Abstract
Context: Ginsenosides are primary active ingredients of ginseng, which are believed to have various health benefits. It is found that the biotransformation of ginsenosides mainly takes place in the gastrointestinal tract and the information about ginsenosides-exerted effects on intestinal contractility is not sufficient.
Aims: The present study proposed that ginsenosides could exert stimulatory or inhibitory effects on intestinal motility depending on the assay condition-related intestinal contractile states and was to characterize the effects of ginsenosides on intestinal motility.
Methods: Jejunal contractility determination, Western blotting analysis, and real-time polymerase chain reaction were performed to test the effects of total ginsenosides isolated from Panax ginseng C. A. Mey (Araliaceae) root.
Results: The results showed that ginsenosides at the fixed concentration of 20 mg/L exerted bidirectional regulation (BR) on the contractility of isolated jejunal segment (IJS), depending on the contractile states. The contractility of IJS was increased by ginsenosides in low contractile states, which were correlated to the cholinergic activation, and the contractility of IJS was decreased by ginsenosides in high contractile states, which were correlated to the adrenergic activation and nitric oxide related mechanisms. Ginsenosides-induced BR was abolished in the absence of Ca2+ or by using tetrodotoxin, implicating the requirement of Ca2+ and the enteric nervous system. Effects of ginsenosides on myosin light chain phosphorylation and the mRNA expression of myosin light chain kinase were also bidirectional.
Discussion and conclusion: Results suggest that ginsenosides may have the potential clinical implication for reliving the symptoms of alternative hypo- and hyper-intestinal motility.
Introduction
The major active components of ginseng are ginsenosides and more than 150 naturally occurring ginsenosides have been isolated (Christensen, Citation2009). Ginsenosides have potential health benefits including protection of the cardiovascular system (Shi et al., Citation2011), anti-tumorigenic (Surh et al., Citation2001), neuroprotective (Gong et al., Citation2011) anti-inflammatory (Han et al., Citation2011), antidiabetic (Kim et al., Citation2011), immunomodulatory, and anti-stress effects (Leung & Wong, Citation2010).
The biotransformation of ginsenosides takes place mainly in the gastrointestinal tract (Tawab et al., Citation2003). Ginsenosides are reported to accelerate mouse small intestinal movement (Furukawa et al., Citation1995) and stimulate smooth muscle contraction in isolated guinea pig ileum and distal colon tissues (Hashimoto, Citation2001). Ginsenosides exerted stimulatory effects on gut motility through modulating the pacemaker activity of interstitial cells of Cajal (Han et al., Citation2012; Kim et al., Citation2007). However, our preliminary assay showed that the effects induced by ginsenosides were not unilateral and were dependent on the assay conditions. The present study proposed that ginsenosides could exert stimulatory or inhibitory effects on intestinal motility depending on the assay condition-related contractile states of jejunal segment. The assays were designed to test the proposal, characterize the effects of ginsenosides on intestinal motility, and provide information to evaluate its potential clinical implication for intestinal contractile dysfunction. Isolated jejunal segment (IJS) was chosen in the assays, based on the fact that the motility of intestinal smooth muscle is modulated by the enteric nervous system (ENS), which is able to fulfill pivotal functions even when isolated from the body (Schemann, Citation2005). Different low and high contractile states of intestinal smooth muscle were established by changing ionic concentration, by using inhibitory and stimulatory neurotransmitters, respectively, by using exogenous inhibitors and stimulators, respectively, and by using jejunal strips isolated from rat models of constipation-prominent (CP) and diarrhea-prominent (DP) bowel dysfunction, respectively. The assays were performed to investigate whether ginsenosides-induced effects were related with muscarinic, adrenergic receptors, and nitric oxide (NO)-mediated relaxing mechanisms, and whether ginsenosides-induced effects were Ca2+ based, ENS dependent and myosin phosphorylation related.
Materials and methods
Animals
Sixty Sprague-Dawley rats, half male and half female, weighing 180–220 g were randomly divided into normal control, CP, DP, ginsenosides-treated CP and ginsenosides-treated DP groups, respectively. Rats were provided by the Experimental Animal Center, Dalian Medical University (Certificate of Conformity: No. SCXK (Liao) 2008-0002). The experimental protocol was approved by Dalian Medical University Animal Care and Ethics Committee, and all experimental procedures described were performed in accordance with the Declaration of Helsinki. Rats used in the study were maintained in accordance with guidance suggestions for the Care and Use of Laboratory Animals (The Ministry of Science and Technology of People’s republic of China, 2006). Rats were housed 1 per cage in a temperature-controlled room with a 12-h light–dark cycle. Food and water were available ad libitum.
