Abstract
Context: Nasturtium officinale R. Br. (watercress) has long been used in Iranian folk medicine to treat hypertension, hyperglycemia, and renal colic. Moreover, anticancer, antioxidant, and hepatoprotective properties of N. officinale have been reported.
Objective: In this study, anti-inflammatory activity of the hydro-alcoholic extract from aerial parts of N. officinale was investigated.
Materials and methods: Oral administration of the hydro-alcoholic extract of N. officinale (250, 500 and 750 mg kg−1) was investigated on two well-characterized animal models of inflammation, including carrageenan- or formalin-induced paw edema in rats. Then, the topical anti-inflammatory effect of N. officinale (2 and 5 mg/ear) was studied on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse ear edema. Finally, biopsy of the paw or ear was performed for pathological evaluation.
Results: Acute toxicity tests of N. officinale in rats established an oral LD50 of >5 g kg−1. The extract of watercress (250, 500 and 750 mg kg−1) significantly inhibited carrageenan-induced paw edema 1, 2, 3 and 4 h after carrageenan challenge (p < 0.001). The extract (500 mg kg−1) also showed considerable activity against formalin-evoked paw edema over a period of 24 h (p < 0.001). Furthermore, topical application of N. officinale (5 mg/ear) reduced TPA-induced ear edema (p < 0.05). Histopathologically, the extract decreased swelling and the tissue damage induced by carrageenan or TPA.
Discussion and conclusion: Our findings indicate potent anti-inflammatory activity of N. officinale in systemic and topical application and propose its potential as an anti-inflammatory agent for treatment of inflammatory conditions.
Introduction
Inflammation is one of the most important physiological reactions of a body to stimuli such as irritation, trauma, tissue injury, and infection, but excessive or persistent inflammation results in a variety of pathological conditions or organ damage (Dinarello, Citation1997). Usually, inflammation develops through infiltration of leukocytes to the injury sites and production of specific cytokines such as IL-1β and TNF-α. Reactive oxygen species (ROS) also are released during the inflammation process to exert a protective effect against invading pathogens (Frode et al., Citation2009; Reuter et al., Citation2010) Nowadays, non-steroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids are the main option for treatment of inflammatory disorders. These drugs exert their anti-inflammatory activities through inhibition of the biosynthesis of prostaglandins and some pro-inflammatory cytokines (Rainsford, Citation2007). However, serious side effects of the available anti-inflammatory agents such as peptic ulcer, renal dysfunction, and cardiovascular problems have complicated their prescription (Da Silva et al., Citation2006; Ng & Chan, Citation2010). Therefore, introducing a new anti-inflammatory drug, especially from a natural origin, with lesser side effects than NSAIDs or glucocorticoids, seems to be necessary.
Nasturtium officinale R. Br. (Brassicaceae) is a perennial plant that grows in Europe and some parts of Asia. Watercress is usually eaten in fresh form in salads, soups and other recipes. The leaves of the plant also are broadly used as a diuretic, expectorant, and anti-diabetic agent (Deni, Citation1995, Citation2003; Launert, Citation1981). Several studies propose hepatoprotective and beneficial effects of watercress in the management of some types of cancer (Boyd et al., Citation2006; Natanzi et al., Citation2010; Xiao et al., Citation2010). Furthermore, Yazdanparast et al. (Citation2008) has reported that watercress supplementation in the diet reduces serum lipids and alters blood antioxidant status in hyper-cholesterolaemic rats (Bahramikia & Yazdanparast, Citation2008). They also have shown that N. officinale has potent antioxidant properties with in vitro conditions (Bahramikia & Yazdanparast, Citation2010). To our knowledge, there is no study about the possible anti-inflammatory effects of N. officinale with either in vivo and in vitro conditions. Therefore, the objective of the present work was to investigate the anti-inflammatory effects of a hydro-alcoholic extract from aerial parts of N. officinale in different experimental models of inflammation.
Materials and methods
Plant materials
Aerial parts of N. officinale were collected from the suburbs of Yasuj (Iran) at the end of spring 2012 and identified by Dr. A. Jafari (Department of Botany, Center for Research in Natural Resource and Animal Husbandry, Yasuj University, Yasuj, Iran) and a voucher specimen (Herbarium No. HYU30230) was deposited there. The aerial parts of the plant were air-dried and protected from direct sunshine.
