on exhaustive swimming times of mice
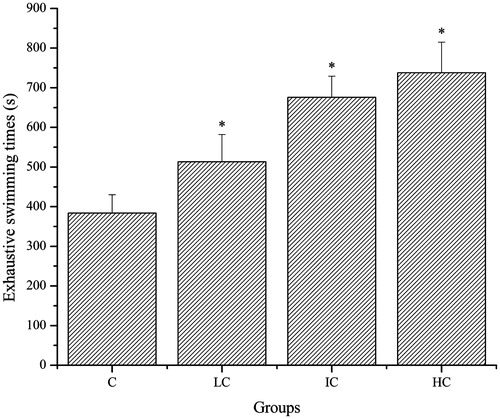
As shown in , the exhaustive swimming time of mice in the LC, IC and HC groups was significantly prolonged compared with that in the C group (p < 0.05).
Figure 1. Effects of CSP on exhaustive swimming times of mice. Data are expressed as mean ± SD. CSP: polysaccharides from Cordyceps sinensis (Berk.) Sacc.; C: control; LC: low-dose CSP treated (100 mg/kg); IC: intermediate-dose CSP treated (200 mg/kg); HC: high-dose CSP treated (400 mg/kg). *, p < 0.05 compared with C group.
Effects of CSP on SOD activities of mice
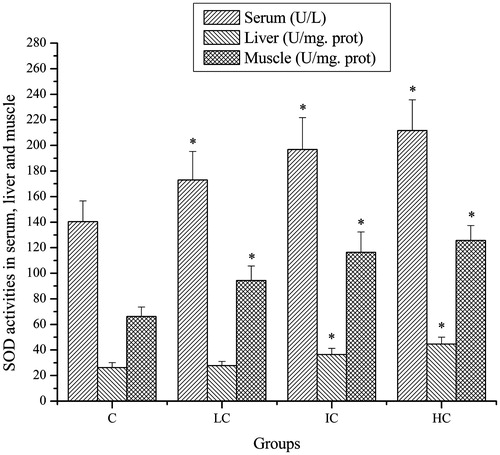
As shown in , after exhaustive swimming exercise, the SOD activities in serum and muscle of mice in the LC, IC and HC groups were significantly higher compared with that in the C group (p < 0.05). The SOD activities in liver of mice in the IC and HC groups were significantly higher compared with that in the C group (p < 0.05). Although the SOD activities in liver of mice in the LC groups were also increased, no significant difference was observed (p > 0.05).
Figure 2. Effects of CSP on SOD activities in serum, liver and muscle of mice. Data are expressed as mean ± SD. CSP: polysaccharides from Cordyceps sinensis; C: control; LC: low-dose CSP treated (100 mg/kg); IC: intermediate-dose CSP treated (200 mg/kg); HC: high-dose CSP treated (400 mg/kg). *, p < 0.05 compared with C group.
Effects of CSP on GPx activities of mice
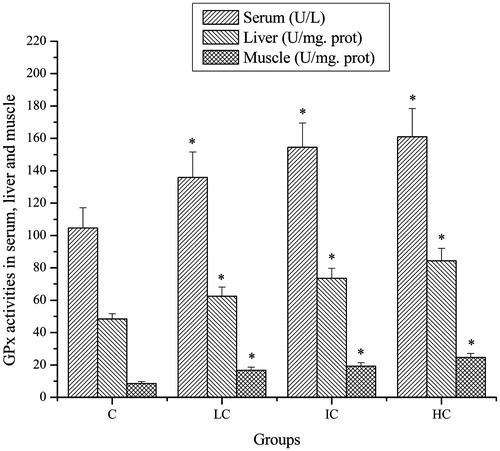
As shown in , after exhaustive swimming exercise, the GPx activities in serum, liver and muscle of mice in the LC, IC and HC groups were significantly higher compared with that in the C group (p < 0.05).
Figure 3. Effects of CSP on GPx activities in serum, liver and muscle of mice. Data are expressed as mean ± SD. CSP: polysaccharides from Cordyceps sinensis; C: control; LC: low-dose CSP treated (100 mg/kg); IC: intermediate-dose CSP treated (200 mg/kg); HC: high-dose CSP treated (400 mg/kg). *, p < 0.05 compared with C group.