Experimental models of constipation and diarrhea
DP rats were established by intracolonic instillation of 4% (V/V) acetic acid and restraint stress, and rats in the control group underwent intracolonic instillation with saline (La et al., 2003). On the fifth day, rats in the treatment DP group were gavaged with ginsenosides (30 mg/kg) until the day 8. CP rats were established by daily gavage with cool water (0–4 °C) for 2 weeks, and rats of the control group were prepared by daily gavage with water at room temperature (Xu et al., Citation2006). On the 11th, the rats in the treatment CP group were gavaged with ginsenosides (30 mg/kg) until the 14th day. The moisture content and granule number of the feces from all groups were measured daily, and the body mass was recorded once every 3 days. The CP model was established when its stool weight and water contents were significantly lower than those in the control group. The DP model was successful when these measurements were significantly higher than those in the control group.
Contractility determination
Jejunal segment isolated from the intact tubular jejunum was prepared according to the method described previously (Barthó & Lefebvre, Citation1994; Mathison & Shaffer, Citation2006). Jejunum was obtained from normal CP and DP rats, respectively. Jejunum was cut into approximately 2 cm in length (tubes) and then allowed to equilibrate in aerated Krebs buffer for 50 min; the buffer was changed every 10 min. The resting tension was set optimally at 1.0 g. Preliminary experiments showed that this load stretched tissues to their optimal length for force development during contraction. Contractile response was recorded using a BL-420F physiological recording system (Chengdou, China). Contractile amplitude of contraction was recorded and identical time-interval of each assay with same start and stop times was chosen to compare the amplitude of contractions before and after drug treatment under different assay conditions. The mean amplitude was calculated from the results of six separate assays.
Ex vivo assay conditions
The contractility of IJS was recorded in Krebs buffer (112.8 mmol/L NaCl, 4.7 mmol/L KCl, 25.0 mmol/L NaHCO3, 1.2 mmol/L KH2PO4, 2.45 mmol/L MgSO4, 2.5 mmol/L CaCl2; pH:7.4) and selected as the normal contractile state. Since spontaneous contractions of intestinal smooth muscle are paralleled to intracellular Ca2+ concentration (Frings et al., Citation2000), jejunal contractility recorded in modified low Ca2+ (1.25 mmol/L) and high Ca2+ (5.0 mmol/L) Krebs buffer was selected as the representative low contractile state (RLCS) and representative high contractile state (RHCS), respectively. One pair of low-high contractile states was established by using IJS obtained from CP and DP rats, respectively. The other four pairs of low-high contractile states were generated by incubating IJS in modified low Na+ (100 mmol/L)-high Na+ (160 mmol/L) Krebs buffer, low K+ (2.5 mmol/L)-high K+ (10.0 mmol/L) Krebs buffer, adrenaline (5.0 µmol/L)-ACh (5.0 µmol/L) Krebs buffer, and NO donor sodium nitroprusside (SNP) (5 μmol/L)-erythromycin (10 μmol/L) Krebs buffer, respectively (Chen et al., Citation2012).
Western blot analysis
Jejunal segments isolated from normal control, CP, DP, ginsenosides-treated CP and ginsenosides-treated DP groups were frozen and stored in liquid nitrogen. Ground product was incubated in an ice-cold homogenization buffer for 30 min. Phosphorylation of the 20-kDa regulatory light chain subunit of myosin (MLC20) in jejunum was determined as described previously (Kobayashi et al., Citation2011; Iwabu et al., Citation2004). The blots on nitrocellulose filter membrane were probed with phosphor-myosin light chain 2 (Ser 19) antibody (1:1000) (Cell Signaling Technology, Inc (CST), Boston, MA) and myosin light chain 2 (total myosin light chain) antibodies (1:1000) (CST) respectively, at 4 °C with gentle shaking over night. For myosin light chain kinase (MLCK) protein content analysis, the blots on nitrocellulose filter membrane were probed with MLCK antibody (1:1000) (Abcam (Hong Kong) Ltd., Cambridge, UK). Anti-rabbit IgG secondary antibodies were used at 1:2500 for 60 min at room temperature and bands were determined and quantified using a Multi Spectral Imaging System (UVP, Cambridge, UK).