Preparation of hydro-alcoholic extract
The powdered plant parts (300 g) were extracted two-times with a l000 mL mixture of EtOH-H2O (7:3) at 45 °C for 48 h. The extract was filtered and organic solvent was completely evaporated under reduced pressure in a rotary evaporator at 60 °C. Then, the concentrated extract was dried at room temperature. The average yield ratio of the ethanol extract was approximately 20.5%.
Experimental animals
Adult male Wistar rats (200–250 g) and adult male Swiss albino mice (25–35 g) were obtained from the Pasteur Institute of Iran (Tehran, Iran). The animals were allowed free access to normal diet and water. They were kept in groups of four per standard cage, under a 12:12 h light–dark cycle at 24 ± 2 °C. The experiments were carried out in accordance with local guidelines for the care of laboratory animals of the Yasuj University of Medical Sciences.
Chemicals
Carrageenan (lambda) was purchased from Fluka Chemical (Switzerland). Indomethacin and 12-O-tetra-decanoyl phorbol-13-acetate (TPA) were purchased from Sigma-Aldrich (St. Louis, MO). Formalin was obtained from Merck Co. (Darmstadt, Germany).
Carrageenan-induced paw edema in rats
The method of inflammation used in the present study was similar to that described in our previous work (Hajhashemi et al., Citation2010; Sadeghi et al., Citation2011). At first, the animals were pretreated with the extract (200, 500 and 750 mg kg−1, p.o.) or indomethacin (10 mg kg−1, p.o. as a reference drug) 45 min before sub-plantar injection of carrageenan. Then, the rats received 100 µl of a 1% (w/v) suspension of carrageenan in the right hind paw (Winter et al., Citation1962). The paw thickness was recorded from the ventral to the dorsal surfaces using a digital caliper (Mitutoyo, Japan) immediately before carrageenan injection and again at 1, 2, 3, and 4 h after that. The data were expressed as the variation in paw thickness (mm) and were compared to pre-injection values (Bawa & Khanum, Citation2009). After measuring the paw thickness, the animals were euthanized by ether and the right paws removed and fixed in 10% formaldehyde solution for histopathological examination.
Formalin-induced edema in the rat paw
Pedal edema was induced by injection of 0.1 ml formalin (2%) into the sub-planter area of right hind paw of the rats (Amresh et al., Citation2007; Fotio et al., Citation2009). The extract (500 mg kg−1, p.o.) and indomethacin (10 mg kg−1, p.o.) were given 45 min prior to formalin challenge. The thickness of the paw was measured 1, 2, 3, 4 and 24 h after formalin injection.
TPA-induced mouse ear edema
Ear edema was induced by topical application of 20 µL of TPA (2.5 mg/ear) dissolved in acetone to both surfaces of the right ear of each mouse (De Young et al., Citation1989). The extract was dissolved in acetone and applied topically (1, 2 and 5 mg/ear) immediately after TPA. Indomethacin (0.5 mg/ear), as a standard drug, was dissolved in acetone and administered immediately after TPA. The animals were euthanized 4 h after the TPA application and two ear punches (6 mm diameter) were taken from each mouse. Edema was measured by the increase in ear weight (mg) because of TPA application. Swelling inhibition was expressed as the decrease in weight compare to the control group. Three ear samples were fixed in formalin 10% for histological examination.
Histological examination
Three samples of ears or paws from the control and extract treated animals were taken and fixed in 10% formaldehyde solution for 1 week. Then, the fixed biopsies were embedded in paraffin and cut into 3–4 μm slices. The slices were mounted on glass slides and stained with hematoxylin and eosin for pathological analysis.
Gastric-ulcerogenic assessment
After anti-inflammatory evaluation in the carrageenan test, the rats were euthanized by ether anesthesia and the stomachs of the rats were collected. Next, the removed stomach of each animal was cut through the greater curvature. Finally, the mucosal surface was washed with normal saline and observed with a convex lens for possible injuries or bleeding (Akkol et al., Citation2008).