Effects of CSP on CAT activities of mice
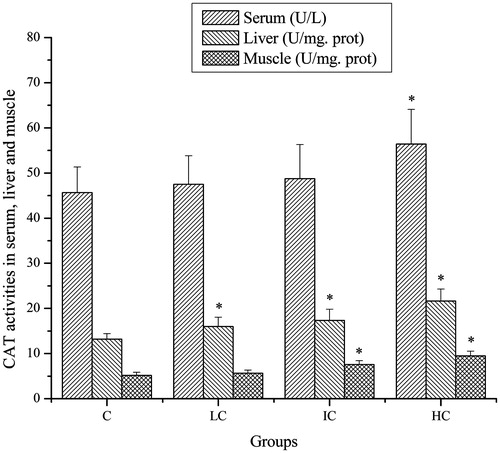
As shown in , after exhaustive swimming exercise, the CAT activities in serum of mice in the HC groups were significantly higher compared with that in the C group (p < 0.05). Although the CAT activities in serum of mice in the LC and IC groups were also increased, no significant difference was observed (p > 0.05). The CAT activities in liver of mice in the LC, IC and HC groups were significantly higher compared with that in the C group (p < 0.05). The CAT activities in muscle of mice in the IC and HC groups were significantly higher compared with that in the C group (p < 0.05). Although the CAT activities in muscle of mice in the LC groups were also increased, no significant difference was observed (p > 0.05).
Figure 4. Effects of CSP on CAT activities in serum, liver and muscle of mice. Data are expressed as mean ± SD. CSP: polysaccharides from Cordyceps sinensis; C: control; LC: low-dose CSP treated (100 mg/kg); IC: intermediate-dose CSP treated (200 mg/kg); HC: high-dose CSP treated (400 mg/kg). *, p < 0.05 compared with C group.
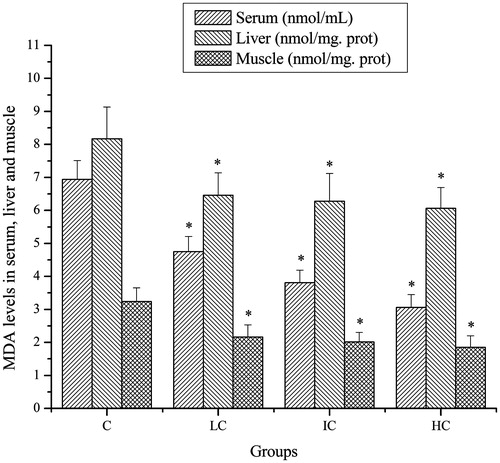
Effects of CSP on MDA levels of mice
As shown in , after exhaustive swimming exercise, the MDA levels in serum, liver and muscle of mice in the LC, IC and HC groups were significantly lower compared with that in the C group (p < 0.05).
Figure 5. Effects of CSP on MDA levels in serum, liver and muscle of mice. Data are expressed as mean ± SD. CSP: polysaccharides from Cordyceps sinensis; C: control; LC: low-dose CSP treated (100 mg/kg); IC: intermediate-dose CSP treated (200 mg/kg); HC: high-dose CSP treated (400 mg/kg). *, p < 0.05 compared with C group.
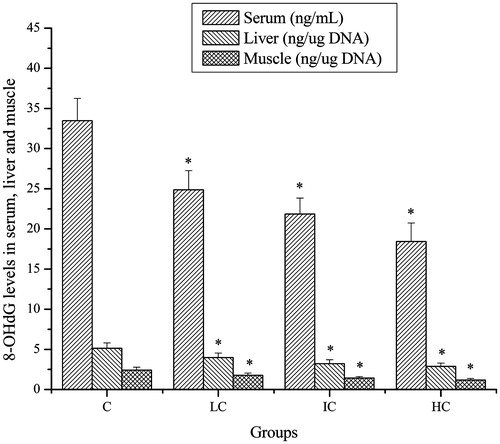
Effects of CSP on 8-OHdG levels of mice
As shown in , after exhaustive swimming exercise, the 8-OHdG levels in serum, liver and muscle of mice in the LC, IC and HC groups were significantly lower compared with that in the C group (p < 0.05).
Figure 6. Effects of CSP on 8-OHdG levels in serum, liver and muscle of mice. Data are expressed as mean ± SD. CSP: polysaccharides from Cordyceps sinensis; C: control; LC: low-dose CSP treated (100 mg/kg); IC: intermediate-dose CSP treated (200 mg/kg); HC: high-dose CSP treated (400 mg/kg). *, p < 0.05 compared with C group.