Drugs
The ginsenosides employed in the present study was total ginsenosides isolated from Panax ginseng C. A. Mey (Araliaceae) root, which is a commercially available standardized ginseng extract (Chengdu Biopurify Phytochemicals Ltd., China.). The ginsenosides mainly includes (w/w) Rg1 (11.3%), Rd (17.27%), Re (23.5%), Rb1 (1.23%), Rb2 (4.3%), Rf (0.01%), and Rc (2.55%). Ginsenosides were dissolved in distilled water and its pH was adjusted to 7.4 before use. The proper volume of ginsenoside solution was chosen to get the final required concentration. Tetrodotoxin (TTX) was obtained from Aladdin Chemistry Co. Ltd (Shanghai, China). Unless otherwise indicated, chemicals were obtained from Sigma (St. Louis, MO).
Statistical analysis
One-way analysis of variance (ANOVA) was used to compare the means of three or more groups of data. Paired Student’s t-test was used to compare the means of two groups of data. Data were expressed as the mean ± SEM. All experiments were repeated for at least three times. p < 0.05 was considered as significantly different.
Results
Dose–response relationship of gisenosides
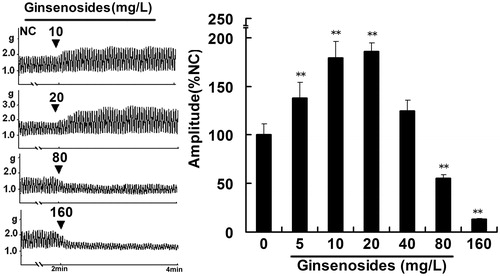
Ginsenoside-exerted effects on jejunal contractility in the normal contractile state were shown by its concentration–response relationship. Ginsenosides increased contractile amplitude of IJS in the concentration range of 5–20 mg/L with an ED50 of 10 mg/L (p < 0.05; n = 6 tissues) and decreased contractile amplitude of IJS in the concentration range of 40–160 mg/L with an ED50 of 80 mg/L (p < 0.05; n = 6 tissues; ).
Figure 1. Representative traces of ginsenosides on the contractile amplitude of isolated jejunal segment (IJS). NC, normal control, the contractility recorded in normal contractile state. Contractile amplitude of IJS in the normal contractile state is set to a relative value of 100% (normal control, NC). Data are expressed as the mean ± SEM. (% NC, n = 6); **p < 0.01 compared with NC.
Ginsenosides-induced bidirectional regulation on jejunal contractility
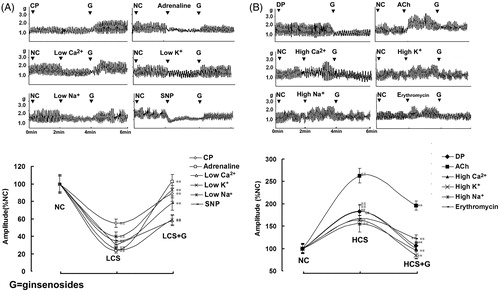
To evaluate ginsenoside-induced effects in different conditions, six low and six high contractile states of jejunal segment were established and used in the study. The contractility of IJS in both low and high contractile states was statistically different from that in normal contractile state (). Ginsenosides exerted bidirectional regulation (BR), i.e., stimulatory effects on the contractility of IJS in all six low contractile states () and inhibitory effects on the contractility of IJS in all six high contractile states (). Ginsenosides at the concentration of 20 mg/L was chosen to exert BR on the contractility of IJS and was used in all the ex vivo assays based on that ginsenosides-induced BR was found in the dose range of 10–40 mg/L.
Figure 2. Ginsenoside-induced bidirectional regulation (BR) on the contractility of isolated jejunal segment (IJS). Representative traces and statistical analysis of total traces from six independent experiments of ginsenosides-induced BR on the contractility of IJS in (A) six low contractile states (LCS) and in (B) six high contractile states (HCS). Contractile amplitude of IJS in the normal contractile state is set to a relative value of 100% (normal control, NC). Low and high contractile states of IJS are the relative values compared with NC. Data are expressed as the mean ± SEM. (% NC, n = 6); ##p < 0.01 compared with nc; **p < 0.01 compared with contractile amplitude of IJS in LCS or HCS before given ginsenosides, respectively. CP: Constipation-prominent rats; DP: Diarrhea-prominent rats; SNP: Sodium nitroprusside.