Acute toxicity
In order to determine the plant LD50, three groups of rats (n = 6) received the hydro-alcoholic extract (1, 3 and 5 g kg−1; p.o.). The animals were observed during 48 h (Lorke, Citation1983).
Statistical analysis
The data were expressed as the means ± SEM. The differences between the control and treatment groups were tested by ANOVA followed by the Tukey post-hoc test, using SPSS 13.0 software. The probability of p < 0.05 was considered to show significant differences for all comparisons made.
Results
Carrageenan-induced paw edema in rat
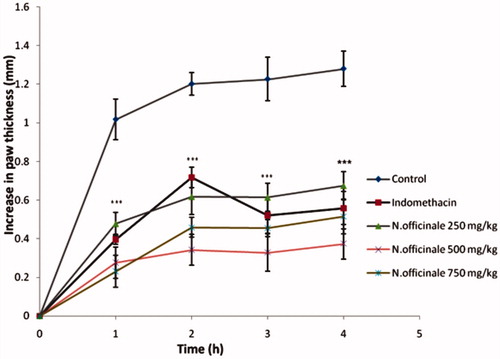
shows that sub-plantar injection of carrageenan produced a prominent increase in paw thickness, beginning immediately after carageenan injection and reaching a peak after 4 h. Oral treatment of the animals with different doses of the ethanol extract of N. officinale (250, 500 and 750 mg kg−1) noticeably inhibited carrageenan-induced paw edema over of a period of 4 h (p < 0.001). The anti-inflammatory activities of the extract at 500 or 750 mg kg−1 were significant compared with indomethacin (10 mg kg−1).
Figure 1. Effect of the hydro-alcoholic extract from the aerial parts of Nasturtium officinale (250, 500 and 750 mg/kg) and indomethacin (10 mg/kg) on carrageenan-induced paw edema in rats. Each point represents the mean ± S.E.M of six animals; ***p < 0.001 statistically significant compared to their respective control.
Formalin-induced paw edema in rat
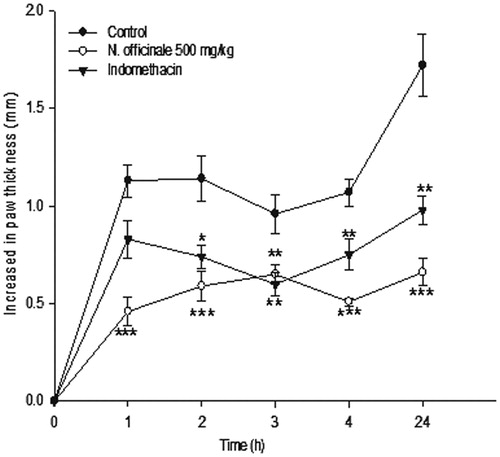
Sub-plantar injection of formalin induced a significant inflammation in the hind paw of the rats. The most effective dose of extract (i.e., 500 mg kg−1) in the carrageenan test was chosen for the formalin test. As shown in , development of formalin-induced paw edema significantly was prevented by the hydro-alcoholic extract of N. officinale at 1, 2, 3, 4 and 24 h after injection of formalin (p < 0.001). As expected, indomethacin (10 mg kg−1) also reduced the paw edema in the formalin test.
Figure 2. Effect of the hydro-alcoholic extract from the aerial parts of Nasturtium officinale (500 mg/kg) and indomethacin (10 mg/kg) on formalin-induced paw edema in rats. Each point represents the mean ± S.E.M of six animals; ***p < 0.001 statistically significant compared to their respective control.