Discussion
It has been generally accepted that oxidative stress is an imbalance between the generation of ROS and the antioxidant defense capacity of the body (Ji, Citation1999). The antioxidant defense systems of the living body consist of enzymatic and non-enzymatic antioxidant, which may be involved in reducing oxidative stress. The main antioxidant enzymes include SOD, GPx and CAT. CAT catalyzes the detoxification of H2O2 to H2O. GPX activates the GSH-scavenging reactions of free radicals (•OH) and singlet oxygen species (1O2), whereas SOD is responsible for the dismutation of two superoxide anion molecules to form H2O2 and O2 (Guizani et al., Citation2011). As antioxidant enzymes play an important role in the protection against free radical damage, a decrease in the activities or expressions of these enzymes may predispose tissues to the free radical damage (Luo et al., Citation2011). In this study, the data showed that the SOD, GPx and CAT activities in serum, liver and muscle of mice in the high-dose CSP-treated groups were significantly higher compared with that in the control group. The results indicated that CSP was able to up-regulate antioxidant enzymes activities to protect against exhaustive exercise-induced oxidative stress.
Lipid peroxidation is one of the major outcomes of free radical-mediated injury, it occurs when the hydroxyl radicals, possibly oxygen, react with the unsaturated lipids of the bio-membranes, resulting in the generation of lipid peroxide radicals (ROO•), lipid hydroperoxide (ROOH) and fragmentation products such as MDA (Cheeseman, Citation1993), and MDA level is commonly known as a marker of oxidative stress. 8-OHdG is a DNA base-modified product generated by reactive oxygen species and is a mutation prone to induce a G-C to T-A transversion during DNA replication and has recently been shown to be a new marker of oxidative damage (Fukushima et al., Citation2010; Huang et al., Citation1999; Ogonovszky et al., 2005a). Over the past two decades, there is growing evidence that exhaustive exercise increased the MDA and 8-OHdG level in blood, liver and muscle tissues in rat and mice (Liu et al., Citation2000; Ochoa et al., Citation2011; Ogonovszky et al., 2005b; Okudan et al., Citation2012; Voces et al., Citation1999; Yu et al., Citation2006). In this study, the data showed that the MDA and 8-OHdG levels in serum, liver and muscle of mice in the CSP-treated groups were significantly lower compared with that in the control group. The results indicated that CSP could effectively reduce lipid peroxidation and prevent exercise-induced oxidative damage in mice.
In conclusion, the present study demonstrated that CSP could ameliorate exhaustive exercise-induced oxidative stress in mice through decreasing MDA and 8-OHdG levels and increasing antioxidant enzymes activities in the serum, liver and muscle of mice.
Declaration of interest
This work was supported by a key program of the Bureau of Science and Technology of Harbin, China.
Acknowledgements
We are grateful to Dr. Yiyang Zhang (Jilin University, China) for his revision and comments on the manuscript.
References
- Bing Y, Zhaobao W. (2010). Effects of Ginkgo biloba extract on free radical metabolism of liver in mice during endurance exercise. Afr J Tradit Complement Altern Med 7:291–5
- Cai RL, Yang MH, Shi Y, et al. (2010). Antifatigue activity of phenylethanoid-rich extract from Cistanche deserticola. Phytother Res 24:313–15
- Cheeseman KH. (1993). Mechanisms and effects of lipid peroxidation. Mol Aspects Med 14:191–7
- Chen YQ, Hu B, Xu F, et al. (2004). Genetic variation of Cordyceps sinensis, a fruit-body-producing entomopathogenic species from different geographical regions in China. FEMS Microbiol Lett 230:153–8
- Fan LD, Zhai F, Shi DX, et al. (2011). Evaluation of antioxidant properties and anti-fatigue effect of green tea polyphenols. Sci Res Essays 6:2624–9
- Fukushima N, Kuromatsu R, Akiba J, et al. (2010). Characteristic expression pattern of oxidative stress in livers with cryptogenic hepatocellular carcinoma. Exp Ther Med 1:809–16
- Guizani N, Waly MI, Ali A, et al. (2011). Papaya epicarp extract protects against hydrogen peroxide-induced oxidative stress in human SH-SY5Y neuronal cells. Exp Biol Med 236:1205–10
- Huang SC, Lee FT, Kuo TY, et al. (2009). Attenuation of long-term Rhodiola rosea supplementation on exhaustive swimming-evoked oxidative stress in the rat. Chin J Physiol 52:316–24