Possible mechanisms involved in ginsenoside-induced bidirectional regulation
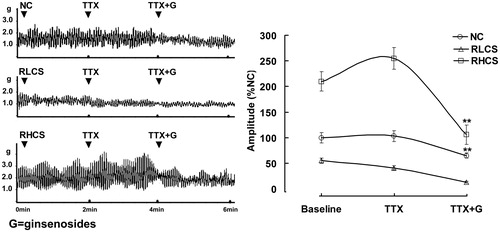
To evaluate the role of ENS in ginsenoside-induced BR, TTX is used as a potent neurotoxin to block action potentials by inhibiting the movement of Na+ across neural membranes, revealing the effects of ginsenosides on the jejunal contractility in the absence of neural control. In the presence of TTX, only inhibitory effects exerted by ginsenoside on the contractility of IJS were observed in the normal contractile state, RLCS and RHCS, respectively ().
Figure 3. Effects of ginsenosides on the contractility of isolated jejunal segement (IJS) pretreated with tetrodotoxin (TTX). Contractile amplitude of IJS in the normal contractile state is set to a relative value of 100% (normal control, NC). Other data are the relative values compared with NC. Data are expressed as the mean ± SEM (% NC, n = 6); *p < 0.05, **p < 0.01 compared with contractile amplitude of IJS after treatment with TTX (0.1 µmol/L). RLCS: Representative low contractile state; RHCS: Representative high contractile state.
Ginsenosides (20 mg/L), did not exert further modulation on the contractility of IJS under Ca2+ free assay condition or pre-incubated with the Ca2+ channel blocker verapamil (1 µmol/L) ().
Figure 4. Effects of ginsenosides on the contractility of isolated jejunal segement (IJS) pretreated with receptor antagonist. Effects of ginsenosides on the contractility of IJS pretreated with (A) Ca2+ free Krebs’ buffer or verapamil (1 µmol/L) (B) 10 μmol/L atropine and 10 μmol/L diphenhydramine (in the representative low contractile state, RLCS); 10 μmol/L phentolamine, 5 μmol/L propranolol and 10 μmol/L l-NG-nitroargenine (l-NNA) (in the representative high contractile state, RHCS).
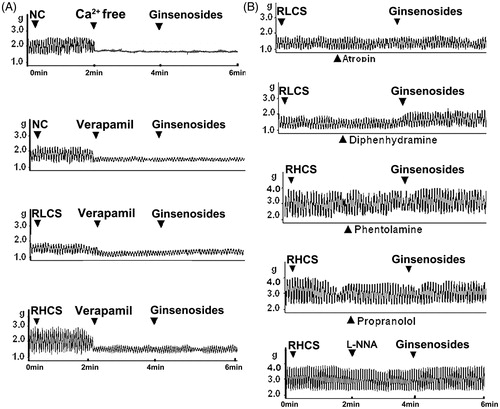
The muscarinic receptor antagonist atropine blocked the stimulatory effects of ginsenosides on the contractility of IJS in RLCS (; ). The histamine H1 receptor antagonist diphenhydramine did not affect ginsenoside-induced stimulatory effect on jejunal contractility in RLCS (; ); α-adrenergic receptor antagonist phentolamine, β-adrenergic receptor antagonist propranolol, and NO synthase inhibitor l-NG-nitro-arginine (l-NNA) blocked ginsenosides-induced inhibitory effects on the contractility of IJS in RHCS, respectively (; ).
Table 1. Effects of ginsenosides on contractile amplitude of jejunal segment pretreated with receptor antagonist.
Effects of ginsenosides on myosin-phosphorylation
Phosphorylation of myosin regulatory light chain (p-MLC20) is primary to initiate smooth muscle contraction. The possible relationship between p-MLC20 and ginsenosides-induced BR was observed at three levels, i.e., p-MLC20, MLCK mRNA expression, and MLCK protein content. As shown in , the p-MLC20 in CP group and DP group was significantly decreased and increased respectively compared with that in the normal control group (p < 0.01, n = 4 rats). Ginsenosides (30 mg/kg once daily for 3 consecutive days) significantly increased p-MLC20 in ginsenosides-treated CP group and significantly decreased p-MLC20 in ginsenosides-treated DP group compared with those of untreated CP and untreated DP groups, respectively (p < 0.01, n = 4 rats).