TPA-induced mouse ear edema
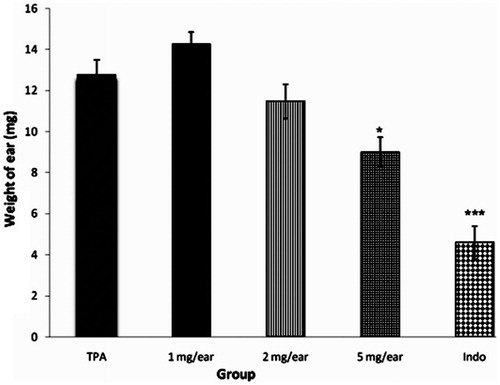
As shown in , ear weight noticeably increased 4 h following topical application of TPA. The extract of N. officinale (1, 2, 5 mg/ear) and indomethacin (0.5 mg/ear) were applied topically immediately after TPA. Although the lower doses of extract (1 and 2 mg/ear) did not produce a noticeable effect on the ear edema, the dose of 5 mg/ear exhibited a prominent inhibitory effect when compared to the control group. As expected, indomethacin also elicited a significant anti-edematogenic effect on the TPA-evoked ear edema.
Figure 3. Effect of hydro-alcoholic extract from the aerial parts of Nasturtium officinale (1 mg/ear, 2 mg/ear and 5 mg/ear) and indomethacin (0.5 mg/ear) on TPA-induced ear edema in mice. Data represent mean ± S.E.M. of six animals; *p < 0.05 and ***p < 0.001 statistically significant compared to their respective control.
Histological examination
As shown in , histological examination of the paw tissues of the control group (carrageenan-treated group), 4 h after carrageenan, revealed epidermal hyperplasia and marked infiltration of inflammatory cells such as lymphocytes and neutrophil associated with congestion of vessels and edema. These inflammatory signs were greatly reduced by oral administration of the hydro-alcoholic extract of N. officinale (250, 500 and 750 mg kg−1) and indomethacin (10 mg kg−1).
Figure 4. (a) Histological changes in rat paws after sub-plantar injection of carrageenan. (A) Normal paw. (B) Carrageenan-induced edema, and infiltration of leukocytes especially neutrophil. (C) Indomethacin (10 mg/kg, oral.) decreased paw swelling and infiltration of neutrophil. Oral pretreatment of rats with Nasturtium officinale at 250 (D), 500 (E) and 750 (F) mg/kg doses inhibited development of edema and migration of neutrophils, compared with carrageenan-treated paw. (b) Pathological examination of ear tissues after topical application of TPA. (A) Normal ear, (B) Control: Topical application of TPA (2.5 mg/ear) induced inflammatory lesion with edema and epidermal hyperplasia. (C) Topical application of the extract from the aerial parts of Nasturtium officinale (5 mg/ear) immediately after TPA reduced edema and epidermal hyperplasia induced by TPA. (D) Indomethacin (0.5 mg/ear) also decreased edema and ear hyperplasia. Sections were stained with hematoxyline and eosin, magnification ×20.
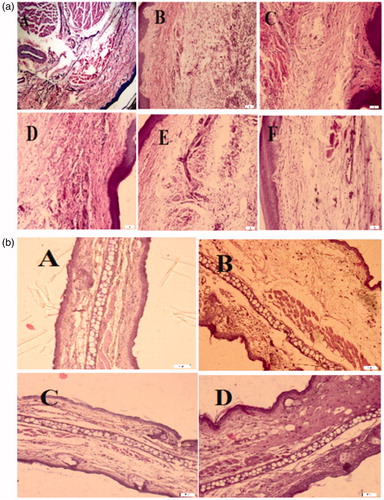
Histological examination of the ears treated with TPA () showed a prominent increase of epidermis thickness and edema, when compared to the control group. Either topical application of the extract (5 mg/ear) or indomethacin (0.5 mg/ear) were able to decrease these inflammatory events.
Gastric-ulcerogenic assessment
Gastric evaluation of the rats treated with the extract at 200, 500 and 750 mg kg−1 (carrageenan test) did not show any gastric lesions or bleeding in comparison to the control group.
Acute toxicity
No animals died during 48 h following oral administration of different doses of N. officinale (1, 3 and 5 g kg−1), nor were any side effects observed in animals treated with the extract. These results indicate that the hydro-alcoholic of the plant was not toxic in rats up to an oral dose of 5 g kg−1.
Discussion
The findings of the present study clearly demonstrate the potent anti-inflammatory effects of the hydro-alcoholic extract from the aerial parts of N. officinale in different animal models of inflammation, i.e., carrageenin- or formaldehyde- induced paw edema in rat, and TPA-evoked ear edema in mice.