- Huang XB, Ito F, Nakazawa H, Toma H. (1999). Increased expression of 8-hydroxydeoxyguanosine in acquired cystic disease of the kidney. Nephron 81:458–9
- Ji LL. (1999). Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med 222:283–92
- Jówko E, Sacharuk J, Balasińska B, et al. (2011). Green tea extract supplementation gives protection against exercise-induced oxidative damage in healthy men. Nutr Res 31:813–21
- Keong CC, Singh HJ, Singh R. (2006). Effects of palm vitamin E supplementation on exercise-induced oxidative stress and endurance performance in the heat. J Sports Sci Med 5:629–39
- Korivi M, Hou CW, Huang CY, et al. (2012). Ginsenoside-Rg1 protects the liver against exhaustive exercise-induced oxidative stress in rats. Evid Based Complement Alternat Med 2012:932165
- Kuo MC, Chang CY, Cheng TL, Wu MJ. (2007). Immunomodulatory effect of exo-polysaccharides from submerged cultured Cordyceps sinensis: Enhancement of cytokine synthesis, CD11b expression, and phagocytosis. Appl Microbiol Biotechnol 75:769–75
- Li SP, Su ZR, Dong TT, Tsim KW. (2002). The fruiting body and its caterpillar host of Cordyceps sinensis show close resemblance in main constituents and anti-oxidation activity. Phytomedicine 9:319–24
- Li SP, Zhang GH, Zeng Q, et al. (2006). Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured Cordyceps mycelia. Phytomedicine 13:428–33
- Li T, Li W. (2009). Impact of polysaccharides from Cordyceps on antifatigue in mice. Sci Res Essay 4:705–9
- Liu J, Yeo HC, Overvik-Douki E, et al. (2000). Chronically and acutely exercised rats: Biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol 89:21–8
- Luo LP, Qian LA, Wang YQ, Yuan GR. (2011). Spirulina platensis extract supplementation attenuates oxidative stress in acute exhaustive exercise: A pilot study. Int J Phys Sci 6:2901–6
- Minato K, Miyake Y, Fukumoto S, et al. (2003). Lemon flavonoid, eriocitrin, suppresses exercise-induced oxidative damage in rat liver. Life Sci 72:1609–16
- Ochoa JJ, Díaz-Castro J, Kajarabille N, et al. (2011). Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J Pineal Res 51:373–80
- Ogonovszky H, Berkes I, Kumagai S, et al. (2005a). The effects of moderate-, strenuous- and over-training on oxidative stress markers, DNA repair, and memory, in rat brain. Neurochem Int 46:635–40
- Ogonovszky H, Sasvári M, Dosek A, et al. (2005b). The effects of moderate, strenuous, and overtraining on oxidative stress markers and DNA repair in rat liver. Can J Appl Physiol 30:186–95
- Okudan N, Revan S, Balci SS, et al. (2012). Effects of CoQ10 supplementation and swimming training on exhaustive exercise-induced oxidative stress in rat heart. Bratisl Lek Listy 113:393–9
- Powers SK, Jackson MJ. (2008). Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243–76
- Tan W, Yu KQ, Liu YY, et al. (2012). Anti-fatigue activity of polysaccharides extract from Radix Rehmanniae Preparata. Int J Biol Macromol 50:59–62
- Urso ML, Clarkson PM. (2003). Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189:41–54
- Voces J, Alvarez AI, Vila L, et al. (1999). Effects of administration of the standardized Panax ginseng extract G115 on hepatic antioxidant function after exhaustive exercise. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 123:175–84
- Vollaard NBJ, Shearman JP, Cooper CE. (2005). Exercise-induced oxidative stress: Myths, realities and physiological relevance. Sports Med 35:1045–62
- Wang Y, Wang M, Ling Y, et al. (2009). Structural determination and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordyceps sinensis. Am J Chin Med 37:977–89
- Wang Y, Yin H, Lv X, et al. (2010). Protection of chronic renal failure by a polysaccharide from Cordyceps sinensis. Fitoterapia 81:397–402
- Wu YL, Sun CR, Pan YJ. (2006). Studies on isolation and structural features of a polysaccharide from the mycelium of an Chinese edible fungus (Cordyceps sinensis). Carbohyd Polym 63:251–6
- Yan F, Zhang Y, Wang B. (2012). Effects of polysaccharides from Cordyceps sinensis mycelium on physical fatigue in mice. Bangladesh J Pharmacol 7:217–21
- Yu F, Lu S, Yu F, et al. (2006). Protective effects of polysaccharide from Euphorbia kansui (Euphorbiaceae) on the swimming exercise-induced oxidative stress in mice. Can J Physiol Pharmacol 84:1071–9
- Zhang QX, Wu JY. (2007). Cordyceps sinensis mycelium extract induces human premyelocytic leukemia cell apoptosis through mitochondrion pathway. Exp Biol Med 232:52–7
- Zhong S, Pan HJ, Fan LF, et al. (2009). Advances in research of polysaccharides in Cordyceps species. Food Tech Biotech 47:304–12