Figure 5. Effects of ginsenosides on myosin phosphorylation, MLCK contents, mRNA expression, and MLCK contents (A) the phosphorylation of 20-kDa regulatory light chain subunit of myosin (p-MLC20), (B) the protein content of myosin light chain kinase (MLCK) and (C) the expression of MLCK mRNA in the normal control group (NC), constipation-prominent group (CP), diarrhea-prominent group (DP), 30 mg/kg ginsenosides treated CP group (CP + C) and 30 mg/kg ginsenosides treated DP group (DP + C). All the data represent mean ± SEM from four independent experiments; **p < 0.01 compared with the NC.
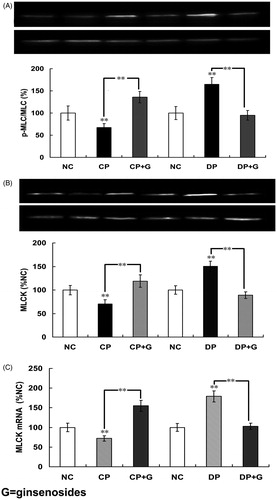
As shown in , the results indicated that the protein content of MLCK in CP group was significantly decreased compared with the normal control group; the protein content of MLCK in the ginsenoside-treated CP group was significantly increased (p < 0.01, n = 10 samples). Results obtained from the DP group indicated that the protein content of MLCK was significantly increased compared with that in the normal control group; the protein content of MLCK in the ginsenoside-treated DP group was significantly decreased (p < 0.01, n = 10 samples). The effects of ginsenosides on the expression of MLCK mRNA were in accordance with the effects of ginsenosides on the protein content of MLCK ().
Effects of ginsenosides on CP and DP rats in vivo
The granules and the moisture content of feces in CP group and DP group rats were significantly decreased and increased, respectively, compared with that in the normal control group. Granules and the moisture contents of feces in the ginsenoside (30 mg/kg) treated CP group was significantly increased compared with that in the untreated CP group. Granules and the moisture contents of feces in the ginsenoside (30 mg/kg) treated DP group were significantly decreased compared with that in the untreated DP group ().
Table 2. Alleviating effects of ginsenosides on constipation and diarrhea symptoms.
Discussion
The results supported our proposal. The effects of ginsenosides on IJS in normal contractile state were dose dependent, namely, ginsenosides increased and decreased the contractility of IJS in the dose range of 5–20 mg/L and 40–80 mg/L, respectively. Ginsenosides in the fixed concentration of 20 mg/L induced BR on the contractility of IJS, i.e., exerted stimulatory effects on the contractility of IJS in all six low contractile states and exerted inhibitory effects on the contractility of IJS in all six high contractile states.
No further stimulatory or inhibitory effects of ginsenosides on IJS were observed when assayed in a Ca2+ free buffer or pretreated with the Ca2+ channel blocker verapamil, implying that the effects of ginsenosides on jejunum were dependent on the influx of extracellular Ca2+. The stimulatory effects of ginsenosides on the contractility of IJS in RLCS were correlated with M-receptor-linked stimulation in jejunum, since they were blocked by the M receptor antagonist atropine. The inhibitory effects of ginsenosides on the contractility of IJS in RHCS were correlated with adrenergic α-, β-receptors, as well as NO-synthase activity-related modulation in jejunum, because phentolamine, propranolol and l-NNA blocked the inhibitory effects of ginsenosides on the contractility of IJS in RHCS, respectively.
Smooth muscle contraction and relaxation are primarily modulated by phosphorylation and dephosphorylation of MLC20 by MLCK and myosin light chain phosphatase, respectively (Jeong et al., Citation2011). In accordance with the modulation on jejunal contractility, the effects of ginsenosides on myosin phosphorylation, MLCK expression, and MLCK contents in jejunal segment determined in low-high contractile states were also bidirectional, indicating that ginsenosides-induced stimulatory and inhibitory effects are related to the phosphorylation and dephosphorylation of MLC20, respectively.
Effects of TTX on the ginsenosides-induced BR were also observed. In the presence of TTX, ginsenosides only exerted inhibitory effect on the contractility of IJS in the normal contractile state, RLCS and RHCS. The results indicated that ginsenoside-induced BR was dependent on the presence of ENS control.
Conclusion
Ginsenoside-induced BR needs the presence of ENS, depends on the influx of extracellular Ca2+, relates to the cholinergic system while IJS is in low contractile states, and relates to the adrenergic system and NO relaxing mechanism while IJS is in high contractile states. The myosin light chain kinase related signaling pathway is also involved in ginsenoside-induced BR.