The carrageenan-induced paw edema is a time-dependent and biphasic inflammation reaction. In the first phase (0–1 h after injection of carrageenan) histamine, bradykinin, and serotonin are involved (Crunkhorn & Meacock, Citation1971; Vinegar et al., Citation1969), while the second phase (after 1 h) is attributed to infiltration of leucocytes, production of oxygen-derived free radicals and release of prostaglandins, protease, and lysosome (Di et al., Citation1971; Halici et al., Citation2007; Vircheva et al., 2011). The ethanol extract of N. officinale exhibited prominent protective effects on the development of paw swelling by carrageenan over a period of 4 h. This finding indicates the inhibitory effects of N. officinale against release of the pro-inflammatory mediators involved in the two phases of the carageenan test (Just et al., Citation1998). Our pathological examination confirmed that the watercress extract reduced edema the migration of leucocytes into carrageenan-treated paws.
Intraplantar injection of formaldehyde also produces a biphasic event. The early phase or neurogenic phase is attributed to the release of substance P and bradykinin, while in the delay phase histamine, serotonin, prostaglandins and bradykinin are involved (Chen et al., Citation1995; Wheeler-Aceto & Cowan, Citation1991). In the formaldehyde test, the ethanol extract of N. officinale elicited a significant anti-inflammatory activity lasting until 24 h, indicating its long duration of action.
It has been reported that phenolic and flavonoid phytochemicals produce strong anti-inflammatory activities (Peluso et al., Citation2013; Sone et al., Citation2011). The presence of phenolic and flavonoid constitutes in the crude extract from N. officinale has been demonstrated (Mazandarani et al., Citation2012). Rutin, chlorogenic, and caffeic acids were quantified in the extract (Boligon et al., Citation2013). Furthermore, anti-inflammatory effects of three indicated components have been reported (Chagas-Paula et al., Citation2011; Gamaro et al., Citation2011; Selloum et al., Citation2003). Therefore, these phenolic components in the pant could play an important role in its anti-inflammatory activity.
Moreover, the ethanol extract of N. officinale (5 mg/ear), like indomethacin, elicited considerable anti-edematogenic activities in TPA-induced ear edema in mice. TPA-induced ear edema is a useful model of skin inflammation for assessment of topical and systemic anti-inflammatory compounds. TPA induces local inflammation with leucocyte infiltration, edema development and the generation of ROS, as a consequence of activation of protein kinase C (PKC). This enzyme activates other enzymatic pathways such as phospholipase A2 (PLA2) and mitogen activated protein kinases (MAPK), resulting in release of platelet activation factor (PAF) and arachidonic acid. These events either induce vasodilatation, vascular permeability, and secretion of serotonin and histamine or stimulate synthesis of prostaglandins and leukotrienes by cyclooxygenase (COX) and 5-lipoxygenase (5-LOX) enzymes, respectively (Carlson et al., Citation1985; Fischer et al., Citation1988; Rao et al., Citation1993). Based on this, one possible mechanism for the anti-inflammatory effects of the extract, in the TPA model, is its interaction with the infiltration of leucocytes (according to the pathological results) and regulation of release or function of inflammatory eicosanoids. Macroscopic evaluation of the gastric mucosa in the extract-treated groups (in the carrageenan test) did not reveal any tissue lesions or bleeding, while peptic ulcer is one of the most important side effect of NSAIDs. This adverse effect of NSAIDs is attributed to their ability to inhibit COX-1, which catalyzes the synthesis of prostaglandins (Lai & Chan, Citation2009; Ng & Chan, Citation2010). Production of prostaglandins by COX-1 is important in the maintenance of normal function of some organs such as kidney, stomach, whereas those generated by COX-2 contribute to the inflammatory development (Mitchell & Warner, Citation1999). Therefore, the anti-inflammatory effects without gastric damage led us to believe that the active ingredient of N. officinale did not inhibit COX-1. Generation of ROS has an important role in the development of both TPA and carrageenan-induced inflammation (Fischer et al., Citation1988; Vircheva et al., 2011). Furthermore, some researchers have established antioxidant effects of N. officinale (Bahramikia & Yazdanparast, Citation2010; Yazdanparast et al., Citation2008), hence another possibility is that antioxidant effects of the plant, at least, play a partial role in its anti-inflammatory activities.