Further study is required since it remains unclear as to the detailed mechanisms for ginsenosides-induced BR, including how dozens of neurotransmitters in the jejunum are interrelated in various contractile states and how they are integrated in regulation. The results suggested the potential clinical implication of ginsenosides for alternating irritable bowel syndrome.
Declaration of interest
The authors wish to acknowledge the financial support of National Natural Science Foundation of China (grant No. 30772601).
Acknowledgements
The authors wish to thank Zhi Lin and Fun Yuan for their valuable comments.
References
- Barthó L, Lefebvre RA. (1994). Nitric oxide causes contraction in the rat isolated small intestine. Eur J Pharmacol 259:101–4
- Chen DP, Xiong YJ, Wang L, et al. (2012). Characteristics of emodin on modulating the contractility of jejunal smooth muscle. Can J Physiol Pharm 90:455–62
- Christensen LP. (2009). Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res 55:1–99
- Frings M, Haschke G, Heinke B, et al. (2000). Spontaneous contractions of intestinal smooth muscle reaggregates from the new born rat triggered by thromboxane A2. J Vet Med A-Physiol Pathol Clin Med 47:469–75
- Furukawa Y, Shiga Y, Hanyu N, et al. (1995). Effect of Chinese herbal medicine on gastrointestinal motility and bowel obstruction. Jpn J Gastroenterol Surg 28:956–60
- Gong Li, Li SL, Li H, Zhang L. (2011). Ginsenoside Rg1 protects primary cultured rat hippocampal neurons from cell apoptosis induced by β-amyloid protein. Pharm Biol 49:501–7
- Han H, Chen Y, Bi H, et al. (2011). In vivo antimalarial activity of ginseng extracts. Pharm Biol 49:283–9
- Han S, Kim JS, Jung BK, et al. (2012). Effects of ginsenoside on pacemaker potentials of cultured interstitial cells of Cajal clusters from the small intestine of mice. Mol Cells 33:243–9
- Hashimoto K, Satoh K, Kase Y, et al. (2001). Modulatory effect of aliphatic acid amides from Zanthoxylum piperitum on isolated gastrointestinal tract. Planta Med 67:179–81
- Iwabu A, Smith K, Allen FD, et al. (2004). Epidermal growth factor induces fibroblast contractility and motility via a protein kinase C δ-dependent pathway. J Biol Chem 279:14551–60
- Jeong SI, Kwon OD, Kwon SC, Jung KY. (2011). Signalling pathways responsible for the methylisogermabullone-induced contraction of ileal longitudinal muscles. J Pharm Pharmacol 63:245–52
- Kim HJ, Kang HJ, Seo JY, et al. (2011). Antiobesity effect of oil extract of ginseng. J Med Food 14:573–83
- Kim HS, Parajuli SP, Yeum CH, et al. (2007). Effects of ginseng total saponins on pacemaker currents of interstitial cells of Cajal from the small intestine of mice. Biol Pharm Bull 30:2037–42
- Kobayashi K, Murata T, Hori M, Ozaki H. (2011). Prostaglandin E2-prostanoid EP3 signal induces vascular contraction via nPKC and ROCK activation in rat mesenteric artery. Eur J Pharmacol 660:375–80
- La JH, Kim TW, Sung TS, et al. (2003). Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol 9:2791–5
- Leung KW, Wong AS. (2010). Pharmacology of ginsenosides: A literature review. Chin Med 5:20
- Mathison R, Shaffer E. (2006). Increased cholinergic contractions of jejunal smooth muscle caused by a high cholesterol diet are prevented by the 5-HT4 agonist-tegaserod. BMC Gastroenterol 6:1–9
- Schemann M. (2005). Control of gastrointestinal motility by the ‘‘Gut Brain’’ -- The enteric nervous system. J Pediatr Gastr Nutr 41:S4–6
- Shi YH, Han B, Yu XF, et al. (2011). Ginsenoside Rb3 ameliorates myocardial ischemia--reperfusion injury in rats. Pharm Biol 49:900–6
- Surh YJ, Na HK, Lee JY, Keum YS. (2001). Molecular mechanisms underlying anti-tumor promoting activities of heat-processed Panax ginseng C.A. Meyer. J Korean Med Sci 16:S38–41
- Tawab MA, Bahr U, Karas M, et al. (2003). Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos 31:1065–71
- Xu JR, Luo J, Shang L, Kong WM. (2006). Effect of change in an inhibitory neurotransmitter of the myenteric plexus on the pathogenetic mechanism of irritable bowel syndrome subgroups in rat models. Chin J Dig Dis 7:89–96