Conclusions
These results clearly prove the anti-inflammatory effects of systemic and topical administration of the hydro-alcoholic extract of watercress in experimental animals and support the potential usage of this plant in the management of inflammatory diseases, without any damage to gastric mucosa.
Declaration of interest
This work was supported by Medicinal Plants Research Center, Yasuj University of Medical Sciences.
References
- Akkol EK, Yalcin FN, Kaya D, et al. (2008). In vivo anti-inflammatory and antinociceptive actions of some Lamium species. J Ethnopharmacol 118:166–72
- Amresh G, Reddy GD, Rao C, Singh PN. (2007). Evaluation of anti-inflammatory activity of Cissampelos pareira root in rats. J Ethnopharmacol 110:526–31
- Bahramikia S, Yazdanparast R. (2008). Effect of hydroalcoholic extracts of Nasturtium officinale leaves on lipid profile in high-fat diet rats. J Ethnopharmacol 115:116–21
- Bahramikia S, Yazdanparast R. (2010). Antioxidant efficacy of Nasturtium officinale extracts using various in vitro assay systems. J Acupunct Meridian Stud 3:283–90
- Bawa AS, Khanum F. (2009). Anti-inflammatory activity of Rhodiola rosea – “A second-generation adaptogen”. Phytother Res 23:1099–102
- Boligon AA, Vanessa Janovik V, et al. (2013). HPLC analysis of polyphenolic compounds and antioxidant activity in Nasturtium officinale. Int J Food Properties 16:61–9
- Boyd LA, McCann MJ, Hashim Y, et al. (2006). Assessment of the anti-genotoxic, anti-proliferative, and anti-metastatic potential of crude watercress extract in human colon cancer cells. Nutr Cancer 55:232–41
- Carlson RP, O'Neill-Davis L, Chang J, Lewis AJ. (1985). Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacologic agents. Agents Actions 17:197–204
- Chagas-Paula DA, Oliveira RB, da Silva VC, et al. (2011). Chlorogenic acids from Tithonia diversifolia demonstrate better anti-inflammatory effect than indomethacin and its sesquiterpene lactones. J Ethnopharmacol 136:355–62
- Chen YF, Tsai HY, Wu TS. (1995). Anti-inflammatory and analgesic activities from roots of Angelica pubescens. Planta Med 61:2–8
- Crunkhorn P, Meacock SC. (1971). Mediators of the inflammation induced in the rat paw by carrageenin. Br J Pharmacol 42:392–402
- Da Silva JA, Jacobs JW, Kirwan JR, et al. (2006). Safety of low dose glucocorticoid treatment in rheumatoid arthritis: Published evidence and prospective trial data. Ann Rheum Dis 65:285–93
- De Young LM, Kheifets JB, Ballaron SJ, Young JM. (1989). Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions 26:335–41
- Deni B. (1995). Encyclopedia of Herbs and Their Uses. London: Dorling Kindersley
- Deni B. (2003). Rhs Encyclopedia of Herbs and Their Uses, 3rd ed. London: Dorling Kindersley
- Di RM, Giroud JP, Willoughby DA. (1971). Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and terpentine. J Pathol 104:15–29
- Dinarello CA. (1997). Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest 112:321S–29S
- Fischer SM, Baldwin JK, Jasheway DW, et al. (1988). Phorbol ester induction of 8-lipoxygenase in inbred SENCAR (SSIN) but not C57BL/6J mice correlated with hyperplasia, edema, and oxidant generation but not ornithine decarboxylase induction. Cancer Res 48:658–64
- Fotio AL, Dimo T, Nguelefack TB, et al. (2009). Acute and chronic anti-inflammatory properties of the stem bark aqueous and methanol extracts of Sclerocarya birrea (Anacardiaceae). Inflammopharmacology 17:229–37
- Frode TS, Buss ZS, dos Reis GO, Medeiros YS. (2009). Evidence of anti-inflammatory effects of pioglitazone in the murine pleurisy model induced by carrageenan. Int Immunopharmacol 9:1394–400
- Gamaro GD, Suyenaga E, Borsoi M, et al. (2011). Effect of rosmarinic and caffeic acids on inflammatory and nociception process in rats. ISRN Pharmacol 2011:451682
- Hajhashemi V, Sadeghi H, Minaiyan M, et al. (2010). The role of central mechanisms in the anti-inflammatory effect of amitriptyline on carrageenan-induced paw edema in rats. Clinics (Sao Paulo) 65:1183–7
- Halici Z, Dengiz GO, Odabasoglu F, et al. (2007). Amiodarone has anti-inflammatory and anti-oxidative properties: An experimental study in rats with carrageenan-induced paw edema. Eur J Pharmacol 566:215–21
- Just MJ, Recio MC, Giner RM, et al. (1998). Anti-inflammatory activity of unusual lupane saponins from Bupleurum fruticescens. Planta Med 64:404–7
- Lai LH, Chan FK. (2009). Nonsteroid anti-inflammatory drug-induced gastroduodenal injury. Curr Opin Gastroenterol 25:544–8
- Launert E. (1981). The Hamlyn Guide to Edible Medicinal Plants of Britain and Northern Europe. London, UK: Hamlyn Publishing Group Limited
- Lorke D. (1983). A new approach to practical acute toxicity testing. Arch Toxicol 54:275–87
- Mazandarani M, Momeji A, Zarghami MP. (2012). Evaluation of phytochemical and antioxidant activities from different parts of Nasturtium officinale R. Br. in Mazandaran. Iranian J Plant Physiol 3:659–64
- Mitchell JA, Warner TD. (1999). Cyclo-oxygenase-2: Pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br J Pharmacol 128:1121–32
- Natanzi ARE, Ghahremani MH, Esfehani HRM, et al. (2010). Evaluation of antihepatotoxic effect of watercress extract and its fractions in rats. Int J Pharmacol 6:896–902
- Ng SC, Chan FK. (2010). NSAID-induced gastrointestinal and cardiovascular injury. Curr Opin Gastroenterol 26:611–17
- Peluso I, Raguzzini A, Serafini M. (2013). Effect of flavonoids on circulating levels of TNF-alpha and IL-6 in humans: A systematic review and meta-analysis. Mol Nutr Food Res 57:784–801
- Rainsford KD. (2007). Anti-inflammatory drugs in the 21st century. Subcell Biochem 42:3–27
- Rao TS, Currie JL, Shaffer AF, Isakson PC. (1993). Comparative evaluation of arachidonic acid (AA)- and tetradecanoylphorbol acetate (TPA)-induced dermal inflammation. Inflammation 17:723–41
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. (2010). Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med 49:1603–16
- Sadeghi H, Hajhashemi V, Minaiyan M, et al. (2011). A study on the mechanisms involving the anti-inflammatory effect of amitriptyline in carrageenan-induced paw edema in rats. Eur J Pharmacol 667:396–401
- Selloum L, Bouriche H, Tigrine C, Boudoukha C. (2003). Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp Toxicol Pathol 54:313–18
- Sone Y, Moon JK, Mai TT, et al. (2011). Antioxidant/anti-inflammatory activities and total phenolic content of extracts obtained from plants grown in Vietnam. J Sci Food Agric 91:2259–64
- Vinegar R, Schreiber W, Hugo R. (1969). Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther 166:96–103
- Vircheva S, Nenkova G, Georgieva A, et al. (2011). Effects of desipramine on the antioxidant status in rat tissues at carrageenan-induced paw inflammation. Cell Biochem Funct 30:18–23
- Wheeler-Aceto H, Cowan A. (1991). Neurogenic and tissue-mediated components of formalin-induced edema: Evidence for supraspinal regulation. Agents Actions 34:264–9
- Winter CA, Risley EA, Nuss GW. (1962). Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 111:544–7
- Xiao D, Powolny AA, Moura MB, et al. (2010). Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. J Biol Chem 285:26558–69
- Yazdanparast R, Bahramikia S, Ardestani A. (2008). Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chem Biol Interact 172:176–84

